Investigation of Normal Tissue Toxicity in Pulsed Low Dose Rate Radiotherapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Test Subjects
2.1.1. Phase I
2.1.2. Phase II.1, II.2, and II.3
2.2. Treatment and Setup
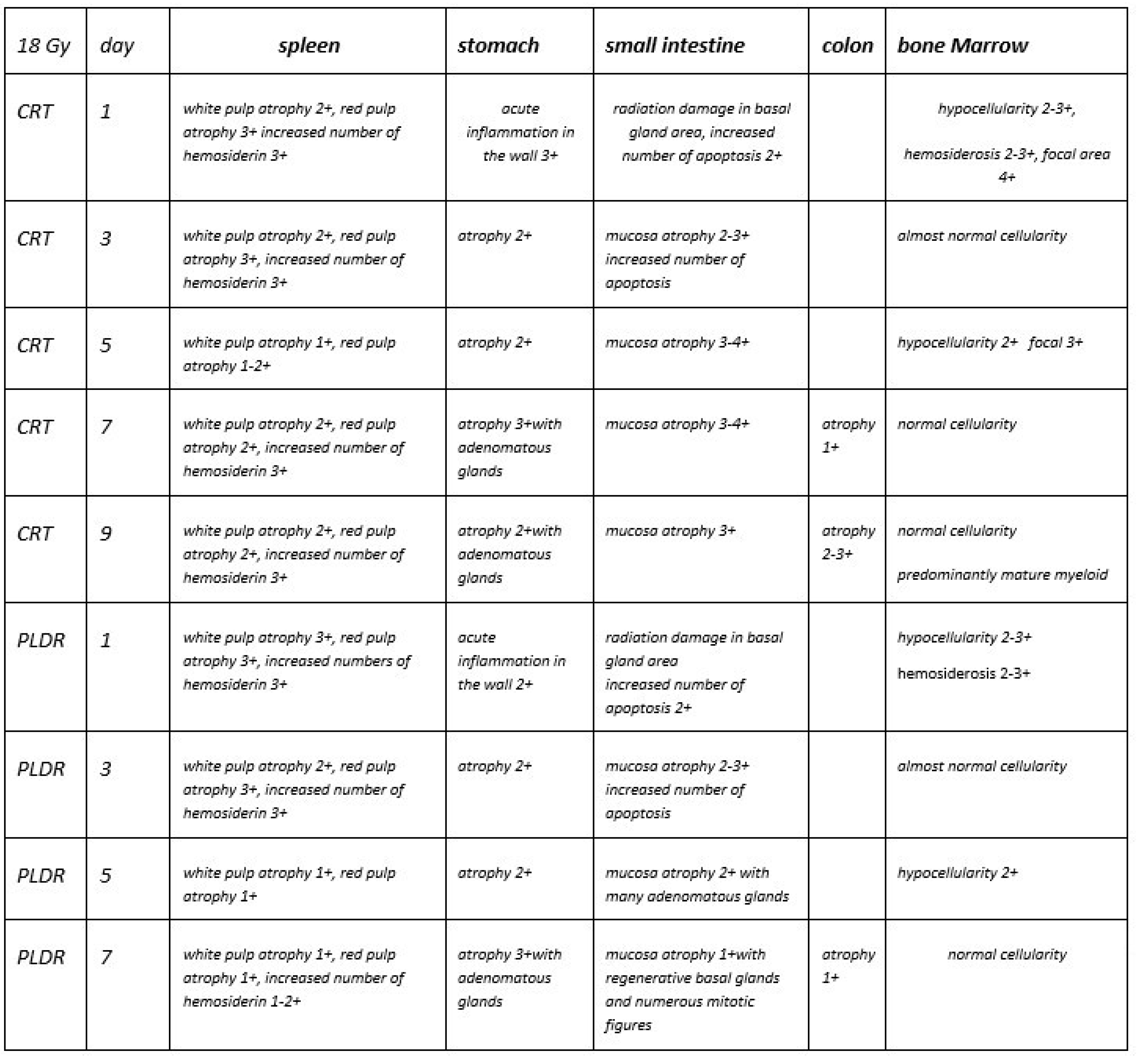
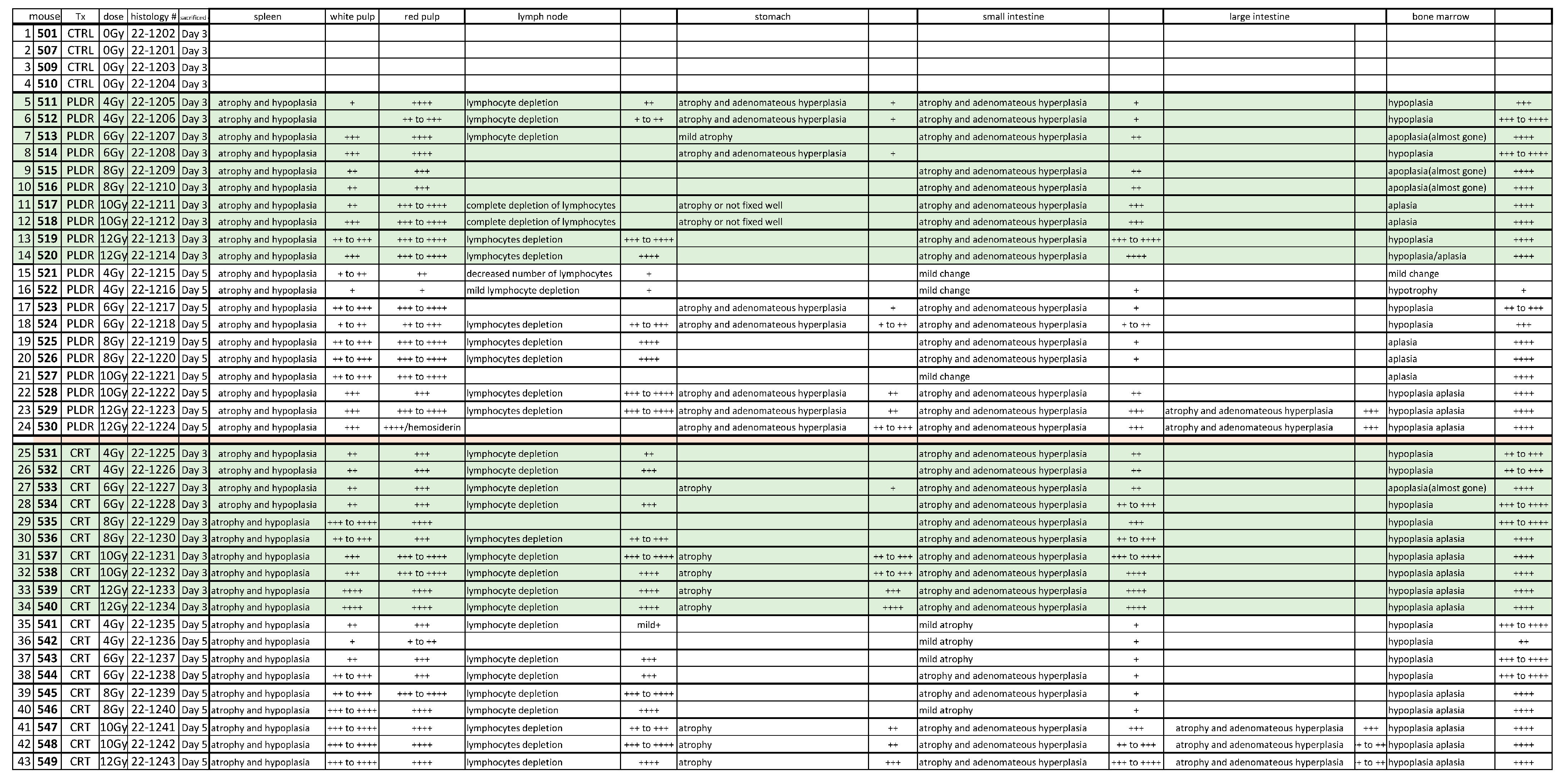
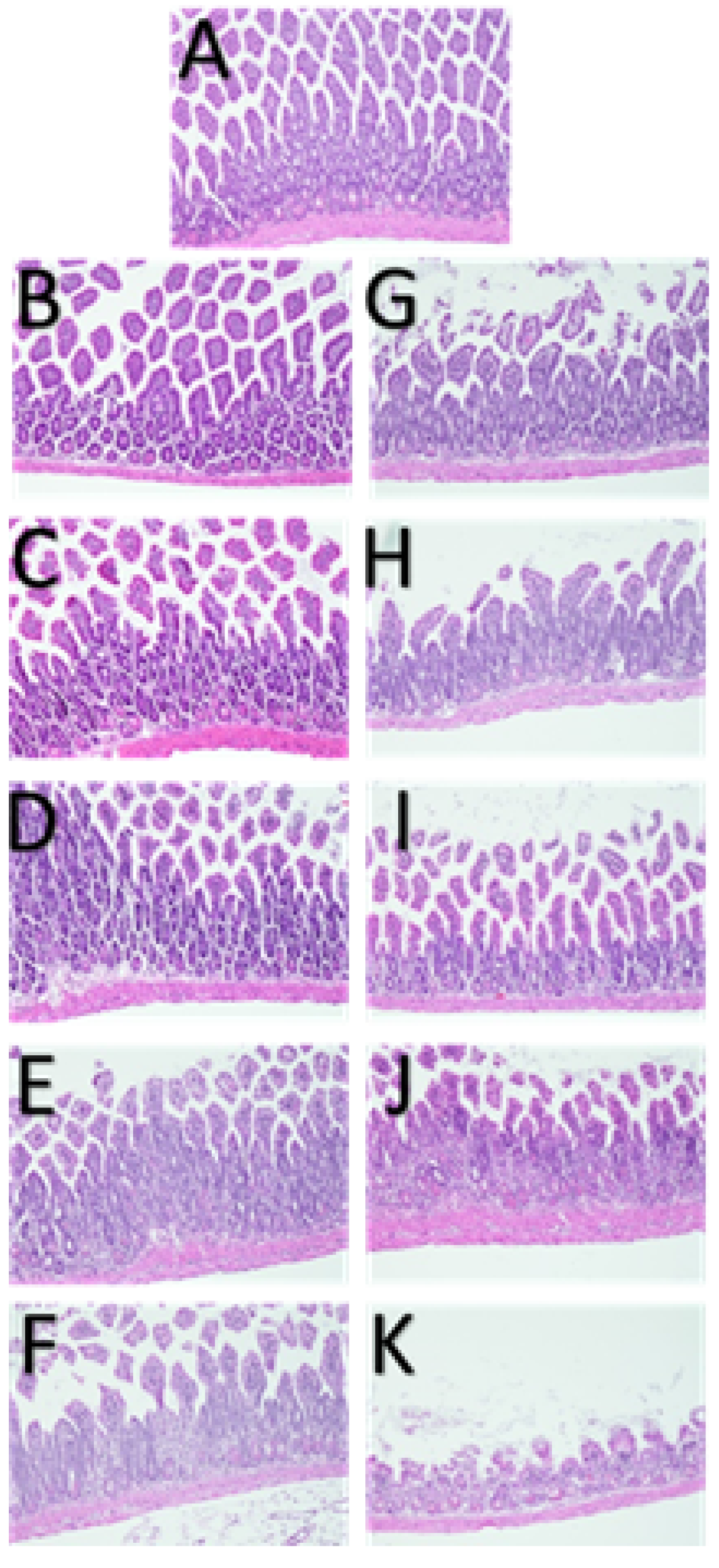
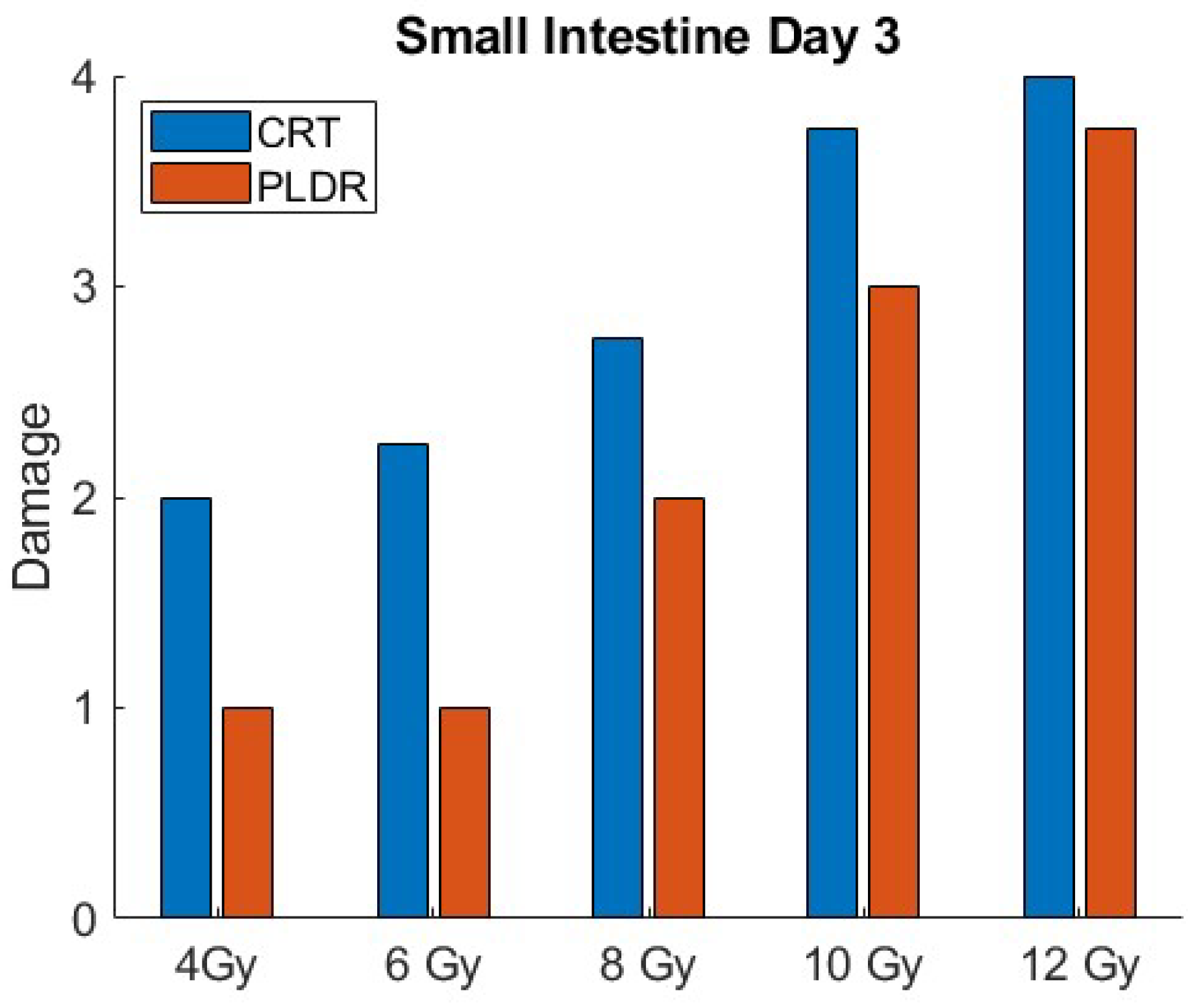
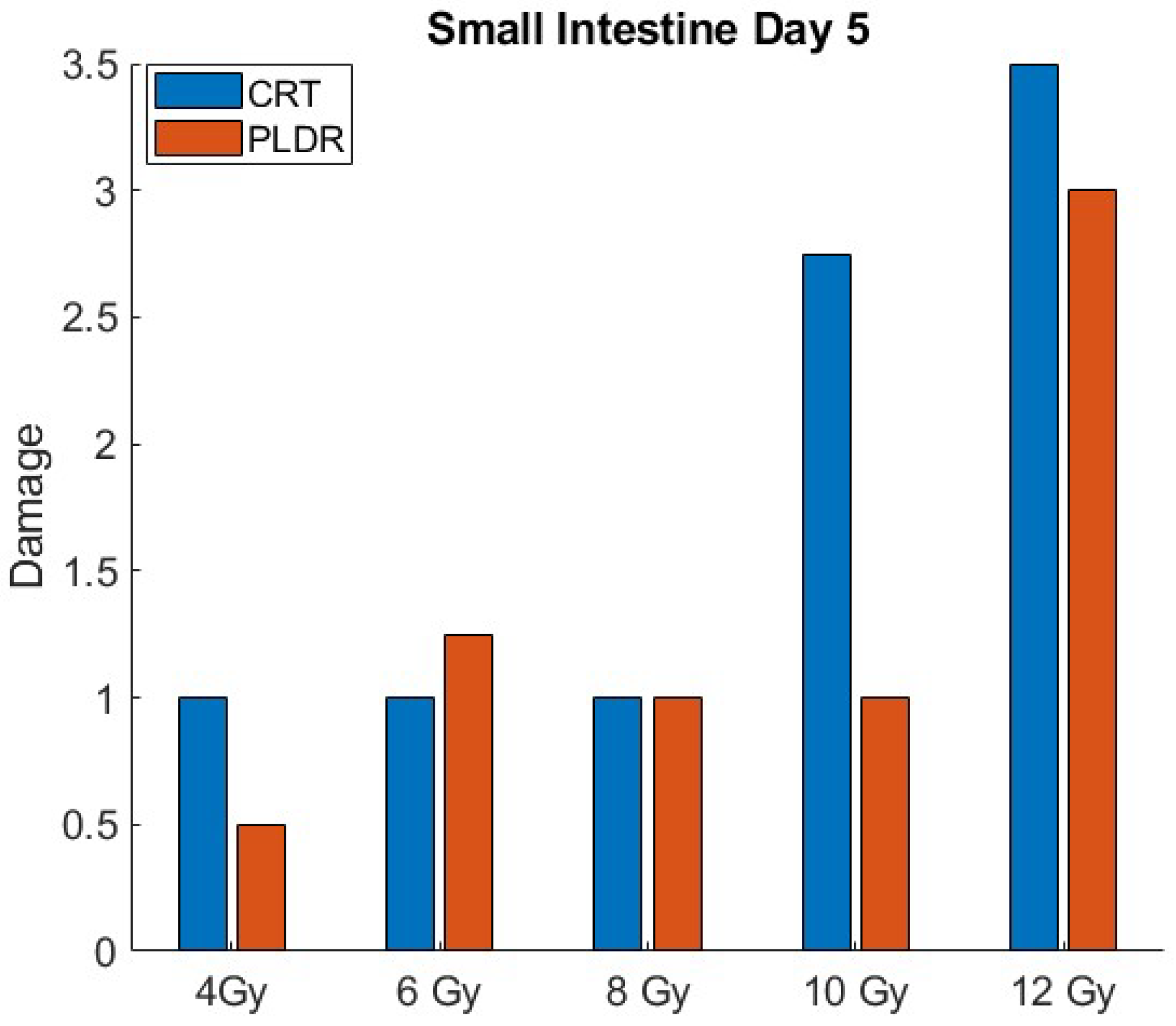
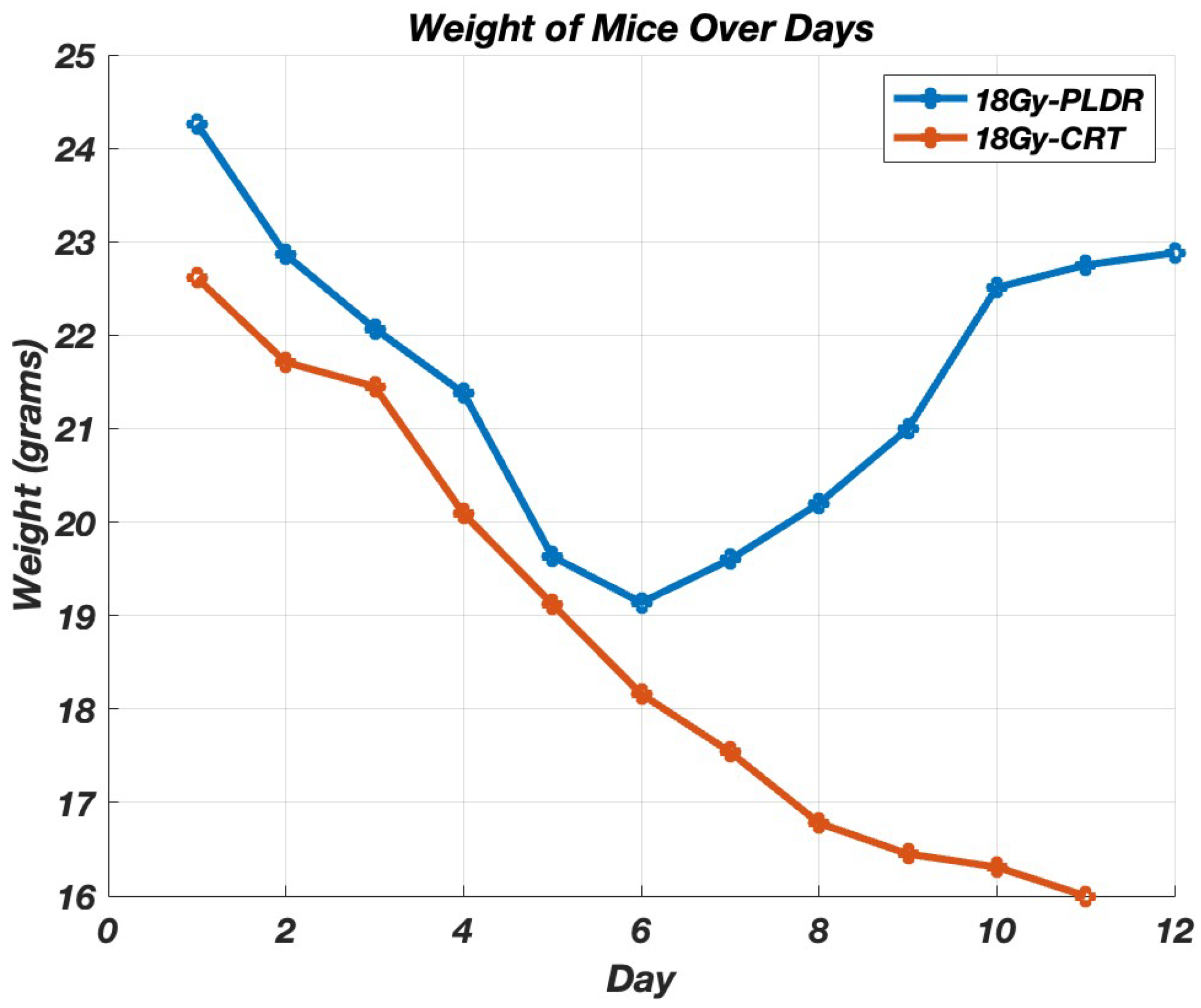
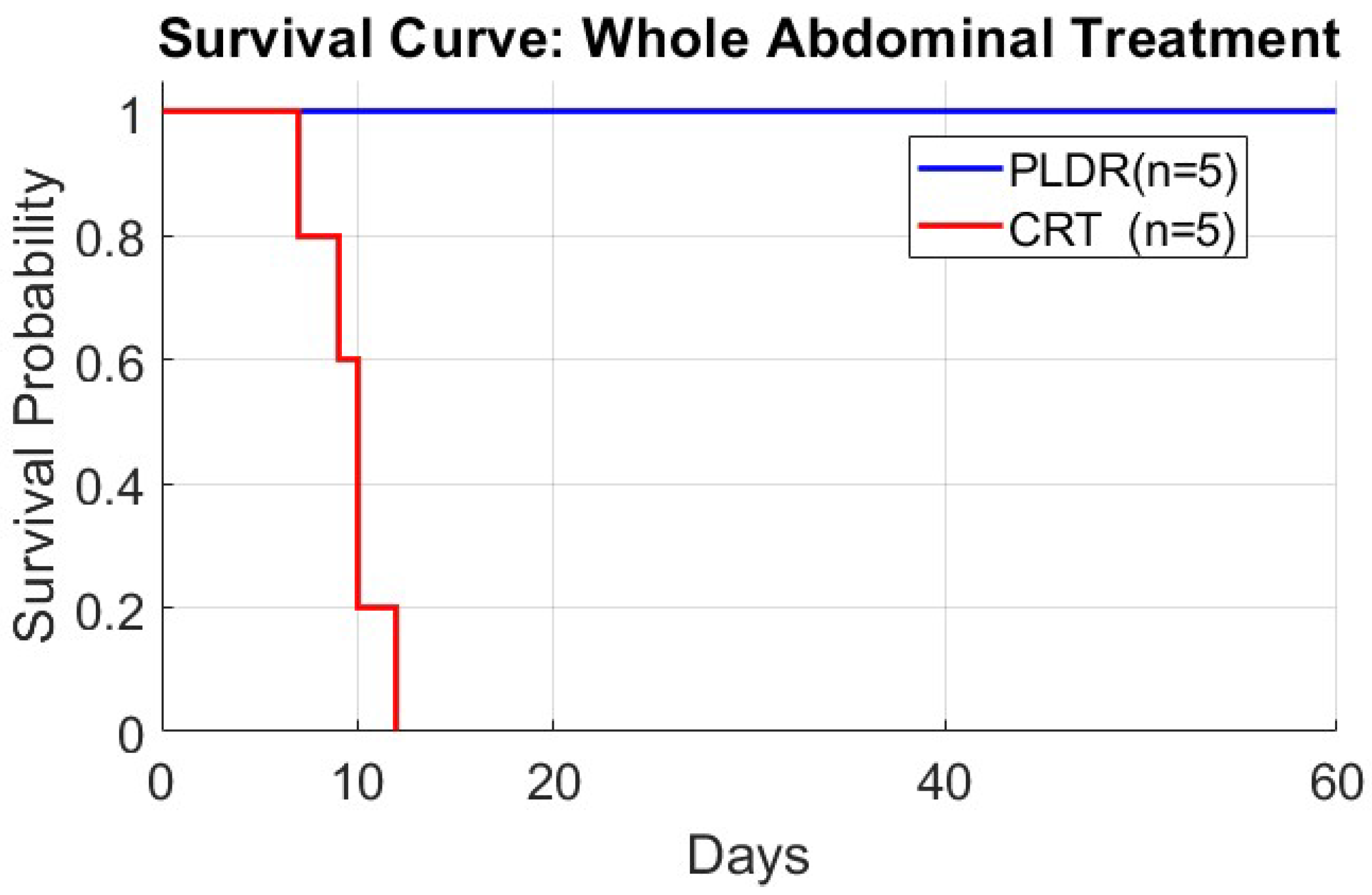
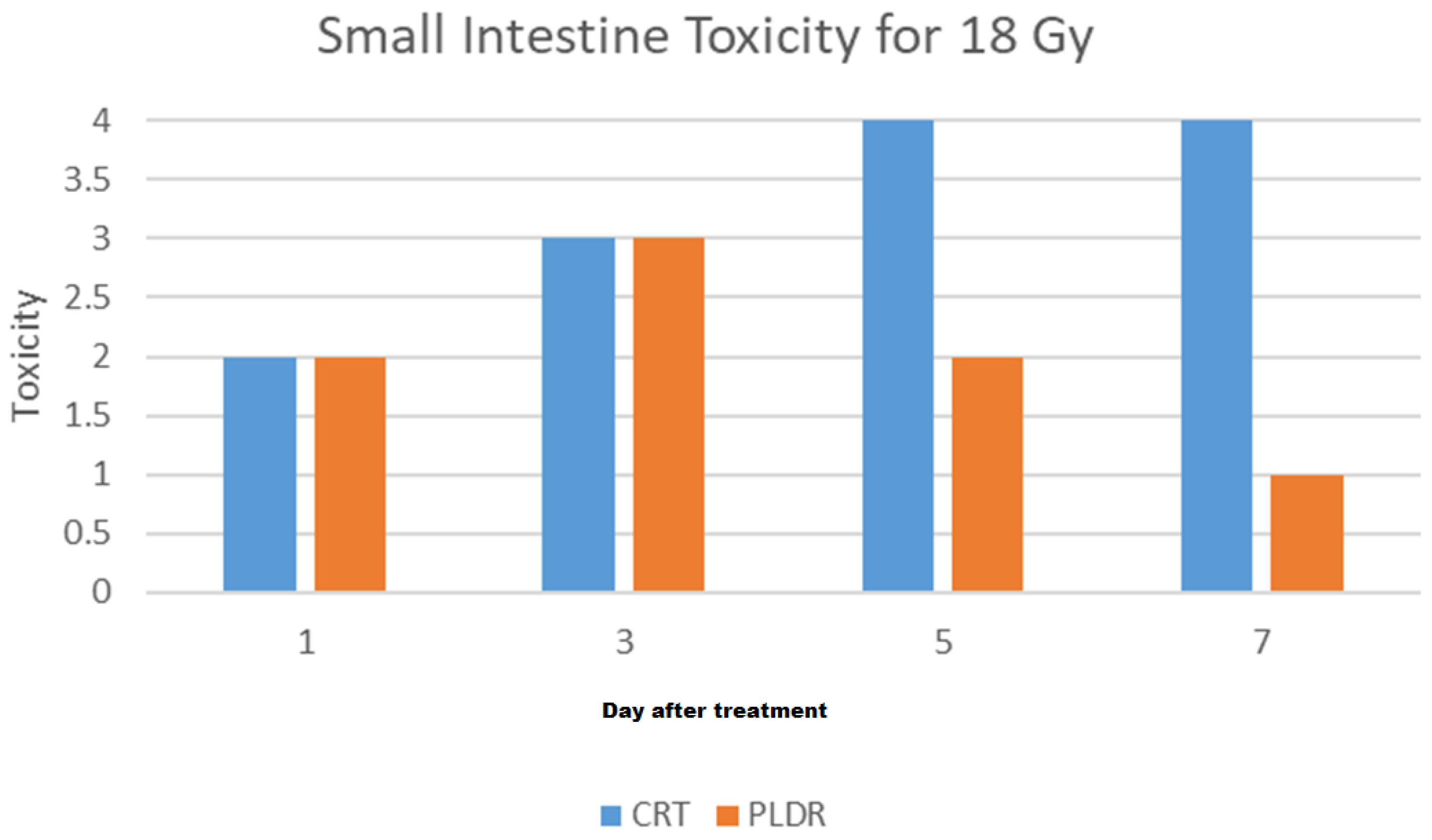
3. Results
3.1. Treatment and Setup
3.1.1. Phase I
3.1.2. Phase II.1
3.1.3. Phase II.2
3.1.4. Phase III
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Koka, K.; Verma, A.; Dwarakanath, B.S.; Papineni, R.V. Technological Advancements in External Beam Radiation Therapy (EBRT): An Indispensable Tool for Cancer Treatment. Cancer Manag. Res. 