Radiological, Pathological, and Surgical Outcomes with Neoadjuvant Cemiplimab for Stage II–IV Cutaneous Squamous Cell Carcinoma in the Deep Sequencing in Cutaneous Squamous Cell Carcinomas (DISCERN) Trial
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Oversight
2.2. Study Population
2.3. Study Design, Treatment and Procedures
2.3.1. Radiological Assessments
2.3.2. Pathological Assessments
2.3.3. Surgical Assessments
2.4. Endpoints
2.5. Statistical Analyses
3. Results
3.1. Patient Disposition and Characteristics
3.2. Clinical Efficacy
3.3. Safety
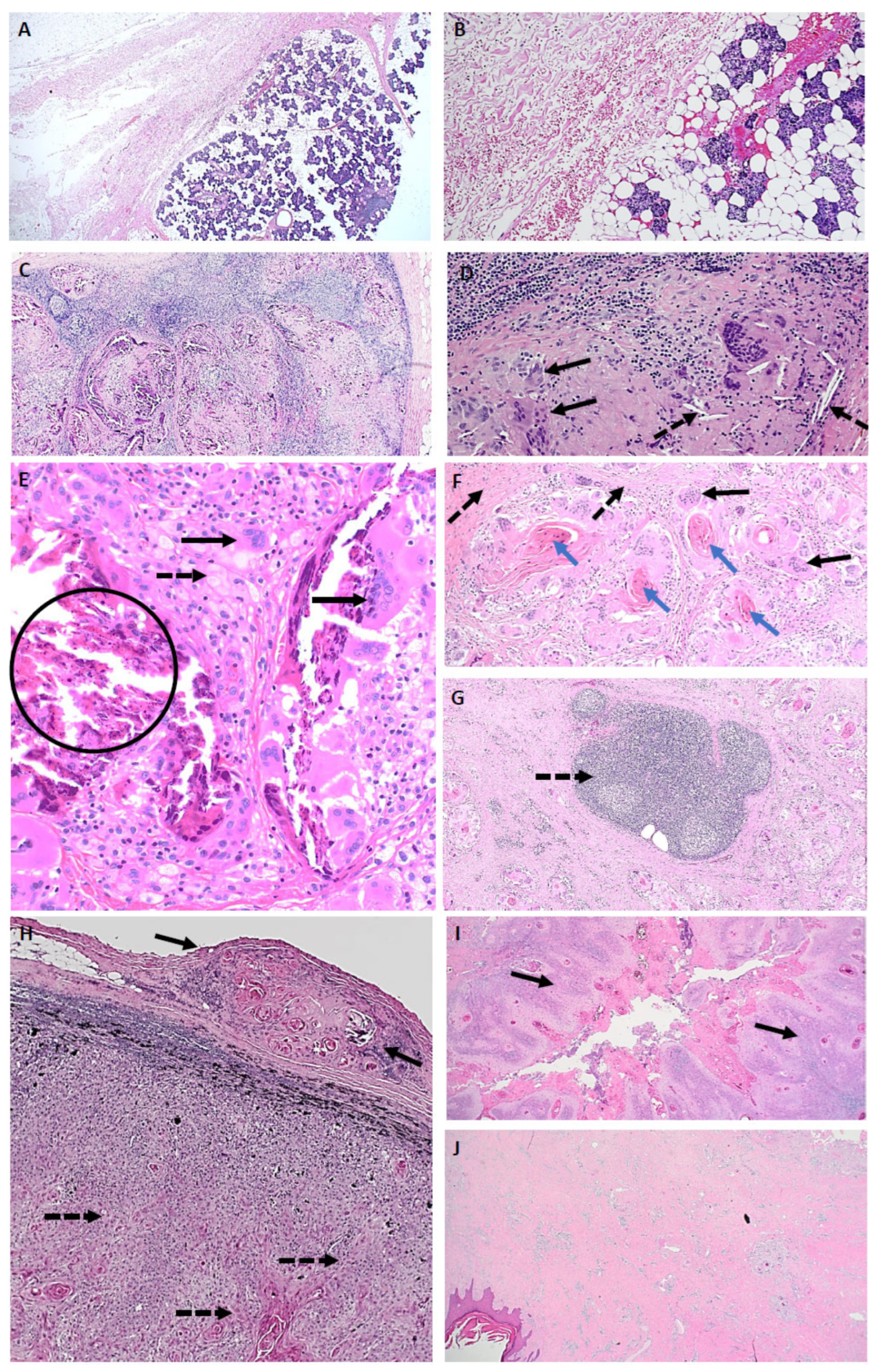
3.4. Pathological Description of Neoadjuvant Response
3.5. Surgical Assessments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse event. |
| CI | Confidence interval. |
| CSCC | Cutaneous squamous cell carcinoma. |
| CMR | Complete metabolic response. |
| CR | Complete response. |
| CT | Computerised tomography. |
| DISCERN | Deep sequencIng in cutaneous Squamous CEll caRciNomas. |
| DFS | Disease-free survival. |
| ECOG | Eastern Cooperative Oncology Group. |
| FDG | Fluorodeoxyglucose. |
| FDG-PET | Fluorodeoxyglucose-positron emission tomography. |
| HIV | Human immunodeficiency virus. |
| ICB | Immune checkpoint blockade. |
| IQR | Interquartile range. |
| irAE | Immune-related adverse event. |
| iPERCIST | Immune PET Response Criteria in Solid Tumours. |
| imRECIST | Immune-Modified Response Evaluation Criteria in Solid Tumours. |
| mPR | Major pathological response. |
| MRI | Magnetic resonance imaging. |
| NTL | Non-target lesion. |
| ORR | Overall response rate. |
| OS | Overall survival. |
| pCR | Pathological complete response. |
| PD | Progressive disease. |
| PD-1 | Programmed Death-1. |
| PD-L1 | Programmed Death Ligand-1. |
| PMR | Partial metabolic response. |
| pNR | Pathological non-responder. |
| PR | Partial response. |
| RECIST | Response Evaluation Criteria in Solid Tumours. |
| RVT | Residual Viable Tumour. |
| SAE | Serious adverse event. |
| SULpeak | Standardised uptake value peak. |
| SUVmax | Standardised uptake value max. |
| TIL | Tumour-infiltrating lymphocytes |
| TL | Target lesion. |
| UPMD | Unconfirmed progressive metabolic disease. |
References
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Perera, E.; Gnaneswaran, N.; Staines, C.; Win, A.K.; Sinclair, R. Incidence and prevalence of non-melanoma skin cancer in Australia: A systematic review. Australas. J. Dermatol. 2015, 56, 258–267. [Google Scholar] [CrossRef]
- Madan, V.; Lear, J.T.; Szeimies, R.M. Non-melanoma skin cancer. Lancet 2010, 375, 673–685. [Google Scholar] [CrossRef]
