The Italian Score for Organ Allocation: A Ten-Year Monocentric Retrospective Analysis in Liver Transplantation for Hepatocellular Carcinoma
Simple Summary
Abstract
1. Introduction
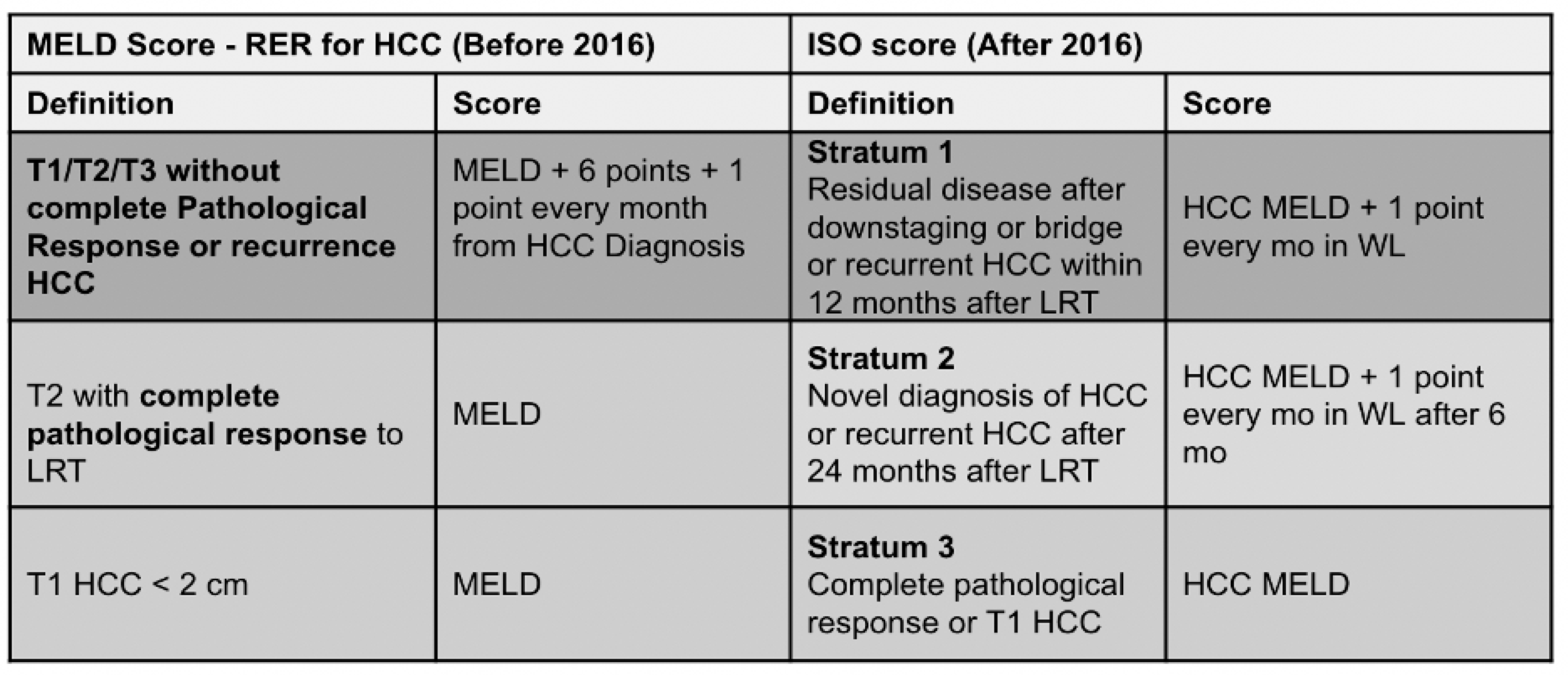
2. Patients and Methods
2.1. Study Design
2.2. Study Endpoints
2.3. Ethics
2.4. Data Collection
2.5. Follow-Up Period
2.6. Statistical Analysis
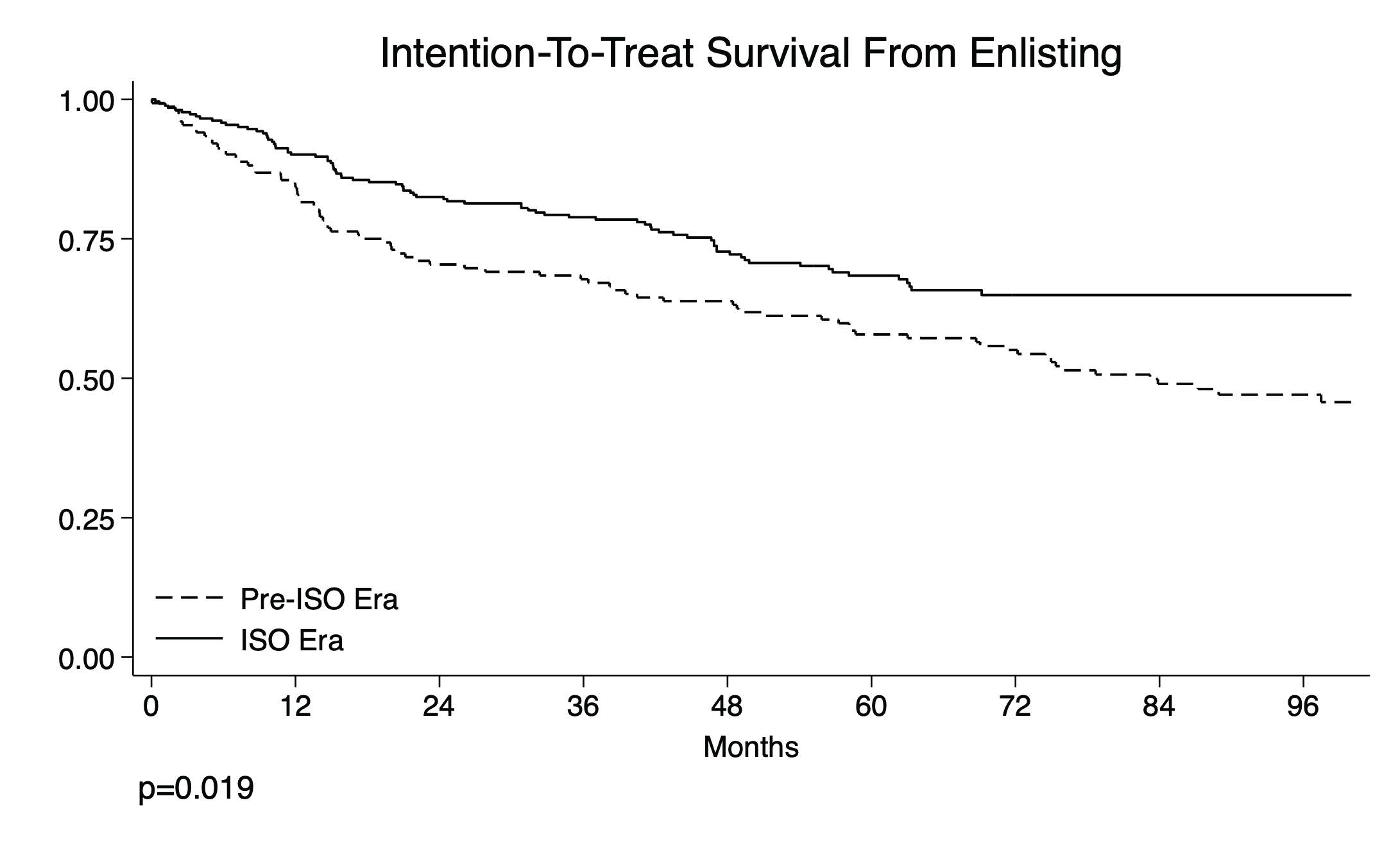
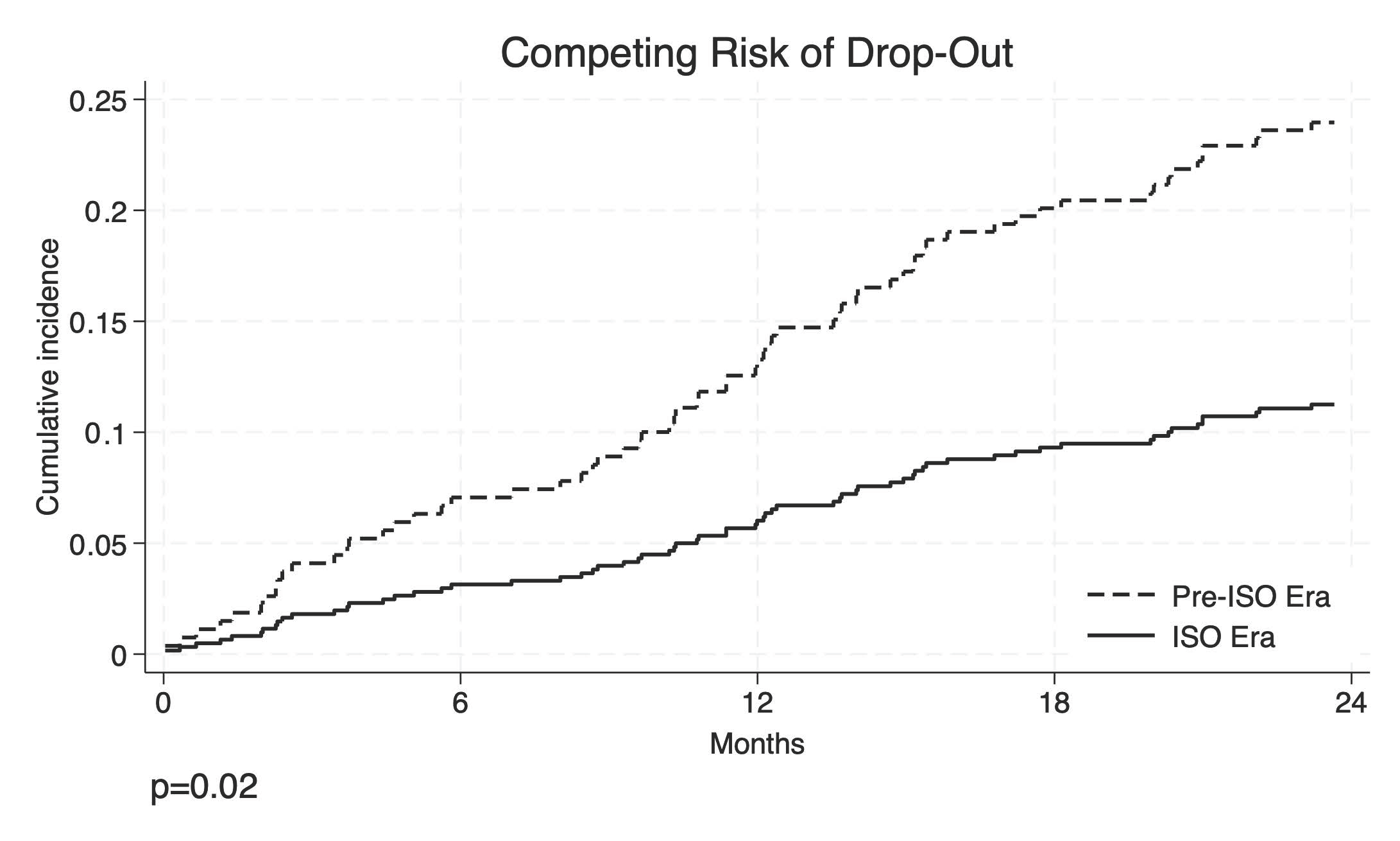
3. Results
3.1. Listing Characteristics
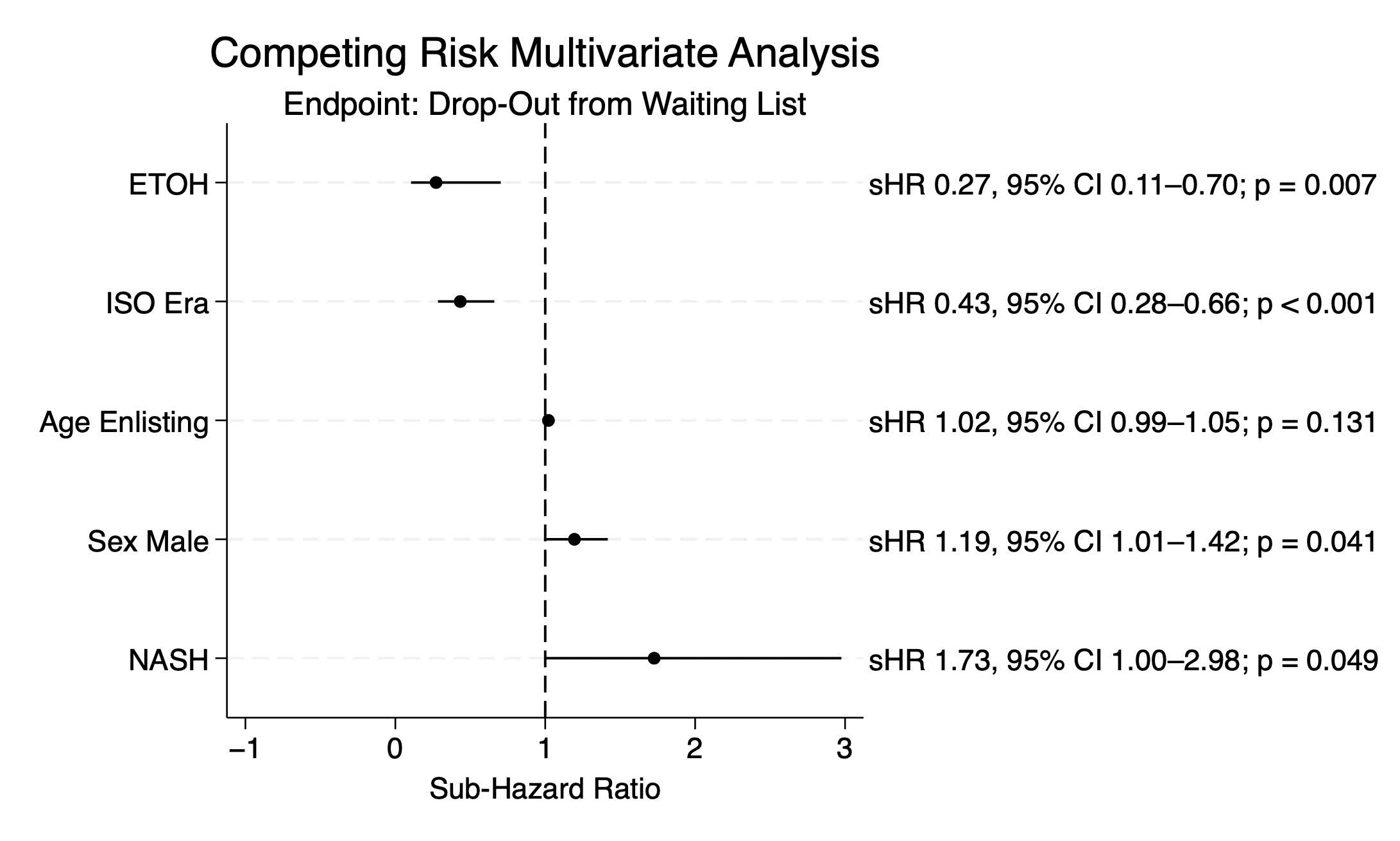
3.2. Risk Factors for Waiting List Drop-Out
3.3. Liver Transplantation Characteristics
3.4. Risk Factors for Post-LT Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACLF | Acute On Chronic Liver Failure |
| AFP | alfa-fetoprotein |
| BCLC | Barcelona Clinic Liver Cancer Staging |
| BMI | body mass index |
| CI | confidence interval |
| cPR | complete pathologic response |
| ceCT | Contrast-Enhanced Computed Tomography |
| ceMRI | Magnetic Resonance Imaging |
| CTP | Child–Pugh Score |
| DCD | Donation After Cardiac Death |
| ECD | Extended Criteria Donor |