2022, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Song, C.W.; Terezakis, S.; Emami, B.; Griffin, R.J.; Sperduto, P.W.; Kim, M.; Hui, S.; Dusenbery, K.; Cho, L. Indirect cell death and the LQ model in SBRT and SRS. J. Radiosurg. SBRT 2020, 7, 1–4. [Google Scholar] [PubMed]
- Brown, J.M.; Carlson, D.J.; Brenner, D.J. The Tumor Radiobiology of SRS and SBRT: Are More than the 5 Rs Involved? Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.J.; Keall, P.; Loo, B.W.; Chen, Z.J.; Brown, J.M. Hypofractionation Results in Reduced Tumor Cell Kill Compared to Conventional Fractionation for Tumors with Regions of Hypoxia. Int. J. Radiat. Oncol. 2011, 79, 1188–1195. [Google Scholar] [CrossRef]
- Guerrero, M.; Li, X.A. Extending the linear-quadratic model for large fraction doses pertinent to stereotactic radiotherapy. Phys. Med. Biol. 2004, 49, 4825–4835. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing Radiation Inhibition of Distant Untreated Tumors (Abscopal Effect) Is Immune Mediated. J. Radiat. Oncol. Biol. Phys. 2004, 58, 862–870. [Google Scholar] [CrossRef]