- Zhang, W.; Zeng, W.; Jiang, A.; He, Z.; Shen, X.; Dong, X.; Feng, J.; Lu, H. Global, regional and national incidence, mortality and disability-adjusted life-years of skin cancers and trend analysis from 1990 to 2019: An analysis of the Global Burden of Disease Study 2019. Cancer Med. 2021, 10, 4905–4922. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, N.; Olsen, C.M.; Whiteman, D.C. The incidence and multiplicity rates of keratinocyte cancers in Australia. Med. J. Aust. 2017, 207, 339–343. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.; Allen, J.; Jones, S.; Nash, R.; Clarke, P. Quality of Life after Orbital Exenteration. J. Neurol. Surg. Part B Skull Base 2014, 75, a143. [Google Scholar] [CrossRef]
- Starkings, R.; Shilling, V.; Jenkins, V.; Fallowfield, L. A structured review of quality of life in advanced and high-risk cutaneous squamous cell carcinoma shows the need for more studies and better measures. Ski. Health Dis. 2021, 1, e39. [Google Scholar] [CrossRef]
- Vaidya, T.S.; Mori, S.; Khoshab, N.; Dusza, S.W.; Bander, T.; Matros, E.; Rossi, A.M.; Nehal, K.S.; Lee, E.H. Patient-reported Aesthetic Satisfaction following Facial Skin Cancer Surgery Using the FACE-Q Skin Cancer Module. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2423. [Google Scholar] [CrossRef]
- Rofail, D.; Ciesluk, A.; Lovell, T.; Marquis, P.; Fury, M.G.; Chen, C.I. A Patient-Relevant Measurement Strategy to Assess Clinical Benefit of Novel Therapies for Non-metastatic Cutaneous Squamous Cell Carcinoma. Oncol. Ther. 2024, 12, 803–815. [Google Scholar] [CrossRef]
- Hughes, B.G.M.; Guminski, A.; Bowyer, S.; Migden, M.R.; Schmults, C.D.; Khushalani, N.I.; Chang, A.L.S.; Grob, J.J.; Lewis, K.D.; Ansstas, G.; et al. A phase 2 open-label study of cemiplimab in patients with advanced cutaneous squamous cell carcinoma (EMPOWER-CSCC-1): Final long-term analysis of groups 1, 2, and 3, and primary analysis of fixed-dose treatment group 6. J. Am. Acad. Dermatol. 2025, 92, 68–77. [Google Scholar] [CrossRef]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef]
- Rischin, D.; Khushalani, N.I.; Schmults, C.D.; Guminski, A.; Chang, A.L.S.; Lewis, K.D.; Lim, A.M.; Hernandez-Aya, L.; Hughes, B.G.M.; Schadendorf, D.; et al. Integrated analysis of a phase 2 study of cemiplimab in advanced cutaneous squamous cell carcinoma: Extended follow-up of outcomes and quality of life analysis. J. Immunother. Cancer 2021, 9, e002757. [Google Scholar] [CrossRef] [PubMed]
- Grob, J.J.; Gonzalez, R.; Basset-Seguin, N.; Vornicova, O.; Schachter, J.; Joshi, A.; Meyer, N.; Grange, F.; Piulats, J.M.; Bauman, J.R.; et al. Pembrolizumab Monotherapy for Recurrent or Metastatic Cutaneous Squamous Cell Carcinoma: A Single-Arm Phase II Trial (KEYNOTE-629). J. Clin. Oncol. 2020, 38, 2916–2925. [Google Scholar] [CrossRef] [PubMed]
