The Role of Nanoparticles in Therapy of Real-World Patients with Pancreatic Cancer: A Scoping Review
Simple Summary
Abstract
1. Introduction
1.1. Pancreatic Cancer—Risk Factors
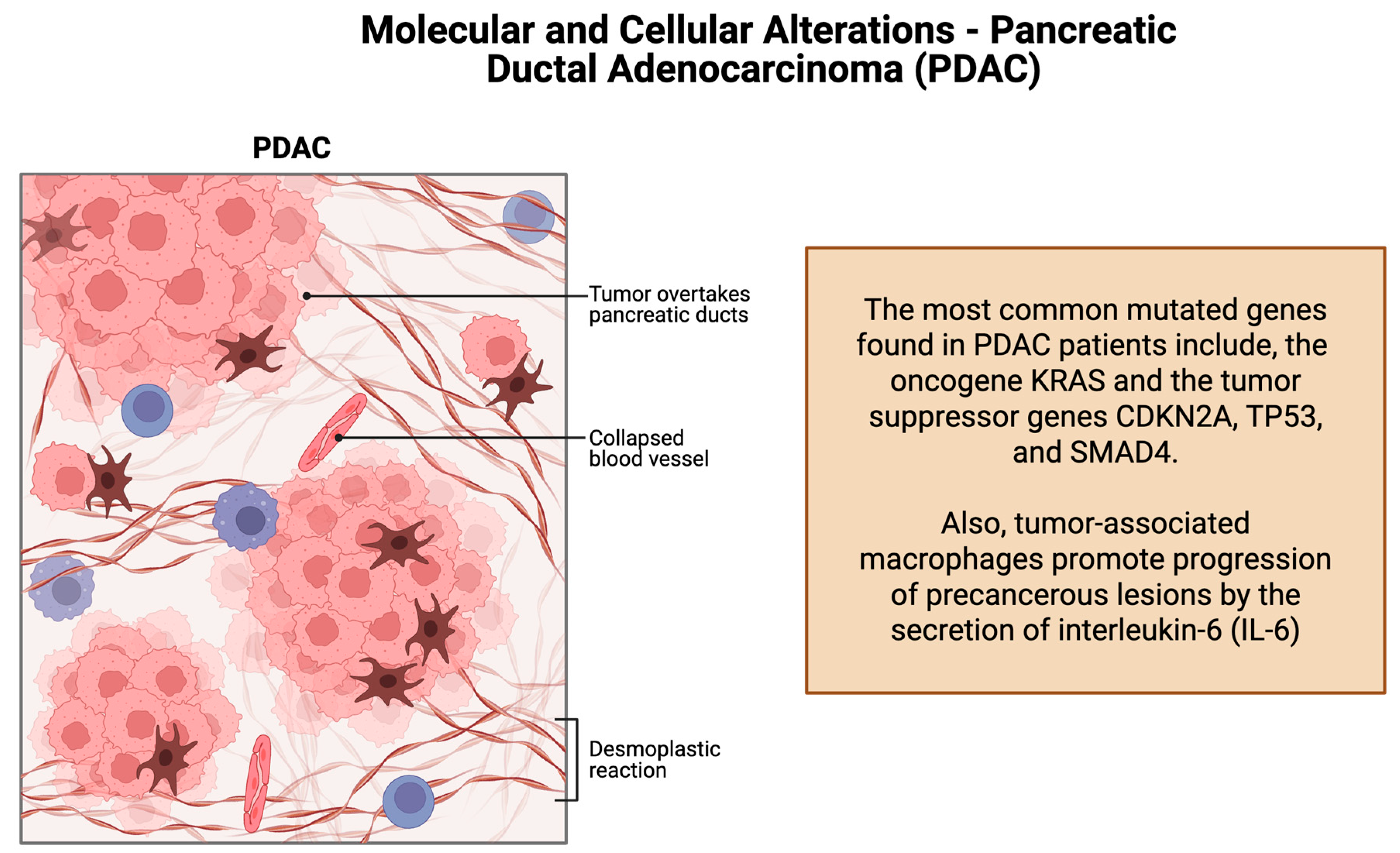
1.2. Molecular and Cellular Alterations–Pancreatic Ductal Adenocarcinoma
1.3. Pancreatic Cancer Therapy
1.4. Nanoparticles in Pancreatic Cancer Therapy
2. Materials and Methods
2.1. Identifying Research Questions
2.2. Identifying Relevant Studies
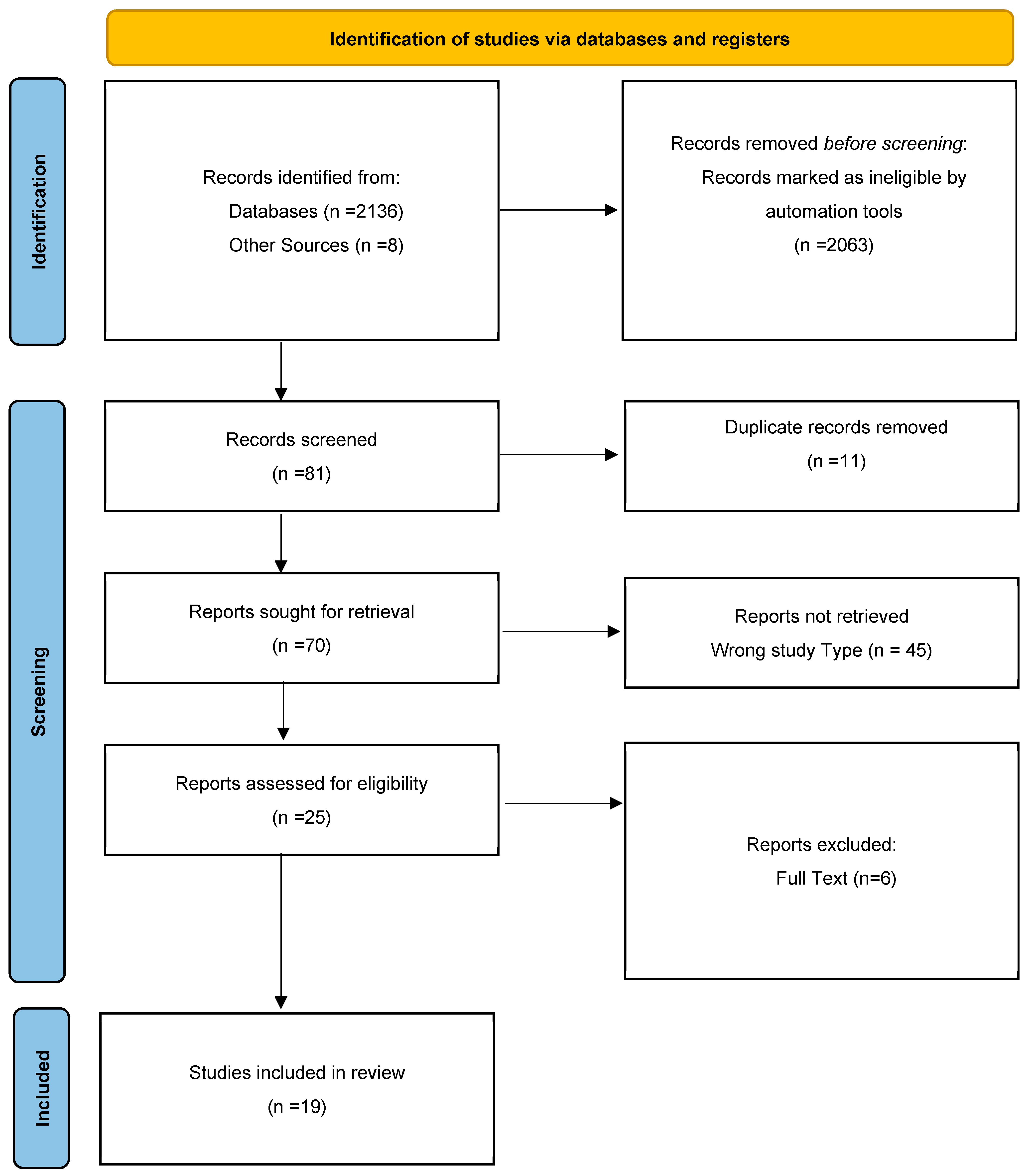
2.3. Study Selection—Eligibility and Screening
2.4. Data Charting
2.5. Collating, Summarizing, and Reporting Results
3. Results
4. Discussion
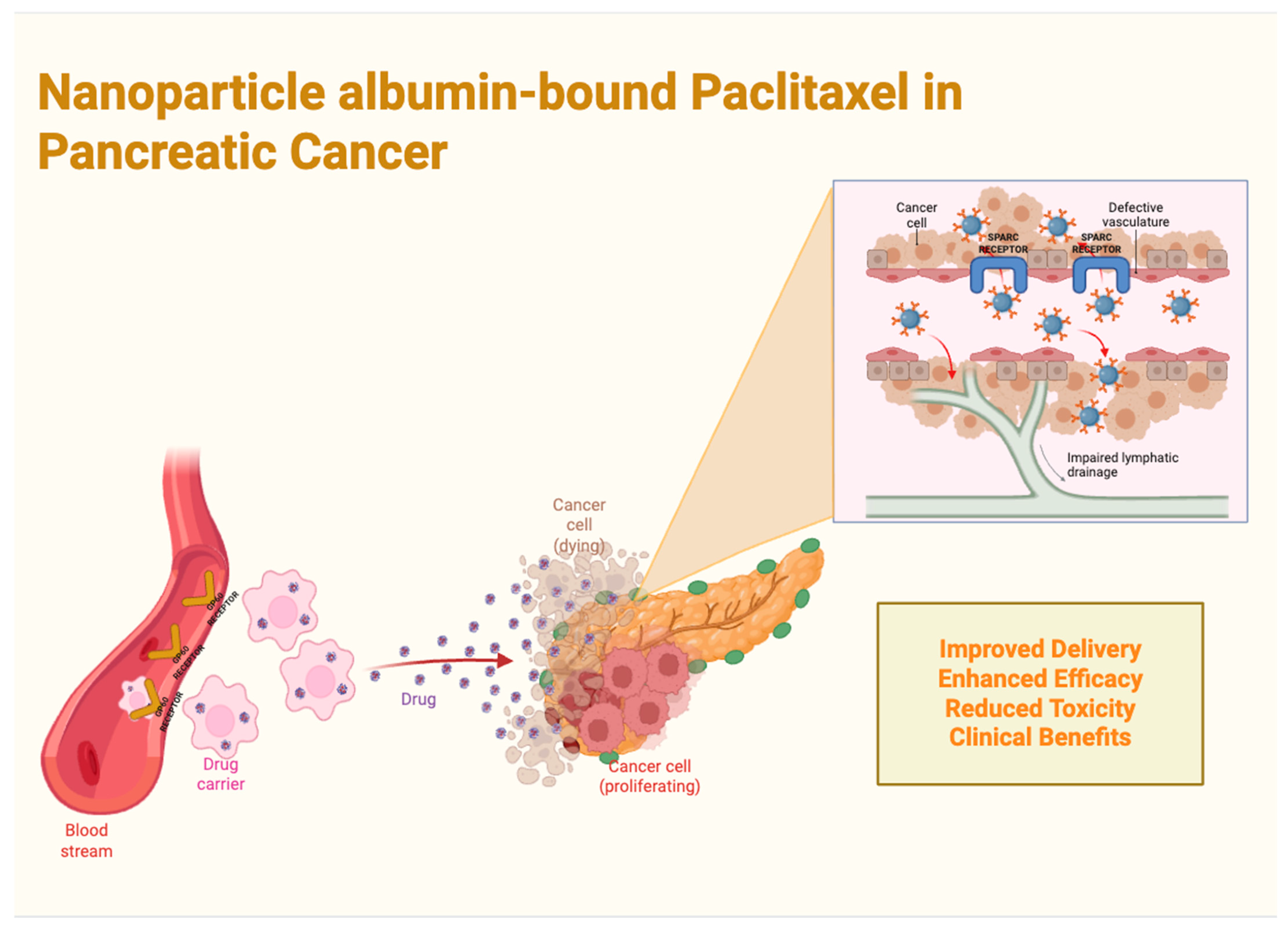
4.1. Nanoparticle Albumin-Bound Paclitaxel (nab-PTX) in Pancreatic Cancer