- Hall, E.J.; Brenner, D.J. The dose-rate effect revisited: Radiobiological considerations of importance in radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 1403–1414. [Google Scholar] [CrossRef]
- Joiner, M.C.; Marples, B.; Lambin, P.; Short, S.C.; Turesson, I. Low-dose hypersensitivity: Current status and possible mechanisms. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 379–389. [Google Scholar] [CrossRef]
- Wykes, S.M.; Piasentin, E.; Joiner, M.C.; Wilson, G.D.; Marples, B. Low-dose hyper-radiosensitivity is not caused by a failure to recognize DNA double-strand breaks. Radiat. Res. 2006, 165, 516–524. [Google Scholar] [CrossRef]
- Matsuya, Y.; McMahon, S.J.; Tsutsumi, K.; Sasaki, K.; Okuyama, G.; Yoshii, Y.; Mori, R.; Oikawa, J.; Prise, K.M.; Date, H. Investigation of Dose-Rate Effects and Cell-Cycle Distribution under Protracted Exposure to Ionizing Radiation for Various Dose-Rates. Sci. Rep. 2018, 8, 8287. [Google Scholar] [CrossRef]
- Ma, C.C. Pulsed low dose-rate radiotherapy: Radiobiology and dosimetry. Phys. Med. Biol. 2022, 67, 03TR01. [Google Scholar] [CrossRef] [PubMed]
- Marples, B.; Collis, S.J. Low-dose hyper-radiosensitivity: Past, present, and future. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Tomé, W.A.; Howard, S.P. On the possible increase in local tumour control probability for gliomas exhibiting low dose hyper-radiosensitivity using a pulsed schedule. Br. J. Radiol. 2007, 80, 32–37. [Google Scholar] [CrossRef]
- Park, S.S.; Chunta, J.L.; Robertson, J.M.; Martinez, A.A.; Wong, C.Y.O.; Amin, M.; Wilson, G.D.; Marples, B. MicroPET/CT imaging of an orthotopic model of human glioblastoma multiforme and evaluation of pulsed low-dose irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 885–892. [Google Scholar] [CrossRef]
