Simple Summary
The present study aimed to analyze and compare potential associations between the usage of general or combined general/epidural anesthesia and postoperative cytokines. Given the association between inflammatory response and various complications after surgery, we narrowed our focus to pro-inflammatory and regulatory cytokines. We performed a meta-analysis on 21 studies, finding that serum levels of IL-6, TNF-α, CRP, and other pro-inflammatory cytokines were significantly less for combined epidural and general anesthesia compared to general anesthesia alone. Our results provide an alternative explanation for the benefit of epidural analgesia on postoperative outcomes. Further studies on the effect of epidural anesthesia on postoperative cytokines are required to assess this relationship reliably, as well as the potential application to clinical settings.
Abstract
Background and Objectives: Local and systemic inflammation is common after surgery and is associated with morbidity and mortality. Inflammatory cytokines have been implicated in cancer metastasis following cancer surgery. The present study aimed to analyze inflammatory cytokines levels after surgery under combined epidural/general anesthesia (EA + GA) vs. general anesthesia (GA). Methods: We systematically searched PubMed, Central, EMBASE, CINAHL, Google Scholar, and Web of Science citation indexes for clinical studies (cancer and non-cancer surgery) comparing the two techniques. We carried out a meta-analysis to evaluate the postoperative plasma levels of cytokines, C-reactive protein (CRP), and cortisol levels. Results: The literature search was last updated on 2 January 2025. We identified a total of 21 studies which compared postoperative inflammatory mediators with EA plus GA compared to GA alone. EA plus GA was associated with significantly lower serum levels of IL-6, TNF-α, CRP, as well as cortisol and other pro-inflammatory cytokines. In cancer surgery, EA plus GA was also associated with lower postoperative cytokines. Conclusions: Our meta-analysis indicates that EA plus GA is associated with diminished postoperative inflammatory response. This offers an alternative explanation for the benefit of epidural analgesia on postoperative outcomes. Considering the link between postoperative inflammation and recurrence after cancer surgery, this is an area that warrants further research.
1. Introduction
Surgical interventions evoke a stress response in patients due to tissue trauma, which is characterized by endocrine alterations, including an increase in plasma cortisol and activation of the sympathetic nervous system with secretion of endogenous catecholamines [1]. The local tissue injury during surgical and perioperative events such as blood transfusion also initiates a systemic inflammatory response [2]. The surge of pro-inflammatory cytokine release, detectable in peripheral blood during the immediate postoperative period has been implicated in the development of various complications, including myocardial injury, acute kidney injury and postoperative delirium [3,4]. The change in postoperative cytokine profiles may also increase the risk of infectious complications [5,6]. Studies have also shown that postoperative inflammation is a risk factor for postoperative mortality, independent from the occurrence of postoperative complications [7,8]. Current research suggests that the choice of anesthetics and postoperative analgesia may alter the profile of the postoperative immune response [9,10] and could affect long term outcomes after surgery [11,12,13].
Epidural anesthesia or analgesia (EA) is a well-established regional anesthesia technique for postoperative analgesia and may be used for surgical anesthesia in selected procedures. Administration of local anesthetic in the epidural space prevents neurotransmission through the spinal nerves, thus preventing transmission pain, as well as other sensory, motor, and sympathetic impulses [14]. It has been suggested that EA may reduce cortisol production and maintain a physiological cytokine balance during postoperative period [15]. Additionally, the use of EA could decrease the demand for systemic opioids, thereby reducing the negative effect of opioids on cell-mediated immunity [16]. Despite several clinical trials investigating the effect of epidural analgesia on perioperative inflammatory mediators, no meta-analysis has been performed on the topic. Evaluation of these changes across various anesthesia methods will help to optimize recovery and guide postoperative care. Therefore, we conducted a meta-analysis on the postoperative inflammatory responses after general anesthesia (GA) compared to GA plus EA.
2. Materials and Methods
2.1. Study Objectives
The objective of the study is to compare the postoperative serum cytokine levels in patients who had general anesthesia plus EA to those who had general anesthesia only with postoperative systemic analgesia. Our primary outcomes of interest are serum levels of interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor-α (TNF-α) 24 h after surgery. Our secondary outcomes of interest include CRP, TNF-α, IL-1β, IL-4, IL-8, IL-10, and cortisol levels immediately after surgery and 24 h postoperatively.