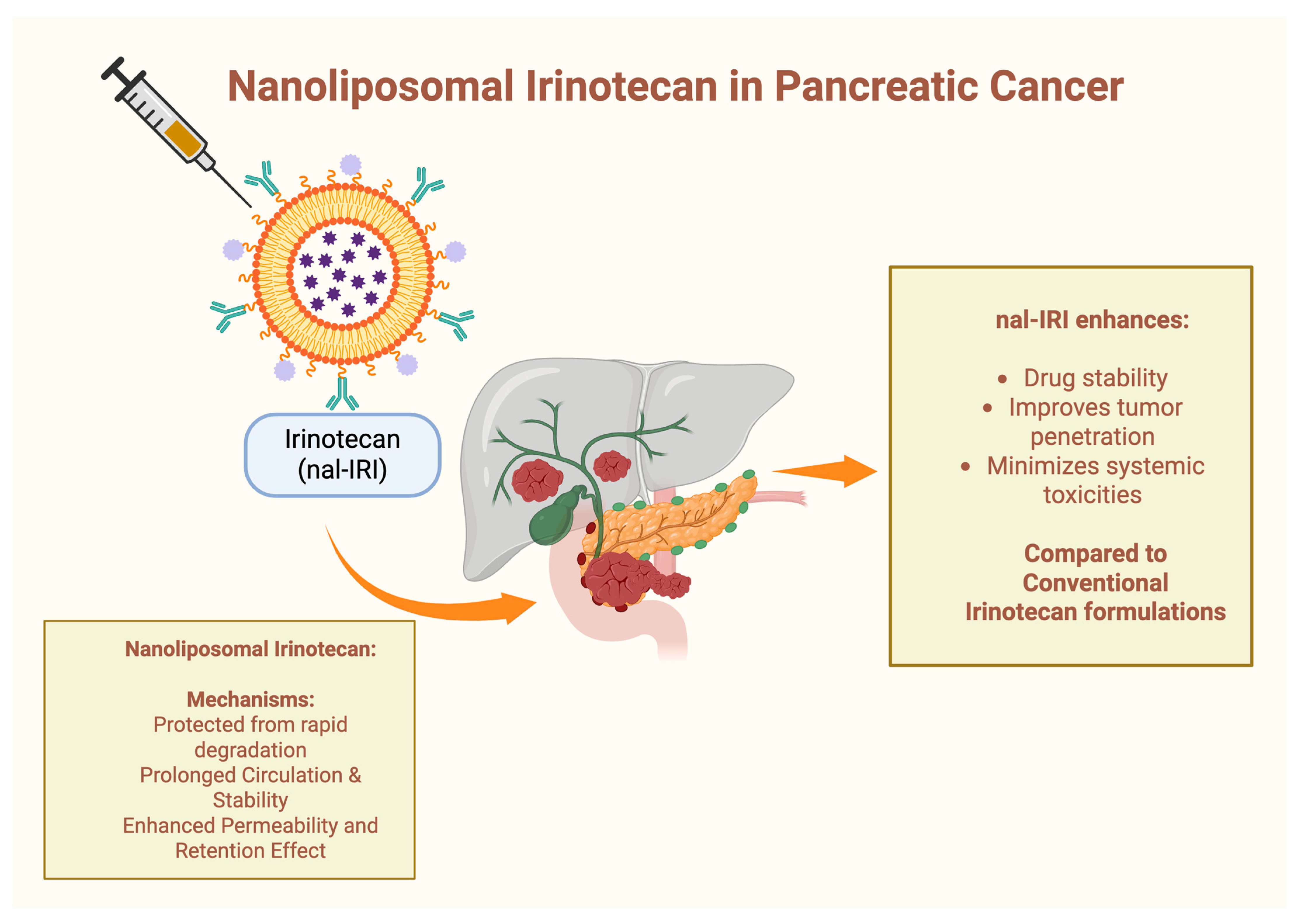
4.2. Nanoliposomal Irinotecan (nal-IRI) in Pancreatic Cancer
4.3. Other Nanoparticle-Based Targeted Therapies
4.4. Limitations, Challenges, and Prospective Views
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stoffel, E.M.; Brand, R.E.; Goggins, M. Pancreatic Cancer: Changing Epidemiology and New Approaches to Risk Assessment, Early Detection, and Prevention. Gastroenterology 2023, 164, 752–765. [Google Scholar] [CrossRef] [PubMed]
- Pancreatic Cancer—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 2 May 2025).
- Gagliardi, J. Mortality Rate of Pancreatic Cancer in Europe in 2022, by Country and Gender; Health Pharma Medtech: Washington, DC, USA, 2024; Available online: https://www.statista.com/statistics/1452352/pancreatic-cancer-mortality-rate-in-europe-by-country-and-gender/ (accessed on 2 May 2025).
- Partyka, O.; Pajewska, M.; Kwaśniewska, D.; Czerw, A.; Deptała, A.; Budzik, M.; Cipora, E.; Gąska, I.; Gazdowicz, L.; Mielnik, A.; et al. Overview of Pancreatic Cancer Epidemiology in Europe and Recommendations for Screening in High-Risk Populations. Cancers 2023, 15, 3634. [Google Scholar] [CrossRef]
- Cancer Site Ranking. Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/13-pancreas-fact-sheet.pdf (accessed on 11 April 2025).
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Puckett, Y.; Garfield, K. Pancreatic Cancer. In StatPearls; StatsPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK518996/ (accessed on 11 April 2025).