- He, K.; Barsoumian, H.B.; Sezen, D.; Puebla-Osorio, N.; Hsu, E.Y.; Verma, V.; Abana, C.O.; Chen, D.; Patel, R.R.; Gu, M.; et al. Pulsed Radiation Therapy to Improve Systemic Control of Metastatic Cancer. Front. Oncol. 2021, 11, 737425. [Google Scholar] [CrossRef]
- Dos-Santos, T.; Cvetkovic, D.; Chen, L.; Liu, S.; Li, M.; Yang, L.; Wu, D.; Ma, C.M. Investigation of Dose-Rate Effects in Pulsed Low Dose Rate Radiotherapy. Mathews J. Cancer Sci. 2022, 7, 1–6. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, B.; Chen, X.; Cvetkovic, D.; Chen, L.; Lang, J.; Ma, C. Local Tumor Control and Normal Tissue Toxicity of Pulsed Low-Dose-Rate Radiation Therapy (PLRT) for Recurrent Lung Cancer: An In Vivo Study. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, S788. [Google Scholar] [CrossRef]
- Wang, B.; Ren, J.; Zhang, P.; Cvetkovic, D.; Chen, X.; Chen, L.; Ma, C.M. An In-Vivo Study on Pulsed Low-Dose-Rate Radiotherapy for Prostate Cancer. Mathews J. Cancer Sci. 2019, 4, 1–7. [Google Scholar] [CrossRef]