| FU | follow-up |
| HCC | hepatocellular carcinoma |
| HCV | hepatitis C virus |
| HBV | hepatitis B virus |
| IQR | interquartile range |
| ISO | Italian Score for Organ Allocation |
| ITT | intention-to-treat survival |
| LRT | locoregional treatment |
| LR | Liver Resection |
| LT | liver transplantation |
| MELD | model for end-stage liver disease |
| mVI | microvascular Invasion |
| MWA | microwave ablation |
| NASH | nonalcoholic steatohepatitis |
| OR | odds ratio |
| OS | overall survival |
| PEI | Percutaneous Ethanol Injection |
| PVT | portal vein thrombosis |
| RECIST | Response Evaluation Criteria In Solid Tumors |
| RER | Regione Emilia-Romagna |
| RFA | radiofrequency ablation |
| RFS | recurrence-free survival |
| sHR | sub-hazard ratio |
| SE | Standard Error |
| SIRT | Emilia-Romagna Donor Manager Software |
| TACE | trans-arterial chemoembolization |
| TIPS | transjugular intrahepatic porto-systemic shunt |
| ULD | underlying liver disease |
| WL | waiting list |
| Y-90 | Yttrium-90 radioembolization |
References
- Wiesner, R.; Edwards, E.; Freeman, R.; Harper, A.; Kim, R.; Kamath, P.; Kremers, W.; Lake, J.; Howard, T.; Merion, R.M.; et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003, 124, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Kamath, P.S. Model for end-stage liver disease score and MELD exceptions: 15 years later. Hepatol. Int. 2015, 9, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Cillo, U.; Burra, P.; Mazzaferro, V.; Belli, L.; Pinna, A.D.; Spada, M.; Costa, A.N.; Toniutto, P.; Italian Board of Experts in the Field of Liver Transplantation. A multistep, consensus-based approach to organ allocation in liver transplantation: Toward a “blended principle model”. Am. J. Transplant. 2015, 15, 2552–2561. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2021, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, J.M.; Lee, J.Y.; Kim, S.H.; Yoon, J.H.; Kim, Y.J.; Han, J.K.; Choi, B.I. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: Long-term results and prognostic factors. Radiology 2014, 270, 900–909. [Google Scholar] [CrossRef]