- Maubec, E.; Boubaya, M.; Petrow, P.; Beylot-Barry, M.; Basset-Seguin, N.; Deschamps, L.; Grob, J.J.; Dreno, B.; Scheer-Senyarich, I.; Bloch-Queyrat, C.; et al. Phase II Study of Pembrolizumab As First-Line, Single-Drug Therapy for Patients With Unresectable Cutaneous Squamous Cell Carcinomas. J. Clin. Oncol. 2020, 38, 3051–3061. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Amit, M.; Nagarajan, P.; Rubin, M.L.; Yuan, Y.; Bell, D.; El-Naggar, A.K.; Johnson, J.M.; Morrison, W.H.; Rosenthal, D.I.; et al. Pilot Phase II Trial of Neoadjuvant Immunotherapy in Locoregionally Advanced, Resectable Cutaneous Squamous Cell Carcinoma of the Head and Neck. Clin. Cancer Res. 2021, 27, 4557–4565. [Google Scholar] [CrossRef]
- Blank, C.U.; Lucas, M.W.; Scolyer, R.A.; van de Wiel, B.A.; Menzies, A.M.; Lopez-Yurda, M.; Hoeijmakers, L.L.; Saw, R.P.M.; Lijnsvelt, J.M.; Maher, N.G.; et al. Neoadjuvant Nivolumab and Ipilimumab in Resectable Stage III Melanoma. N. Engl. J. Med. 2024, 391, 1696–1708. [Google Scholar] [CrossRef] [PubMed]
- Chalabi, M.; Verschoor, Y.L.; Tan, P.B.; Balduzzi, S.; Van Lent, A.U.; Grootscholten, C.; Dokter, S.; Buller, N.V.; Grotenhuis, B.A.; Kuhlmann, K.; et al. Neoadjuvant Immunotherapy in Locally Advanced Mismatch Repair-Deficient Colon Cancer. N. Engl. J. Med. 2024, 390, 1949–1958. [Google Scholar] [CrossRef]
- Gross, N.D.; Miller, D.M.; Khushalani, N.I.; Divi, V.; Ruiz, E.S.; Lipson, E.J.; Meier, F.; Su, Y.B.; Swiecicki, P.L.; Atlas, J.; et al. Neoadjuvant Cemiplimab for Stage II to IV Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 387, 1557–1568. [Google Scholar] [CrossRef]
- Gross, N.D.; Miller, D.M.; Khushalani, N.I.; Divi, V.; Ruiz, E.S.; Lipson, E.J.; Meier, F.; Su, Y.B.; Swiecicki, P.L.; Atlas, J.; et al. Neoadjuvant cemiplimab and surgery for stage II-IV cutaneous squamous-cell carcinoma: Follow-up and survival outcomes of a single-arm, multicentre, phase 2 study. Lancet Oncol. 2023, 24, 1196–1205. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH E6 (R2) Good Clinical Practice—Scientific Guideline; European Medicines Agency: Amsterdam, Netherlands, 2016. [Google Scholar]
- Amin, M.B.; Edge, S.B. (Eds.) AJCC Cancer Staging Manual, 8th ed.; American Joint Committee on Cancer: Chicago, IL, USA; Springer: Cham, Switzerland, 2017; p. 1024. [Google Scholar]
- O’Sullivan, B.; Brierley, J. (Eds.) UICC Manual of Clinical Oncology, 9th ed.; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2015; p. 1. [Google Scholar]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0; United States Department of Health and Human Services: Washington, DC, USA, 2017.