- Dilworth, J.T.; Krueger, S.A.; Dabjan, M.; Grills, I.S.; Torma, J.; Wilson, G.D.; Marples, B. Pulsed low-dose irradiation of orthotopic glioblastoma multiforme (GBM) in a pre-clinical model: Effects on vascularization and tumor control. Radiother. Oncol. 2013, 108, 149–154. [Google Scholar] [CrossRef]
- Cannon, G.M.; Tomé, W.A.; Robins, H.I.; Howard, S.P. Pulsed reduced dose-rate radiotherapy: Case report: A novel re-treatment strategy in the management of recurrent glioblastoma multiforme. J. Neurooncol. 2007, 83, 307–311. [Google Scholar] [CrossRef]
- Adkison, J.B.; Tomé, W.; Seo, S.; Richards, G.M.; Robins, H.I.; Rassmussen, K.; Welsh, J.; Mahler, P.; Howard, S.P.S. Reirradiation of large-volume recurrent glioma with pulsed reduced-dose-rate radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-M.; Mu, Z.M.; Tafo, A.; Chen, L. Variation of cytotoxic effect with pulsed dose sequence and low dose rate radiation. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, S629–S630. [Google Scholar] [CrossRef]
- Schoenherr, D.; Krueger, S.A.; Martin, L.; Marignol, L.; Wilson, G.D.; Marples, B. Determining if low dose hyper-radiosensitivity (HRS) can be exploited to provide a therapeutic advantage: A cell line study in four glioblastoma multiforme (GBM) cell lines. Int. J. Radiat. Biol. 2013, 89, 1009–1016. [Google Scholar] [CrossRef]
- Terashima, S.; Hosokawa, Y.; Tsuruga, E.; Mariya, Y.; Nakamura, T. Impact of time interval and dose rate on cell survival following low-dose fractionated exposures. J. Radiat. Res. 2017, 58, 782–790. [Google Scholar] [CrossRef]
- Todorovic, V.; Prevc, A.; Zakelj, M.N.; Savarin, M.; Bucek, S.; Groselj, B.; Strojan, P.; Cemazar, M.; Sersa, G. Pulsed low dose-rate irradiation response in isogenic HNSCC cell lines with different radiosensitivity. Radiol. Oncol. 2020, 54, 168–179. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslmarand, S.M.; Dos Santos, T.; Yang, D.