- Brace, C.L.; Ziemlewicz, T.J.; Hinshaw, J.L.; Lee, F.T.; Lubner, M.G. Microwave ablation of hepatic malignancy. Semin. Interv. Radiol. 2013, 30, 56–66. [Google Scholar] [CrossRef][Green Version]
- Shiina, S.; Teratani, T.; Obi, S.; Sato, S.; Tateishi, R.; Fujishima, T.; Ishikawa, T.; Koike, Y.; Yoshida, H.; Kawabe, T.; et al. A randomized controlled trial of radiofrequency ablation with ethanol injection. Gastroenterology 2005, 129, 122–130. [Google Scholar] [CrossRef]
- Lencioni, R.; Petruzzi, P.; Crocetti, L. Chemoembolization of hepatocellular carcinoma. Semin. Interv. Radiol. 2013, 30, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Gabr, A.; Kulik, L.; Mouli, S.; Riaz, A.; Ali, R.; Desai, K.; Mora, R.A.; Ganger, D.; Maddur, H.; Flamm, S.; et al. Liver transplantation following Yttrium-90 radioembolization: 15-year experience. Hepatology 2021, 73, 998–1010. [Google Scholar] [CrossRef]
- Centro Nazionale Trapianti. Valutazione esiti Trapianti di Fegato. SIED-CNT. 2023. Available online: https://www.trapianti.salute.gov.it/imgs/C_17_cntPubblicazioni_581_allegato.pdf (accessed on 15 July 2023).