- Hodi, F.S.; Ballinger, M.; Lyons, B.; Soria, J.C.; Nishino, M.; Tabernero, J.; Powles, T.; Smith, D.; Hoos, A.; McKenna, C.; et al. Immune-Modified Response Evaluation Criteria In Solid Tumors (imRECIST): Refining Guidelines to Assess the Clinical Benefit of Cancer Immunotherapy. J. Clin. Oncol. 2018, 36, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, L.; Duchemann, B.; Chouahnia, K.; Zelek, L.; Soussan, M. Monitoring anti-PD-1-based immunotherapy in non-small cell lung cancer with FDG PET: Introduction of iPERCIST. EJNMMI Res. 2019, 9, 8. [Google Scholar] [CrossRef]
- Lopci, E.; Hicks, R.J.; Dimitrakopoulou-Strauss, A.; Dercle, L.; Iravani, A.; Seban, R.D.; Sachpekidis, C.; Humbert, O.; Gheysens, O.; Glaudemans, A.; et al. Joint EANM/SNMMI/ANZSNM practice guidelines/procedure standards on recommended use of [(18)F]FDG PET/CT imaging during immunomodulatory treatments in patients with solid tumors version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2323–2341. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, T.R.; Thompson, E.D.; Forde, P.M.; Stein, J.E.; Duffield, A.S.; Anagnostou, V.; Rekhtman, N.; Anders, R.A.; Cuda, J.D.; Illei, P.B.; et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: A proposal for quantitative immune-related pathologic response criteria (irPRC). Ann. Oncol. 2018, 29, 1853–1860. [Google Scholar] [CrossRef]
- Stein, J.E.; Lipson, E.J.; Cottrell, T.R.; Forde, P.M.; Anders, R.A.; Cimino-Mathews, A.; Thompson, E.D.; Allaf, M.E.; Yarchoan, M.; Feliciano, J.; et al. Pan-Tumor Pathologic Scoring of Response to PD-(L)1 Blockade. Clin. Cancer Res. 2020, 26, 545–551. [Google Scholar] [CrossRef]
- Tetzlaff, M.T.; Messina, J.L.; Stein, J.E.; Xu, X.; Amaria, R.N.; Blank, C.U.; van de Wiel, B.A.; Ferguson, P.M.; Rawson, R.V.; Ross, M.I.; et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann. Oncol. 2018, 29, 1861–1868. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inf. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Migden, M.R.; Khushalani, N.I.; Chang, A.L.S.; Lewis, K.D.; Schmults, C.D.; Hernandez-Aya, L.; Meier, F.; Schadendorf, D.; Guminski, A.; Hauschild, A.; et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: Results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020, 21, 294–305. [Google Scholar] [CrossRef]
- Ladwa, R.; Lee, J.H.J.; Porceddu, S.V.; McGrath, M.L.; Cooper, C.; Liu, H.; Gupta, R.; Cuscaden, C.; Nottage, M.K.; Clark, J.; et al. A phase 2 study of de-escalation in resectable, locally advanced cutaneous squamous cell carcinoma (cSCC) with the use of neoadjuvant pembrolizumab: De-Squamate. J. Clin. Oncol. 2024, 42, 9514. [Google Scholar] [CrossRef]
- Divi, V.; Aasi, S.; Khan, S.A.; Lam, L.; Martinez Ramirez, L.; Sunwoo, J.B.; Rahman, M.; Ma, Y.; Kurzymski, M.; Baik, F.M.; et al. Neoadjuvant Atezolizumab in Patients With Surgically Resectable Advanced Cutaneous Squamous Cell Carcinoma. JCO Oncol. Adv. 2024, 1, e2400058. [Google Scholar] [CrossRef]