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Bandrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef]
- Tsokkou, S.; Konstantinidis, I.; Georgaki, M.N.; Kavvadas, D.; Papadopoulou, K.; Keramas, A.; Sioga, A.; Papamitsou, T.; Karachrysafi, S. Gestational Diabetes Mellitus and Its Correlation in the Development of Pancreatic Cancer: A 10-Year Systematic Review. Cancers 2024, 16, 1840. [Google Scholar] [CrossRef]
- Wood, L.D.; Canto, M.I.; Jaffee, E.M.; Simeone, D.M. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology 2022, 163, 386. [Google Scholar] [CrossRef]
- Hayat, U.; Croce, P.S.; Saadeh, A.; Desai, K.; Appiah, J.; Khan, S.; Khan, Y.I.; Kumar, K.; Hanif, A. Current and Emerging Treatment Options for Pancreatic Cancer: A Comprehensive Review. J. Clin. Med. 2025, 14, 1129. [Google Scholar] [CrossRef]
- Dallavalle, S.; Campagnoli, G.; Pastena, P.; Martinino, A.; Schiliró, D.; Giovinazzo, F. New Frontiers in Pancreatic Cancer Management: Current Treatment Options and the Emerging Role of Neoadjuvant Therapy. Medicina 2024, 60, 1070. [Google Scholar] [CrossRef]
- Gall, T.M.H.; Tsakok, M.; Wasan, H.; Jiao, L.R. Pancreatic cancer: Current management and treatment strategies. Postgrad. Med. J. 2015, 91, 601–607. [Google Scholar] [CrossRef]
- Nanotechnology Cancer Therapy Treatment—NCI. Available online: https://www.cancer.gov/nano/cancer-nanotechnology/treatment (accessed on 11 April 2025).
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Au, M.; Emeto, T.I.; Power, J.; Vangaveti, V.N.; Lai, H.C. Emerging Therapeutic Potential of Nanoparticles in Pancreatic Cancer: A Systematic Review of Clinical Trials. Biomedicines 2016, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef]
- Sethna, Z.; Guasp, P.; Reiche, C.; Milighetti, M.; Ceglia, N.; Patterson, E.; Lihm, J.; Payne, G.; Lyudovyk, O.; Rojas, L.A.; et al. RNA neoantigen vaccines prime long-lived CD8+ T cells in pancreatic cancer. Nature 2025, 639, 1042–1051. [Google Scholar] [CrossRef]
- Yang, Y.; Bteich, J.; Li, S.D. Current Update of a Carboxymethylcellulose-PEG Conjugate Platform for Delivery of Insoluble Cytotoxic Agents to Tumors. AAPS J. 2017, 19, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Stainthorpe, A.; Greenhalgh, J.; Bagust, A.; Richardson, M.; Boland, A.; Beale, S.; Duarte, R.; Kotas, E.; Banks, L.; Palmer, D. Paclitaxel as Albumin-Bound Nanoparticles with Gemcitabine for Untreated Metastatic Pancreatic Cancer: An Evidence Review Group Perspective of a NICE Single Technology Appraisal. Pharmacoeconomics 2018, 36, 1153–1163. [Google Scholar] [CrossRef]
- Roy, H.; Nayak, B.S.; Nandi, S. Chitosan Anchored Nanoparticles in Current Drug Development Utilizing Computer-Aided Pharmacokinetic Modeling: Case Studies for Target Specific Cancer Treatment and Future Prospective. Curr. Pharm. Des. 2020, 26, 1666–1675. [Google Scholar] [CrossRef]
- Song, S.Y.; Kim, K.P.; Jeong, S.Y.; Park, J.; Park, J.; Jung, J.; Chung, H.K.; Lee, S.-W.; Seo, M.H.; Lee, J.-S.; et al. Polymeric nanoparticle-docetaxel for the treatment of advanced solid tumors: Phase I clinical trial and preclinical data from an orthotopic pancreatic cancer model. Oncotarget 2016, 7, 77348–77357. [Google Scholar] [CrossRef]
- Sloat, B.R.; Sandoval, M.A.; Li, D.; Chung, W.-G.; Lansakara-P, D.S.; Proteau, P.J.; Kiguchi, K.; DiGiovanni, J.; Cui, Z. In vitro and in vivo anti-tumor activities of a gemcitabine derivative carried by nanoparticles. Int. J. Pharm. 2011, 409, 278–288. [Google Scholar] [CrossRef]
- Bagley, A.F.; Ludmir, E.B.; Maitra, A.; Minsky, B.D.; Smith, G.L.; Das, P.; Koong, A.C.; Holliday, E.B.; Taniguchi, C.M.; Katz, M.H.; et al. NBTXR3, a first-in-class radioenhancer for pancreatic ductal adenocarcinoma: Report of first patient experience. Clin. Transl. Radiat. Oncol. 2022, 33, 66–69. [Google Scholar] [CrossRef]
- Chen, N.; Brachmann, C.; Liu, X.; Pierce, D.W.; Dey, J.; Kerwin, W.S.; Li, Y.; Zhou, S.; Hou, S.; Carleton, M.; et al. Albumin-bound nanoparticle (nab) paclitaxel exhibits enhanced paclitaxel tissue distribution and tumor penetration. Cancer Chemother. Pharmacol. 2015, 76, 699–712. [Google Scholar] [CrossRef]
- Daman, Z.; Ostad, S.; Amini, M.; Gilani, K. Preparation, optimization and in vitro characterization of stearoyl-gemcitabine polymeric micelles: A comparison with its self-assembled nanoparticles. Int. J. Pharm. 2014, 468, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Appidi, T.; China, D.; Ștefan, G.R.; Moreau, M.; Mao, S.; Velarde, E.; Toyang, N.; Lowe, H.; Rengan, A.K.; Ding, K.; et al. Engineered multifunctional nanoparticles for enhanced radiation therapy: Three-in-one approach for cancer treatment. Mol. Cancer 2025, 24, 68. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Melisi, D.; Macarulla, T.; Cid, R.P.; Chandana, S.R.; De La Fouchardière, C.; Dean, A.; Kiss, I.; Lee, W.J.; Goetze, T.O.; et al. NALIRIFOX versus nab-paclitaxel and gemcitabine in treatment-naive patients with metastatic pancreatic ductal adenocarcinoma (NAPOLI 3): A randomised, open-label, phase 3 trial. Lancet 2023, 402, 1272–1281. [Google Scholar] [CrossRef]