-M.; Cvetkovic, D.; Chen, L.; Ma, C.-M.C. Investigation of Normal Tissue Toxicity in Pulsed Low Dose Rate Radiotherapy. Cancers 2025, 17, 1701. https://doi.org/10.3390/cancers17101701
Aslmarand SM, Dos Santos T, Yang D-M, Cvetkovic D, Chen L, Ma C-MC. Investigation of Normal Tissue Toxicity in Pulsed Low Dose Rate Radiotherapy. Cancers. 2025; 17(10):1701. https://doi.org/10.3390/cancers17101701
Chicago/Turabian StyleAslmarand, Shahabeddin M., Troy Dos Santos, Dae-Myoung Yang, Dusica Cvetkovic, Lili Chen, and C.-M. Charlie Ma. 2025. "Investigation of Normal Tissue Toxicity in Pulsed Low Dose Rate Radiotherapy" Cancers 17, no. 10: 1701. https://doi.org/10.3390/cancers17101701
APA StyleAslmarand, S. M., Dos Santos, T., Yang, D.-M., Cvetkovic, D., Chen, L., & Ma, C.-M. C. (2025). Investigation of Normal Tissue Toxicity in Pulsed Low Dose Rate Radiotherapy. Cancers, 17(10), 1701. https://doi.org/10.3390/cancers17101701