- Zuur, C.L.; Breukers, S.; Machuca-Ostos, M.; Boere, T.; Smit, L.; De Boer, J.P.; Cornelissen, S.; Navran, A.; van Houdt, W.J.; Westerink, B.; et al. Towards organ preservation and cure via 2 infusions of immunotherapy only, in patients normally undergoing extensive and mutilating curative surgery for cutaneous squamous cell carcinoma: An investigator-initiated randomized phase II trial—The MATISSE trial. J. Clin. Oncol. 2023, 41, 9507. [Google Scholar] [CrossRef]
- Amatore, F.; Sridharan, S.; Karunamurthy, A.; Wang, H.; Patel, R.; Pugliano-Mauro, M.; Kim, S.; Choudry, M.H.A.; Pingpank, J.F.; Holtzman, M.P.; et al. Pathologic response rates to neoadjuvant pembrolizumab in locally advanced (LA) resectable cutaneous squamous cell carcinoma (cSCC). J. Clin. Oncol. 2024, 42, 9591. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Bossi, P.; Mandalà, M.; Queirolo, P.; Spagnolo, F.; Bassetto, F.; Rampinelli, V.; Giovacchini, F.; Pennacchioli, E.; Caraco, C.; et al. NEO-CESQ study: Neoadjuvant plus adjuvant treatment with cemiplimab in surgically resectable, high risk stage III/IV (M0) cutaneous squamous cell carcinoma. J. Clin. Oncol. 2023, 41, 9576. [Google Scholar] [CrossRef]
- Mercieca, S.; Belderbos, J.; van Loon, J.; Gilhuijs, K.; Julyan, P.; van Herk, M. Comparison of SUVmax and SUVpeak based segmentation to determine primary lung tumour volume on FDG PET-CT correlated with pathology data. Radiother. Oncol. 2018, 129, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Abgral, R.; Keromnes, N.; Robin, P.; Le Roux, P.Y.; Querellou, S.; Rousset, J.; Valette, G.; Gobel, Y.; Marianowski, R.; Salaun, P. Comparison of prognostic value of tumor SUL-peak and SUV-max on pretreatment FDG-PET/CT in patients with HNSCC. J. Nucl. Med. 2013, 54, 513. [Google Scholar]
- Chen, X.; Bai, G.; Zang, R.; Song, P.; Bie, F.; Huai, Q.; Li, Y.; Liu, Y.; Zhou, B.; Bie, Y.; et al. Utility of (18)F-FDG uptake in predicting major pathological response to neoadjuvant immunotherapy in patients with resectable non-small cell lung cancer. Transl. Oncol. 2023, 35, 101725. [Google Scholar] [CrossRef]
- O, J.H.; Jacene, H.; Luber, B.; Wang, H.; Huynh, M.H.; Leal, J.P.; Wahl, R.L. Quantitation of Cancer Treatment Response by (18)F-FDG PET/CT: Multicenter Assessment of Measurement Variability. J. Nucl. Med. 2017, 58, 1429–1434. [Google Scholar] [CrossRef][Green Version]
- Vanderhoek, M.; Perlman, S.B.; Jeraj, R. Impact of the definition of peak standardized uptake value on quantification of treatment response. J. Nucl. Med. 2012, 53, 4–11. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry: Pathologic Complete Response in Neoadjuvant Treatment of High-Risk Early-Stage Breast Cancer: Use as an Endpoint to Support Accelerated Approval; Food and Drug Administration: Silver Spring, MD, USA, 2012.