- Bockorny, B.; Macarulla, T.; Semenisty, V.; Borazanci, E.; Feliu, J.; Ponz-Sarvise, M.; Abad, D.G.; Oberstein, P.; Alistar, A.; Muñoz, A.; et al. Motixafortide and Pembrolizumab Combined to Nanoliposomal Irinotecan, Fluorouracil, and Folinic Acid in Metastatic Pancreatic Cancer: The COMBAT/KEYNOTE-202 Trial. Clin. Cancer Res. 2021, 27, 5020–5027. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, H.; Shin, K.; Hong, T.H.; Suh, J.H.; Lee, M.A. Nanoliposomal irinotecan plus fluorouracil and folinic acid as a second-line treatment option in patients with metastatic pancreatic ductal adenocarcinoma: A retrospective cohort study. BMC Cancer 2021, 21, 1176. [Google Scholar] [CrossRef]
- Glassman, D.C.; Palmaira, R.L.; Covington, C.M.; Desai, A.M.; Ku, G.Y.; Li, J.; Harding, J.J.; Varghese, A.M.; O’reilly, E.M.; Yu, K.H. Nanoliposomal irinotecan with fluorouracil for the treatment of advanced pancreatic cancer, a single institution experience. BMC Cancer 2018, 18, 693. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Li, C.P.; Bodoky, G.; Dean, A.; Shan, Y.-S.; Jameson, G.; Macarulla, T.; Lee, K.-H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef]
- Ceelen, W.; Sandra, L.; Van de Sande, L.; Graversen, M.; Mortensen, M.B.; Vermeulen, A.; Gasthuys, E.; Reynders, D.; Cosyns, S.; Hoorens, A.; et al. Phase I study of intraperitoneal aerosolized nanoparticle albumin based paclitaxel (NAB-PTX) for unresectable peritoneal metastases. EBioMedicine 2022, 82, 104151. [Google Scholar] [CrossRef]
- Sohal, D.P.S.; Duong, M.; Ahmad, S.A.; Gandhi, N.S.; Beg, M.S.; Wang-Gillam, A.; Wade, J.L.; Chiorean, E.G.; Guthrie, K.A.; Lowy, A.M.; et al. Efficacy of Perioperative Chemotherapy for Resectable Pancreatic Adenocarcinoma. JAMA Oncol. 2021, 7, 421. [Google Scholar] [CrossRef]
- Azmi, A.S.; Khan, H.Y.; Muqbil, I.; Aboukameel, A.; Neggers, J.E.; Daelemans, D.; Mahipal, A.; Dyson, G.; Kamgar, M.; Al-Hallak, M.N.; et al. Preclinical Assessment with Clinical Validation of Selinexor with Gemcitabine and Nab-Paclitaxel for the Treatment of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2020, 26, 1338–1348. [Google Scholar] [CrossRef]
- Hasegawa, R.; Okuwaki, K.; Kida, M.; Yamauchi, H.; Kawaguchi, Y.; Matsumoto, T.; Kaneko, T.; Miyata, E.; Uehara, K.; Iwai, T.; et al. A clinical trial to assess the feasibility and efficacy of nab-paclitaxel plus gemcitabine for elderly patients with unresectable advanced pancreatic cancer. Int. J. Clin. Oncol. 2019, 24, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Macarulla, T.; Pazo-Cid, R.; Guillén-Ponce, C.; López, R.; Vera, R.; Reboredo, M.; Martin, A.M.; Rivera, F.; Beveridge, R.D.; La Casta, A.; et al. Phase I/II Trial to Evaluate the Efficacy and Safety of Nanoparticle Albumin-Bound Paclitaxel in Combination with Gemcitabine in Patients with Pancreatic Cancer and an ECOG Performance Status of 2. J. Clin. Oncol. 2019, 37, 230–238. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Hosein, P.J.; de Lima Lopes, G.; Pastorini, V.H.; Gomez, C.; Macintyre, J.A.; Zayas, G.C.; Reis, I.; Montero, A.J.; Merchan, J.R.; Lima, C.M.R. A Phase II Trial of nab-Paclitaxel as Second-line Therapy in Patients with Advanced Pancreatic Cancer. Am. J. Clin. Oncol. 2013, 36, 151–156. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ramanathan, R.K.; Borad, M.J.; Laheru, D.A.; Smith, L.S.; Wood, T.E.; Korn, R.L.; Desai, N.; Trieu, V.; Iglesias, J.L.; et al. Gemcitabine Plus nab-Paclitaxel Is an Active Regimen in Patients with Advanced Pancreatic Cancer: A Phase I/II Trial. J. Clin. Oncol. 2011, 29, 4548–4554. [Google Scholar] [CrossRef]
- Libutti, S.K.; Paciotti, G.F.; Byrnes, A.A.; Alexander, H.R.; Gannon, W.E.; Walker, M.; Seidel, G.D.; Yuldasheva, N.; Tamarkin, L. Phase I and Pharmacokinetic Studies of CYT-6091, a Novel PEGylated Colloidal Gold-rhTNF Nanomedicine. Clin. Cancer Res. 2010, 16, 6139–6149. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Kato, K.; Yasui, H.; Morizane, C.; Ikeda, M.; Ueno, H.; Muro, K.; Yamada, Y.; Okusaka, T.; Shirao, K.; et al. A phase I and pharmacokinetic study of NK105, a paclitaxel-incorporating micellar nanoparticle formulation. Br. J. Cancer 2007, 97, 170–176. [Google Scholar] [CrossRef]
- Stathopoulos, G.; Boulikas, T.; Vougiouka, M.; Rigatos, S.; Stathopoulos, J. Liposomal cisplatin combined with gemcitabine in pretreated advanced pancreatic cancer patients: A phase I-II study. Oncol. Rep. 2006, 15, 1201–1204. [Google Scholar] [CrossRef]