- Emilia-Romagna Region. 2012 Circular on the Adjustment of the Regional Liver Transplant Network to the National Guidelines for the Management of Waiting Lists and Allocation of Organs in Deceased Donor Liver Transplantation; Regional Department of Health Policies: Bologna, Italy, 2012. (In Italian) [Google Scholar]
- Ravaioli, M.; Odaldi, F.; Cucchetti, A.; Trevisani, F.; Piscaglia, F.; De Pace, V.; Bertuzzo, V.R.; Neri, F.; Golfieri, R.; Cappelli, A.; et al. Long term results of down-staging and liver transplantation for patients with hepatocellular carcinoma. Sci. Rep. 2019, 9, 3781. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, D. The 8th Edition AJCC staging for hepato-pancreato-biliary cancer: A review. Arch. Pathol. Lab. Med. 2020, 145, 543–553. [Google Scholar] [CrossRef]
- Vitale, A.; Volk, M.L.; De Feo, T.M.; Burra, P.; Frigo, A.C.; Morales, R.R.; De Carlis, L.; Belli, L.; Colledan, M.; Fagiuoli, S.; et al. A method for establishing allocation equity. J. Hepatol. 2014, 60, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Gorgen, A.; Rosales, R.; Sadler, E.; Beecroft, R.; Knox, J.; Dawson, L.A.; Ghanekar, A.; Grant, D.; Greig, P.; Sapisochin, G. Mortality after waitlist dropout in HCC patients. Transplantation 2019, 103, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- Straś, W.A.; Wasiak, D.; Łągiewska, B.; Tronina, O.; Hreńczuk, M.; Gotlib, J.; Lisik, W.; Małkowski, P. Recurrence of HCC after liver transplantation. Ann. Transplant. 2022, 27, e934924. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Forner, A.; Zeuzem, S.; Galle, P.R.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Uemura, T.; Kirichenko, A.; Bunker, M.; Vincent, M.; Machado, L.; Thai, N. Stereotactic body radiation therapy in HCC while awaiting LT. World J. Surg. 2019, 43, 886–893. [Google Scholar] [CrossRef]
- Mehta, N.; Dodge, J.L.; Roberts, J.P.; Yao, F.Y. A novel waitlist dropout score for hepatocellular carcinoma. J. Hepatol. 2021, 74, 829–837. [Google Scholar] [CrossRef]
- Volk, M.; Marrero, J.A. Liver transplantation for hepatocellular carcinoma: Who benefits and who is harmed? Gastroenterology 2008, 134, 1612–1614. [Google Scholar] [CrossRef]
- Al-Ameri, A.; Yu, X.; Zheng, S. Predictors of post-recurrence survival in hepatocellular carcinoma. Transplant. Rev. 2022, 36, 100676. [Google Scholar] [CrossRef]
- Mazzaferro, V. Selection and allocation in liver transplantation for HCC. Hepatology 2015, 63, 1707–1717. [Google Scholar] [CrossRef]
- Wedd, J.P.; Nordstrom, E.; Nydam, T.; Durham, J.; Zimmerman, M.; Johnson, T.; Purcell, W.T.; Biggins, S.W. Hepatocellular carcinoma in patients listed for liver transplantation. Liver Transplant. 2015, 21, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Perkins, J.D.; Carithers, R.L. Liver transplantation for hepatocellular carcinoma. Gastroenterology 2008, 134, 1342–1351. [Google Scholar] [CrossRef]
- Freeman, R.B.; Edwards, E.B.; Harper, A.M. Waiting list removal rates. Am. J. Transplant. 2006, 6, 1416–1421. [Google Scholar] [CrossRef]