- Fumagalli, D.; Bedard, P.L.; Nahleh, Z.; Michiels, S.; Sotiriou, C.; Loi, S.; Sparano, J.A.; Ellis, M.; Hylton, N.; Zujewski, J.A.; et al. A common language in neoadjuvant breast cancer clinical trials: Proposals for standard definitions and endpoints. Lancet Oncol. 2012, 13, e240–e248. [Google Scholar] [CrossRef]
| Characteristic | N (%) | |
|---|---|---|
| Age (range) years | 67 (55–83) | |
| Sex Female: Male | 1:10 | |
| Primary tumour site | Head and neck | 9 (82) |
| Hand | 2 (18) | |
| Overall Stage | III | 3 (27) |
| IV (M0) | 8 (73) | |
| Tumour stage | T1 | 1 (9) |
| T2 | 2 (18) | |
| T3 | 4 (36) | |
| Tx | 4 (36) | |
| Nodal stage | N0 | 2 (18) |
| N1 | 1 (9) | |
| N2a | 1 (9) | |
| N2b | 2 (18) | |
| N2c | 2 (18) | |
| N3a | 1 (9) | |
| N3b | 2 (18) | |
| ECOG performance status score | 0 | 8 (73) |
| 1 | 3 (27) | |
| Median duration of follow-up (IQR) months | 10.2 (6.7–16.4) | |
| Tumour Response | Number (%) | 95% CI |
|---|---|---|
| Pathological Response | ||
| Complete Response | 8/11 (73) | 0.39, 0.93 |
| Non-Responder * | 3/11 (27) | 0.07, 0.61 |
| Imaging Response by RECIST 1.1 | ||
| Partial response | 8/11 (73) | 0.39, 0.93 |
| Progressive disease | 3/11 (27) | 0.07, 0.61 |
| ID | Timepoint | RECIST 1.1 | imRECIST | iPERCIST | Pathological Response |
|---|---|---|---|---|---|
| 001 | Day 75 Overall | PR (−30%) | PR (−30%) | PMR (−61%) Responder | pCR |
| 002 | Day 75 Overall | PR (−58%) | PR (−58%) | * PMR (TL−100%, NTL PMR) Responder | pCR |
| 003 | Day 75 Overall | PR (−31%) | PR (−31%) | CMR (−100%) Responder | pCR |
| 004 | Day 75 Overall | PR (−42%) | PR (−42%) | PMR (−70%) Responder | pCR |
| 005 | Day 75 Overall | PR (−29%) | PR (−29%) | PMR (−44%) Responder | pCR |
| 006 | Day 75 Overall | PR (−43%) | PR (−43%) | PMR (TL−100%, NTL SMD) Responder | pCR |
| 008 | Day 75 Overall | PR (−57%) | PR (−57%) | # CMR (−74%) Responder | pCR |
| 010 | Day 75 Overall | PR (−43%) | PR (−43%) | PMR (−63%) Responder | pCR |
| 009 | $ Day 43 TL NTL New Overall | NE Non-CR/Non-PD No ^ PD | NE Non-CR/Non-PD No ^ PD | UPMD (+87%) UPMD No UPMD Non-responder | pNR & Primary 95% RVT, Epitrochlear node 60% RVT, Axillary nodal 90% RVT |
| 011 | Day 43 TL NTL New Overall | PR (−45%) Non-CR/Non-PD Yes ^^ PD | PR (−45%) Non-CR/Non-PD Yes ^^ PD | SMD (+5%) UPMD Yes UPMD Non-responder | pNR & Primary 15% RVT, Axillary node >50% RVT, New level V nodal disease 100% viable |
| 012 | $ Day 43 TL NTL New Overall | PD (+57%) NA No PD | PD (+57%) NA No PD | UPMD (+31%) NA No UPMD Non-responder | pNR/ progressive disease & Primary 100% viable |
| PATHOLOGICAL COMPLETE RESPONDERS | ||||||
|---|---|---|---|---|---|---|
| ID | Pathological Response (% RVT) | Largest Regression Bed Size | Closest Resection Margin (mm) | Extra-Nodal Extension? | ||
| Primary | Nodal | Primary | Nodal | Regression Bed | Regression Bed | |