- Huang, X.; Wang, C.; Ma, T.; Huang, Z.; Zhou, H.; Xu, L.; Zhang, R.; Zhao, J.; Zhang, Y.; Huang, Z.; et al. The Efficacy of Combined Cisplatin and Nanoparticle Albumin-Bound Paclitaxel in a Stage IV Pancreatic Squamous Cell Carcinoma Patient with a Somatic BRCA2 Mutation: A Case Report. Front. Oncol. 2021, 11, 585983. [Google Scholar] [CrossRef]
- Otsubo, M.; Kinouchi, R.; Kamiya, T.; Yoshida, A. Regression of taxane-related cystoid macular edema after topical dorzolamide treatment: Two case reports. J. Med. Case Rep. 2021, 15, 355. [Google Scholar] [CrossRef]
- Assi, H.A.; Shikdar, S.; Alhyari, L.; Aljumaily, R. Long-Term Survival with Nanoliposomal Irinotecan in Pancreatic Cancer. Pancreas 2020, 49, e95–e96. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; De La Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Abal, M.; Bras-Goncalves, R.; Judde, J.G.; Fsihi, H.; de Cremoux, P.; Louvard, D.; Magdelenat, H.; Robine, S.; Poupon, M.-F. Enhanced sensitivity to irinotecan by Cdk1 inhibition in the p53-deficient HT29 human colon cancer cell line. Oncogene 2004, 23, 1737–1744. [Google Scholar] [CrossRef] [PubMed]

| P (Population) | Adult (≥18 years) patients with pancreatic cancer |
| I (Intervention) | Nanoparticle-based pharmacotherapy |
| C (Comparator) | Conventional pharmacological regimens |
| O (Outcome) | OS, PFS, DCR, PR, CR, SD, and TEAEs |
| T (Time) | 20 Years (2005–2025) |
| S (Study design) | Clinical trials, cohorts (retrospective or prospective), case reports |
| Author, Year | Type of Study | N | Patients | NPs | Regimens | Age (Years) | mOS (mo; 95% CI) | mPFS (mo; 95% CI) | DCR (%, 95% CI) | PR (%, 95% CI) | CR (%, 95% CI) | SD (%, 95% CI) | TEAEs | Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nanoliposomal Irinotecan and Nanoparticle Albumin-Bound Paclitaxel | ||||||||||||||
| Wainberg ZA et al., 2023 [28] | Phase III randomized clinical trial (NAPOLI 3; NCT04083235) | 770 | Previously untreated mPDAC | Nanoliposomal irinotecan and nanoparticle albumin-bound paclitaxel | NALIRIFOX (N = 383) vs. nab-PTX + gemcitabine (N = 387) | 64 (20–85) vs. 65 (36–82) | 11.1 (10.0–12.1) vs. 9.2 (8.3–10.6) | 7.4 (6.0–7.7) vs. 5.6 (5.3–5.8) | 41.8 (36.8–46.9) vs. 36.2 (21.4–41.2) | 42 vs. 36 | <1 vs. <1 | 26 vs. 26 | Grade 3–4: neutropenia; diarrhea; hypokalemia in the NALIRIFOX group Grade 3–4: neutropenia; anemia; peripheral neuropathy in the nab-PTX + gemcitabine group | NALIRIFOX promising treatment for mPDAC; improved survival and tolerability; fewer hematological TEAEs |
| Nanoliposomal Irinotecan | ||||||||||||||
| Bockorny B et al., 2021 [29] | Phase IIa clinical trial (COMBAT/KEYNOTE-202; NCT02826486) | 43 | De novo mPDAC with documented radiographic progression after treatment with first-line gemcitabine-based chemotherapy | Nanoliposomal irinotecan | Motixafortide + pembrolizumab + nal-IRI + 5-FU + LV | 68 (40–85) | 6.6 (4.5–8.7) | 3.8 (1.6–5.1) | 63.2 (47.8–78.5) | N/A | N/A | 42.1 (26.4–57.8) | Grade 3–4: nausea and vomiting (18.6%); asthenia (16.3%); diarrhea (14%); serious neutropenia (7%); febrile neutropenia (2.3%); reactions at the injection site (4.7%) | Safe; well tolerated; promising efficacy; lower-than-expected rates of neutropenia and infections |
| Park SJ et al., 2021 [30] | Retrospective cohort study | 51 | mPDAC previously treated with gemcitabine-based therapy | Nanoliposomal irinotecan | nal-IRI + 5-FU + LV | 67 (50–78) | 7.0 (6.0–7.9) | 2.8 (1.8–3.7) | 60.8 | 5.9 | 0 | 54.9 | Anemia (84.3%); neutropenia (84.3%; Grade 3–4: 58.8%); nausea (43.1%); diarrhea (23.5%); fatigue (21.6%); febrile neutropenia (7.8%) | Clinical benefits; gemcitabine plus nab-PTX remains a viable first-line treatment option for a significant portion of patients with mPDAC |
| Glassman DC et al., 2018 [31] | Retrospective study | 56 | mPDAC previously treated with gemcitabine-based therapy | Nanoliposomal irinotecan | FOLFIRINOX or FOLFOX, followed by nab-PTX + gemcitabine and nal-IRI + 5-FU + LV (Sequence 1) vs. gemcitabine alone or nab-PTX + gemcitabine followed by nal-IRI + 5-FU + LV (Sequence 2) | 68 (42–88) | 4.1 vs. 9.0 | 2.2 vs. 4.8 | N/A | 5 | 0 | 41 | GI toxicities (nausea and vomiting; diarrhea); fatigue; anorexia; neutropenia; anemia (Grade 3/4) | Confirmed safety and efficacy of nal-IRI + 5-FU/LV for advanced PDAC after gemcitabine-based therapy; earlier use in treatment and absence of irinotecan-refractory disease correlated with improved progression-free survival; dose reductions did not compromise outcomes; genetic predictors of response require further validation; promising OS with sequential integration into combination chemotherapy |