- Washburn, K.; Edwards, E.; Harper, A.; Freeman, R. HCC patients are advantaged in liver transplant allocation. Am. J. Transplant. 2010, 10, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- Toso, C.; Dupuis-Lozeron, E.; Majno, P.; Berney, T.; Kneteman, N.M.; Perneger, T.; Morel, P.; Mentha, G.; Combescure, C. A model for dropout assessment. Hepatology 2012, 56, 149–156. [Google Scholar] [CrossRef]
- Marvin, M.R.; Ferguson, N.; Cannon, R.M.; Jones, C.M.; Brock, G.N. MELDEQ: An alternative MELD score. Liver Transplant. 2015, 21, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Firl, D.J.; Sasaki, K.; Agopian, V.G.; Gorgen, A.; Kimura, S.; Dumronggittigule, W.; McVey, J.C.; Iesari, S.; Mennini, G.; Vitale, A.; et al. International validation of HALTHCC. Hepatology 2019, 71, 569–582. [Google Scholar] [CrossRef]
- Manzia, T.M.; Trapani, S.; Nardi, A.; Ricci, A.; Lenci, I.; Milana, M.; Angelico, R.; De Feo, M.T.; Agnes, S.; Andorno, E.; et al. Temporal trends in liver transplantation in Italy. Dig. Liver Dis. 2022, 54, 1664–1671. [Google Scholar] [CrossRef]
- Ravaioli, M.; Lai, Q.; Sessa, M.; Ghinolfi, D.; Fallani, G.; Patrono, D.; Di Sandro, S.; Avolio, A.; Odaldi, F.; Bronzoni, J.; et al. Impact of MELD 30-allocation policy on liver transplant outcomes. J. Hepatol. 2021, 76, 619–627. [Google Scholar] [CrossRef]
- DiNorcia, J.; Florman, S.S.; Haydel, B.; Tabrizian, P.; Ruiz, R.M.; Klintmalm, G.B.; Senguttuvan, S.; Lee, D.D.; Taner, C.B.; Verna, E.C.; et al. Pathologic response to pretransplant therapy. Ann. Surg. 2020, 271, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Vitale, A.; Iesari, S.; Finkenstedt, A.; Mennini, G.; Spoletini, G.; Hoppe-Lotichius, M.; Vennarecci, G.; Manzia, T.M.; Nicolini, D.; et al. Intention-to-treat survival benefit of liver transplantation. Hepatology 2017, 66, 1910–1919. [Google Scholar] [CrossRef]
- Cucchetti, A.; Serenari, M.; Sposito, C.; Di Sandro, S.; Mosconi, C.; Vicentin, I.; Garanzini, E.; Mazzaferro, V.; De Carlis, L.; Golfieri, R.; et al. Including mRECIST in Metroticket 2.0. J. Hepatol. 2020, 73, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Vitale, A.; Iesari, S.; Finkenstedt, A.; Mennini, G.; Onali, S.; Maria Hoppe-Lotichius, M.; Manzia, T.M.; Nicolini, D.; Avolio, A.W.; et al. Bridging treatments and Milan Criteria. Liver Transplant. 2019, 25, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
| Pre-ISO Era (n = 152) | ISO Era (n = 258) | Overall (N = 410) | p | |
|---|---|---|---|---|
| Median (IQR) or n (%) | ||||
| Delisting Cause | ||||
| Liver transplantation | 103 (67.8%) | 217 (84.1%) | 320 (78.0%) | <0.001 |
| Drop-Out | 49 (32.2%) | 41 (15.9%) | 90 (22.0%) | |
| Age at enlisting, years | 57.1 (50.5–61.4) | 57.5 (52.5–63.3) | 57.4 (51.8–62.6) | 0.04 |
| Age at delisting, years | 58.2 (51.8–62.8) | 58.9 (53.2–64.6) | 58.6 (52.7–63.7) | 0.07 |
| BMI | 25.6 (23–27.8) | 26.2 (24.1–29) | 26 (23.9–28.6) | 0.02 |
| Sex, male | 126 (82.9%) | 218 (84.5%) | 344 (83.9%) | 0.67 |