| 001 | 0 | 0 | 40 | 18 | <0.1 | Yes |
| 002 | NA | 0 | NA | 16 | 0.2 | No |
| 003 | 0 | NA | 40 | NA | Involved | NA |
| 004 | NA | 0 | NA | 45 | Involved | Yes |
| 005 | NA | 0 | NA | No * | NA * | No * |
| 006 | 0 | NA | NE # | - | Involved | NA |
| 008 | 0 | 0 | 22 | 11 | Involved | No |
| 010 | NA | 0 | NA | 42 | Involved | No |
| PATHOLOGICAL NON-RESPONDERS | ||||||
| ID | Pathological response (% RVT) | Largest area of RVT/total tumour bed (mm) | Closest resection margin (mm) | Extra-nodal extension? | ||
| Primary | Nodal | Primary | Nodal | Viable tumour | Viable tumour | |
| 009 | 95% | Axilla 90% Epitrochlear 60% | 43/50 | Axilla 110/125 Epitrochlear 55/60 | Primary involved Axilla 1 mm Epitrochlear involved | Present |
| 011 | 15% | Axilla > 50% New level V 100% | 55/55 | New Level V 20/20 | Margins clear | Present |
| 012 | 100% | NA | 43/43 | NA | 0.2 | NA |
| Patient ID | Regression Pattern | TIL | Tertiary Lymphoid Structure | Dense Plasma Cells | Cholesterol Clefts | Foamy Macrophages | Neo-Vascularisation | Proliferative Fibrosis | Granulomas | Giant Cells | Immune Exclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 001 | Mixed & | No | Yes | No | No | No | No | Yes | Yes | Yes | No |
| 002 | Mixed | No | No | No | Yes | Yes | No | No | Yes | Yes | No |
| 003 | Fibrotic | No | Yes | No | No | No | No | Yes | Yes | Yes | No |
| 004 | Mixed | No | No | No | No | No | No | Yes | Yes | Yes | No |
| 005 | # Normal | No | No | No | No | No | No | No | No | No | No |
| 006 | Mixed | No | Yes | No | No | No | No | No | Yes | Yes | No |
| 008 | Mixed | No | No | No | No | No | No | No | Yes | Yes | No |
| 010 | Mixed | No | Yes | No | Yes | Yes | No | Yes | Yes | Yes | No |
| 009 | Inflamed | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | No |
| 011 | Mixed | Yes | No | No | No | Yes | No | Yes | Yes | Yes | No |
| 012 | * None | No | No | Yes | No | No | No | No | No | No | Yes |
| Legend | |||||||||||
| Minimal | |||||||||||
| Moderate | |||||||||||
| Dense | |||||||||||
| ID | Planned Surgery | Actual Surgery | PORT Recommended |
|---|---|---|---|
| Complete Pathological Responders | |||
| 001 | WLE of skin left occiput, bilateral SLND +/− local flap reconstruction + PORT | WLE of skin left occiput + rotational cervical flap + right posterolateral neck dissection | No |
| 002 | Parotidectomy and radical neck dissection +/− excision of facial nerve +/− accessory nerve sacrifice + PORT | Parotidectomy + SLND II/III | No |
| 003 | WLE + free flap +/− local flaps (full thickness cheek defect) + PORT | WLE DRAPE 1 (DRAPE 2 not required) | No |
| 004 | SLND + PORT | SLND IIa,b + Va | No |
| 005 | Subtotal parotidectomy +/− facial nerve sacrifice, right selective neck dissection (II/III/Va) + local flaps + PORT | Subtotal parotidectomy with facial nerve preservation + SLND I/II/III/upper V | No |