| Wang-Gillam A et al., 2016 [32] | Phase III randomized clinical trial (NAPOLI-1; NCT01494506) | 417 | mPDAC previously treated with gemcitabine-based therapy | Nanoliposomal irinotecan | nal-IRI + 5-FU + LV (N = 117) vs. 5-FU + LV (N = 149) vs. nal-IRI monotherapy (N = 151) | 63 (57–70) vs. 65 (58–70) vs. 63 (55–69) | 6.1 (4.8–8.9) vs. 4.2 (3.3–5.3) vs. 4.9 (4.2–5.6) | 3.1 (2.7–4.2) vs. 1.5 (1.4–1.8) vs. 2.7 (2.1–2.9) | N/A | N/A | N/A | N/A | Grade 3–4 neutropenic sepsis and febrile neutropenia (3% vs. 0 vs. 4%); Grade 4 (10% vs. 7% vs. 16%); resulting in death: gastrointestinal toxicity, infectious enterocolitis, septic shock, and disseminated intravascular coagulation with pulmonary embolism | Improves survival and other key efficacy measures in metastatic PC patients previously treated with gemcitabine-based therapy; manageable and mostly reversible safety profile; new treatment option, although its applicability to patients with low performance status remains uncertain |
| Nanoparticle albumin-bound paclitaxel | ||||||||||||||
| Ceelen W et al., 2022 [33] | Phase I clinical trial (NCT03304210) | 20 | Peritoneal metastases from ovarian, breast, gastric, hepatobiliary, or pancreatic origin | Nanoparticle albumin-bound paclitaxel | Pressurized intraperitoneal aerosol chemotherapy (PIPAC) nab-PTX | 57 (49–65) | N/A | N/A | N/A | N/A | N/A | N/A | Hematological toxicity (moderate); Grade 3 neutropenia | Safety and potential effectiveness for advanced, unresectable peritoneal metastases; well-tolerated dosing; stable patient quality of life; promising anticancer activity |
| Sohal DPS et al., 2021 [34] | Phase II randomized clinical trial (NCT02562716) | 102 | Treatment-naïve PDAC with no metastases | Nanoparticle albumin-bound paclitaxel | mFOLFIRINOX (N = 55) vs. nab-PTX + gemcitabine (N = 47) | 66 (44–76) vs. 64 (46–75) | 23.2 (17.6–45.9) vs. 23.6 (17.8–31.7) | N/A | N/A | N/A | N/A | N/A | Neoadjuvant: neutropenia (19% vs 27%); (11% vs 4%) | Safety and efficacy; no improved OS compared to historical adjuvant trials |
| Azmi AS et al., 2020 [35] | Phase Ib study (NCT02178436) | 5 | mPDAC not treated with chemotherapy | Nanoparticle albumin-bound paclitaxel | Selinexor + gemcitabine + nab-PTX | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Synergy between selinexor and GEM-nab-PTX in PDAC models, including stem cells and patient-derived xenografts |
| Hasegawa R et al., 2019 [36] | Clinical trial (UMIN000018907) | 27 | Unresectable advanced PC + age ≥ 75 years | Nanoparticle albumin-bound paclitaxel | nab-PTX + gemcitabine | 77 (75–85) | 10.3 (8.2–12.5) | 7.0 (6.0–8.1) | N/A | 44.4 | N/A | 48.1 | Grade 3–4: hemotoxic (51.9%); non-hemotoxic (59.3%): peripheral nerve disorder (22.2%) | Improved progression-free survival in elderly patients; favorable disease control even in stage IV cases; feasible with proper dose management; elderly patients more susceptible to non-hemotoxic TEAEs |
| Macarulla T et al., 2019 [37] | Phase I trial | 24 | Locally advanced or advanced PDAC; ECOG PS of 2 | Nanoparticle albumin-bound paclitaxel | nab-PTX at 150 mg/m2 (arm A) or 125 mg/m2 (arm C) + gemcitabine; nab-PTX at 100 mg/m2 (arm B) or 125 mg/m2 (arm D) + gemcitabine | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Not designed for direct comparison; both arms showed similar efficacy and toxicity; potential treatment option in clinical practice |
| Phase II randomized trial (NCT02382263) | 221 | Locally advanced or advanced PDAC; ECOG PS of 2 | nab-PTX at 100 mg/m2 (arm B; N = 111) or 125 mg/m2 (arm D; N = 110) + gemcitabine | 71 (43–89) vs. 68 (35–84) | 7.7 (6.3–9.1) vs. 9.8 (7.5–11.8) | 5.4 (4–6.9) vs. 6.6 (5.6–7.6) | 64.9 (56–73.7) vs. 71.8 (63.4–80.2) | 20.7 vs. 21.8 | 0 vs. 0.9 | N/A | Hematological toxicity (neutropenia); fatigue; peripheral neuropathy | |||
| Von Hoff DD et al., 2013 [38] | Phase III Randomized control trial (MPACT; NCT00844649) | 861 | mPDAC not treated with chemotherapy | Nanoparticle albumin-bound paclitaxel | nab-PTX + gemcitabine (N = 431) vs. gemcitabine monotherapy (N = 430) | 63 (27–88) | 8.5 (7.89–9.53) vs. 6.7 (6.01–7.23) | 5.5 (4.5–5.9) vs. 3.7 (3.6–4.0) | 48 (43–53) vs. 33 (28–37) | 23 vs. 7 | <1 vs. 0 | 20 vs. 26 | Grade 3–4: neutropenia; leukopenia; fatigue; peripheral neuropathy (mostly in nab-PTX cohort) | Significantly improved survival; benefits observed across multiple time points and subgroups; increased myelosuppression and peripheral neuropathy, reversible; potential as an effective treatment option |
| Hosein PJ et al., 2013 [39] | Phase II clinical trial (NCT00691054) | 19 | Advanced PC that progressed on gemcitabine-based therapy with unresectable locally advanced or metastatic disease | Nanoparticle albumin-bound paclitaxel | nab-PTX monotherapy | 61 (24–80) | 7.3 (2.8–15.8) | 1.7 (1.5–3.5) | 5 | 5 | 0 | 32 | Grades 3–4 neutropenia (26%); Grades 3–4 anemia (11%); neutropenic fever (11%); hypocalcemia | The nab-PTX monotherapy demonstrated preliminary activity in a subset of patients and was well tolerated |