| HCV-related cirrhosis | 84 (55.3%) | 130 (50.4%) | 214 (52.2%) | 0.34 |
| HBV-related cirrhosis | 47 (30.9%) | 64 (24.8%) | 111 (27.1%) | 0.18 |
| Alcohol-related cirrhosis | 23 (15.1%) | 41 (15.9%) | 64 (15.6%) | 0.84 |
| NASH-related cirrhosis | 18 (11.8%) | 35 (13.6%) | 53 (12.9%) | 0.62 |
| Cryptogenic-related cirrhosis | 2 (1.3%) | 7 (2.7%) | 9 (2.2%) | 0.35 |
| Other | 4 (2.6%) | 6 (2.4%) | 10 (2.5%) | 0.91 |
| Blood group | 0.20 | |||
| 0 | 53 (34.9%) | 113 (43.8%) | 166 (40.5%) | |
| A | 71 (46.7%) | 94 (36.4%) | 165 (40.2%) | |
| B | 19 (12.5%) | 37 (14.3%) | 56 (13.7%) | |
| AB | 9 (5.9%) | 14 (5.4%) | 23 (5.6%) | |
| Previous surgery | 96 (63.2%) | 171 (66.3%) | 267 (65.1%) | 0.52 |
| PVT | 0.39 | |||
| Absent | 116 (76.3%) | 201 (77.9%) | 317 (77.3%) | |
| Partial | 28 (18.4%) | 37 (14.3%) | 65 (15.9%) | |
| Complete | 8 (5.3%) | 20 (7.8%) | 28 (6.8%) | |
| Diabetes | 54 (35.5%) | 64 (24.8%) | 118 (28.8%) | 0.02 |
| Waiting list time, months | 10.9 (4.6–19.9) | 10.2(4.4–17.9) | 10.6(4.4–18.1) | 0.52 |
| Pre-ISO Era (n = 152) | ISO Era (n = 258) | Overall (N = 410) | p | |
|---|---|---|---|---|
| Median (IQR) or n (%) | ||||
| Child–Pugh Score | ||||
| A | 142 (39.0) | 43 (29.3) | 99 (45.6) | 0.007 |
| B | 130 (35.7) | 60 (40.8) | 70 (32.3) | |
| C | 92 (25.3) | 44 (29.9) | 48 (22.1) | |
| Number of HCC tumors at diagnosis | 0.06 | |||
| 1 | 81 (54.4%) | 110 (42.6%) | 191 (46.9%) | |
| 2 | 31 (20.8%) | 78 (30.2%) | 109 (26.8%) | |
| 3 | 26 (17.4%) | 41 (15.9%) | 67 (16.5%) | |
| >3 | 11 (7.4%) | 29 (11.2%) | 40 (9.8%) | |
| Maximum HCC diameter at diagnosis | 0.41 | |||
| ≤20 mm | 59 (39.3%) | 115 (44.6%) | 174 (42.6%) | |
| 20–50 mm | 85 (56.7%) | 129 (50.0%) | 214 (52.5%) | |
| ≥50 mm | 6 (4.0%) | 14 (5.4%) | 20 (4.9%) | |
| Milan Out at diagnosis | 32 (21.1%) | 65 (25.2%) | 97 (23.7%) | 0.34 |
| AFP diagnosis, ng/mL | 7.0 (4.0–26.0) | 6.1 (3.5–14.1) | 6.8 (3.7–17) | 0.35 |
| LRT | 141 (92.8%) | 232 (89.9%) | 373 (91.0%) | 0.33 |
| LR | 34 (22.4%) | 54 (20.9%) | 88 (21.5%) | 0.73 |
| Ablation | 77 (51.0%) | 132 (52.0%) | 209 (51.6%) | 0.85 |
| LR + ablation | 97 (64.7%) | 33 (44.6%) | 130 (58.0%) | 0.004 |
| TACE | 93 (61.6%) | 135 (53.1%) | 228 (56.3%) | 0.10 |
| Y-90 | 2 (1.3%) | 14 (5.5%) | 16 (4.0%) | 0.04 |
| Other treatments | 2 (1.3%) | 19 (7.3%) | 21 (5.1%) | 0.007 |
| Variables | Pre-ISO Era (n = 103) | ISO Era (n = 217) | Overall (N = 320) | p |
|---|---|---|---|---|
| Median (IQR) or n (%) | ||||
| LRT | 97 (94.2%) | 197 (90.8%) | 294 (91.9%) | 0.3 |
| LR | 22 (21.4%) | 47 (21.7%) | 69 (21.6%) | 0.95 |
| Ablation | 52 (50.5%) | 113 (52.8%) | 165 (52.1%) | 0.70 |
| LR + ablation | 64 (62.7%) | 11 (32.4%) | 75 (55.1%) | 0.002 |
| TACE | 66 (64.1%) | 114 (53.3%) | 180 (56.8%) | 0.07 |
| Y-90 | 2 (1.9%) | 12 (5.6%) | 14 (4.4%) | 0.14 |
| Other treatments | 1 (1%) | 14 (6.4%) | 15 (4.7%) | 0.031 |
| Number of HCC tumors at diagnosis | 0.07 | |||
| 1 | 60 (58.3%) | 95 (43.8%) | 155 (48.4%) | |
| 2 | 22 (21.4%) | 66 (30.4%) | 88 (27.5%) | |