| 006 | Total lower lip resection (full thickness) + free flap reconstruction with bilateral (levels I–III) neck dissections + PORT | Wedge resection of the lip with SLND (level Ia, Ib bilateral) | No |
| 008 | WLE skin, parotidectomy +/− facial nerve dissection + SLND + PORT | WLE skin, parotidectomy + SLND (no flap required) | No |
| 010 | Parotidectomy +/- facial nerve resection + SLND + PORT | Superficial parotidectomy + SLND levels I/II/III/upper V | No |
| Pathological Non-Responders | |||
| 009 | WLE, metastatectomy, ALND + free flap reconstruction of hand with tendon reconstruction + PORT | WLE with free flap reconstruction and tendon reconstruction, metastatectomy, ALND + flap reconstruction | Yes |
| 011 | WLE first web space + muscles +/− bone burring + regional flap + ALND (two levels) + PORT | Surgery 1: DRAPE 1 hand + ALND. Surgery 2: Re-excision. Surgery 3. Free flap reconstruction hand + right level V SLND | Yes |
| 012 | WLE (full thickness resection) lower lip with SLND, local flap reconstruction + PORT | WLE (full thickness lip) + free flap with bilateral I–III neck dissection | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, A.M.; Baker, B.; Lion, P.; Angel, C.M.; Simmons, J.; Jackson, B.; Magarey, M.; Webb, A.; Nguyen, K.; Hudson, J.; et al. Radiological, Pathological, and Surgical Outcomes with Neoadjuvant Cemiplimab for Stage II–IV Cutaneous Squamous Cell Carcinoma in the Deep Sequencing in Cutaneous Squamous Cell Carcinomas (DISCERN) Trial. Cancers 2025, 17, 1727. https://doi.org/10.3390/cancers17101727
Lim AM, Baker B, Lion P, Angel CM, Simmons J, Jackson B, Magarey M, Webb A, Nguyen K, Hudson J, et al. Radiological, Pathological, and Surgical Outcomes with Neoadjuvant Cemiplimab for Stage II–IV Cutaneous Squamous Cell Carcinoma in the Deep Sequencing in Cutaneous Squamous Cell Carcinomas (DISCERN) Trial. Cancers. 2025; 17(10):1727. https://doi.org/10.3390/cancers17101727
Chicago/Turabian StyleLim, Annette M., Benjamin Baker, Peter Lion, Christopher M. Angel, Jennifer Simmons, Bryce Jackson, Matthew Magarey, Angela Webb, Kevin Nguyen, Jo Hudson, and et al. 2025. "Radiological, Pathological, and Surgical Outcomes with Neoadjuvant Cemiplimab for Stage II–IV Cutaneous Squamous Cell Carcinoma in the Deep Sequencing in Cutaneous Squamous Cell Carcinomas (DISCERN) Trial" Cancers 17, no. 10: 1727. https://doi.org/10.3390/cancers17101727
APA StyleLim, A. M., Baker, B., Lion, P., Angel, C. M., Simmons, J., Jackson, B., Magarey, M., Webb, A., Nguyen, K., Hudson, J., Chin, K. Y., Cardin, A., Ravi, R., Morrison, E., Quinn, T., Hunt, I., & Rischin, D. (2025). Radiological, Pathological, and Surgical Outcomes with Neoadjuvant Cemiplimab for Stage II–IV Cutaneous Squamous Cell Carcinoma in the Deep Sequencing in Cutaneous Squamous Cell Carcinomas (DISCERN) Trial. Cancers, 17(10), 1727. https://doi.org/10.3390/cancers17101727