| Von Hoff DD et al., 2011 [40] | Phase I/II clinical trial (NCT00398086.) | 67 | Previously untreated advanced PC | Nanoparticle albumin-bound paclitaxel | 100 (N = 20), 125 (N = 44; Results), or 150 (N = 3) mg/m2 nab-PTX + gemcitabine | 62 (30–86) vs. 61 (28–78) vs. 69 (53–72) | 12.2 (8.9–17.9) | 7.9 (5.8–11.0) | 68 | 48 | 0 | 20 | Grade 3–4 fatigue (21%); sensory neuropathy (15%); neutropenia (67%); leukopenia (44%); thrombocytopenia (23%) | Favorable safety and encouraging antitumor activity; patient selection may influence outcomes |
| Other Nanoparticle-Based Targeted Therapies | ||||||||||||||
| Libutti SK et al., 2010 [41] | Phase I clinical trial | 3 | Advanced-stage PC patients | CYT-6091 | PEGylated colloidal gold nanoparticle carrying rhTNF-a | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Grade 3–4 lymphopenia (89%); hypoalbuminemia (17%); hypokalemia (17%); hypophosphatemia (17%); hyperbilirubinemia (17%), increased AST (17%) | Promising tumor targeting; potential benefits when administered systemically before chemotherapy or surgery, particularly for solid tumors |
| Hamaguchi T et al., 2007 [42] | Phase I clinical trial | 11 | PC refractory to conventional chemotherapy | NK105 | Polymeric micellar nanoparticle paclitaxel | 57 (43–72) | N/A | N/A | N/A | N/A | N/A | N/A | Grade 3–4: neutropenia; Grade 1–2: fever; nausea; fatigue; stomatitis; rash; alopecia | Reduction in the size of metastatic lesions; minimal severity of adverse events; favorable therapeutic response and manageable safety profile |
| Stathopoulos GP et al., 2006 [43] | Phase I/II clinical trial | 24 | Advanced PDAC after chemotherapy pretreatment and recurrent or non-responsive disease | Nanoliposomal cisplatin | Lipoplatin + gemcitabine | 66 (47–80) | 4 (2–8) | N/A | N/A | 8.3 | N/A | 58.3 | Grade 3 myelotoxicity (50%) | Well tolerated in advanced pretreated PC patients; promising efficacy with symptom relief and disease stability |
| Author, Year | Type | Disease | Gender | Age | NPs | Regimen | Conclusions |
|---|---|---|---|---|---|---|---|
| Huang X et al., 2021 [44] | Case report | Stage IV primary SCC of the pancreas harboring a deleterious BRCA2 somatic mutation | F | 52 | NP albumin-bound paclitaxel | nab-PTX + cisplatin | Significant tumor reduction; prolonged survival; improved tumor resectability; the longest reported survival for metastatic pancreatic SCC to date |
| Otsubo M et al., 2021 [45] | Case report | Stage IV PC with hepatic and lymph node metastases | M | 70 | NP albumin-bound paclitaxel | nab-PTX + gemcitabine | Taxane-related cystoid macular edema (CME) as a rare but notable side effect of nab-PTX, affecting bilateral central vision; CME resolved after stopping nab-PTX, with no intervention other than topical dorzolamide |
| Assi HA et al., 2020 [46] | Case report | T4, N1, and M1 primary pancreatic adenocarcinoma | F | 57 | Nanoliposomal irinotecan and NP albumin-bound paclitaxel | nab-PTX + gemcitabine followed by nal-IRI + 5-FU + LV | Rare instance of long-term survival in an mPDAC patient treated with nal-IRI + 5-FU/LV; despite initial disease progression, sustained response for over two years, receiving 58 cycles without dose adjustments; PFS and OS reached 31 and 40 months, respectively; potential clinical benefits |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantinidis, I.; Tsokkou, S.; Katsikeros, D.; Chatzikomnitsa, P.; Papakonstantinou, M.; Liampou, E.; Toutziari, E.; Giakoustidis, D.; Bageas, P.; Papadopoulos, V.; et al. The Role of Nanoparticles in Therapy of Real-World Patients with Pancreatic Cancer: A Scoping Review. Cancers 2025, 17, 1726. https://doi.org/10.3390/cancers17101726
Konstantinidis I, Tsokkou S, Katsikeros D, Chatzikomnitsa P, Papakonstantinou M, Liampou E, Toutziari E, Giakoustidis D, Bageas P, Papadopoulos V, et al. The Role of Nanoparticles in Therapy of Real-World Patients with Pancreatic Cancer: A Scoping Review. Cancers. 2025; 17(10):1726. https://doi.org/10.3390/cancers17101726
Chicago/Turabian StyleKonstantinidis, Ioannis, Sophia Tsokkou, Dimitrios Katsikeros, Paraskevi Chatzikomnitsa, Menelaos Papakonstantinou, Eftychia Liampou, Evdokia Toutziari, Dimitrios Giakoustidis, Petros Bageas, Vasileios Papadopoulos, and et al. 2025. "The Role of Nanoparticles in Therapy of Real-World Patients with Pancreatic Cancer: A Scoping Review" Cancers 17, no. 10: 1726. https://doi.org/10.3390/cancers17101726
APA StyleKonstantinidis, I., Tsokkou, S., Katsikeros, D., Chatzikomnitsa, P., Papakonstantinou, M., Liampou, E., Toutziari, E., Giakoustidis, D., Bageas, P., Papadopoulos, V., Giakoustidis, A., & Papamitsou, T. (2025). The Role of Nanoparticles in Therapy of Real-World Patients with Pancreatic Cancer: A Scoping Review. Cancers, 17(10), 1726. https://doi.org/10.3390/cancers17101726