| 3 | 16 (15.5%) | 35 (16.1%) | 51 (15.9%) | |
| >3 | 5 (4.9%) | 21 (9.7%) | 26 (8.1%) | |
| Maximum HCC diameter at diagnosis | 0.37 | |||
| ≤20 mm | 41 (39.8%) | 99 (45.6%) | 140 (43.8%) | |
| 20–50 mm | 58 (56.3%) | 105 (48.4%) | 163 (50.9%) | |
| ≥50 mm | 4 (3.9%) | 13 (6.0%) | 17 (5.3%) | |
| Milan Out at diagnosis | 16 (15.5%) | 55 (25.3%) | 71 (22.2%) | 0.048 |
| AFP diagnosis, ng/mL | 9.8 (4.0–30.0) | 6.1 (3.8–15.0) | 7.1 (4.0–19.0) | 0.03 |
| Last HCC number before LT | 0.12 | |||
| 0 | 32 (31.1%) | 101 (46.5%) | 133 (41.6%) | |
| 1 | 35 (34.0%) | 57 (26.3%) | 92 (28.7%) | |
| 2 | 15 (14.6%) | 28 (12.9%) | 43 (13.4%) | |
| 3 | 12 (11.7%) | 17 (7.8%) | 29 (9.1%) | |
| >3 | 9 (8.7%) | 14 (6.5%) | 23 (7.2%) | |
| Last maximum HCC diameter before LT | ||||
| ≤20 mm | 49 (69%) | 88 (76%) | 137 (73.3%) | 0.57 |
| 20–50 mm | 20 (28.2%) | 26 (22.4%) | 46 (24.6%) | |
| ≥50 mm | 2 (2.8%) | 2 (1.7%) | 4 (2.1%) | |
| AFP at LT, ng/mL | 9.0 (4.0–31.0) | 5.105 (3.0–12.1) | 6.0 (3.1–18.2) | 0.001 |
| Radiological cRPR | 32 (31.1%) | 101 (46.5%) | 133 (41.6%) | 0.009 |
| MELD pre-LT | 14 (9–19) | 12 (9–16) | 12 (9–17) | 0.01 |
| Histological cPR | 17 (16.8%) | 65 (30.5%) | 82 (26.1%) | 0.01 |
| Last imaging before LT, months | 3.0 (1.3–5.4) | 2.1 (1.2–3.8) | 2.3 (1.2–4.4) | 0.009 |
| Waiting list time, months | 8.7 (4.2–17.6) | 9.2 (4.1–16.8) | 9.02 (4.2–17.3) | 0.81 |
| Waiting list > 6 months | 68 (66.0%) | 142 (65.4%) | 210 (65.6%) | 0.92 |
| Recurrence | 15 (14.7%) | 25 (11.5%) | 40 (12.5%) | 0.414 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prosperi, E.; Cescon, M.; Lai, Q.; Bonatti, C.; Prosperi, E.; Rizzo, F.; Maroni, L.; Laurenzi, A.; Serenari, M.; Morelli, M.C.; et al. The Italian Score for Organ Allocation: A Ten-Year Monocentric Retrospective Analysis in Liver Transplantation for Hepatocellular Carcinoma. Cancers 2025, 17, 1720. https://doi.org/10.3390/cancers17101720
Prosperi E, Cescon M, Lai Q, Bonatti C, Prosperi E, Rizzo F, Maroni L, Laurenzi A, Serenari M, Morelli MC, et al. The Italian Score for Organ Allocation: A Ten-Year Monocentric Retrospective Analysis in Liver Transplantation for Hepatocellular Carcinoma. Cancers. 2025; 17(10):1720. https://doi.org/10.3390/cancers17101720
Chicago/Turabian StyleProsperi, Enrico, Matteo Cescon, Quirino Lai, Chiara Bonatti, Edoardo Prosperi, Francesca Rizzo, Lorenzo Maroni, Andrea Laurenzi, Matteo Serenari, Maria Cristina Morelli, and et al. 2025. "The Italian Score for Organ Allocation: A Ten-Year Monocentric Retrospective Analysis in Liver Transplantation for Hepatocellular Carcinoma" Cancers 17, no. 10: 1720. https://doi.org/10.3390/cancers17101720
APA StyleProsperi, E., Cescon, M., Lai, Q., Bonatti, C., Prosperi, E., Rizzo, F., Maroni, L., Laurenzi, A., Serenari, M., Morelli, M. C., & Ravaioli, M. (2025). The Italian Score for Organ Allocation: A Ten-Year Monocentric Retrospective Analysis in Liver Transplantation for Hepatocellular Carcinoma. Cancers, 17(10), 1720. https://doi.org/10.3390/cancers17101720