2.2. Search Strategy and Study Selection Criteria
We followed the Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) statement for conducting and reporting the meta-analysis [17]. The study was registered in the “International Prospective Register of Systematic Reviews” (PROSPERO) in 2019 (CRD42019145596). We systematically searched the PubMed, Central, EMBASE, CINAHL, Google Scholar, and Web of Science citation indexes for relevant studies. Used terms included ‘epidural’, ‘interleukin’, and ‘cytokine’, and their Boolean combinations, the full search strategy is included in the Electronic Supplementary Material (ESM). We did not impose any language restriction at the time of the literature search. Two authors independently conducted all the searches and discrepancies were discussed after the search process. The last search was carried out on 2 January 2025.
Studies were included if they fulfill the following criteria:
Study design and patients: randomized control trials in adult patients undergoing cancer or non-cancer surgery under general anesthesia.
Intervention: epidural catheter for perioperative analgesia.
Control: general anesthesia only without epidural analgesia.
Outcome: studies need to report at least one of the primary outcomes to be included.
Comparison must be reported as mean ± standard deviation. Alternatively, it must be possible to derive mean ± standard deviation from the reported data. Studies which did not include data in a suitable format were excluded from the analysis. Exclusion criteria were studies with children under 18, ongoing clinical trials, study designs other than RCTs, studies on subjects who did not have surgery under general anesthesia, and studies where the patient received regional anesthesia other than EA.
2.3. Data Extraction
The following information was extracted from each study: bibliographical information (family name of the first author, year of publication, and PubMed ID), study design (number of participants in the GA + EA and EA cohort and time points for outcome measurement), and outcomes (plasma level of cytokine, interleukin, and stress hormone). When study results are only displayed as graphical form, two authors independently extracted the data using WebPlot Digitizer [18]. For the studies where the plasma cytokine values were expressed as “medium” and no standard deviation values were reported, the mean ± standard deviation values were obtained using ‘Deep Meta tool Version 1.0’ [19]. Data extraction was conducted using standardized pro-forma and checked by a second author (RL and EL).
We used the Cochrane Collaboration tool 2.0 for assessing the risk of bias in each included study [20]. Two authors performed the assessment independently at the same time and any disagreements were discussed with and resolved by a third author. Studies were assessed on randomization, deviation from intended intervention, missing outcome data, outcome measurement, and selective reporting. Each category of the study was assigned ‘low risk’, ‘high risk’, or ‘some concerns’.
2.4. Statistical Analyses
We conducted meta-analysis for outcomes reported in more than five studies. Review Manager V5.3. (Cochrane Collaboration, Copenhagen, Denmark) was used for the pooled analysis. As the effect size for the outcomes are of clinical relevance, for continuous variables, we calculated the standardized mean differences (SMD) based on the random effect model. The random effect model was used in the analysis due to varying effect sizes between studies and the inherent heterogeneous nature of the surgical procedure and patient population involved. In addition, we conducted subgroup analyses of the primary outcomes, including only cancer surgeries. For outcomes that reported positive findings, Duval and Tweedie’s trim and fill was used to assessed the publication bias and Egger’s regression was used to assess for small study effect; both were conducted using methods described by Suurmond et al. [21]. For all outcomes, the statistical significance was set to p < 0.05 and with 95% confidence intervals. We used GRADEpro Guideline Development Tool (GRADEpro GDT, McMaster University, 2015) to assess the quality of the meta-analysis findings.
3. Results
3.1. Description of Included Studies
Following the search criteria, a total of 355 studies were shortlisted, of which 34 passed the title and abstract screening (Figure 1). Fourteen were removed on further reading since four studies did not report the primary outcome we selected, two studies did not report the details of epidural procedure, one study reported LPS-induced TNF-α rather than serum level, one was a retrospective study, one study designed to compare different local anesthetics in epidural procedure, and one study did not report cytokine levels at the time point we chose. Finally, we included 21 studies in our meta-analysis and their characteristics are described in Table 1 [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. Among them, 15 are cancer surgeries and 7 are non-cancer surgeries. The risk of bias assessment for each study is shown in Figure 2. The most common source of bias came from the randomization process since most studies did not report information about how the allocation sequence was concealed.
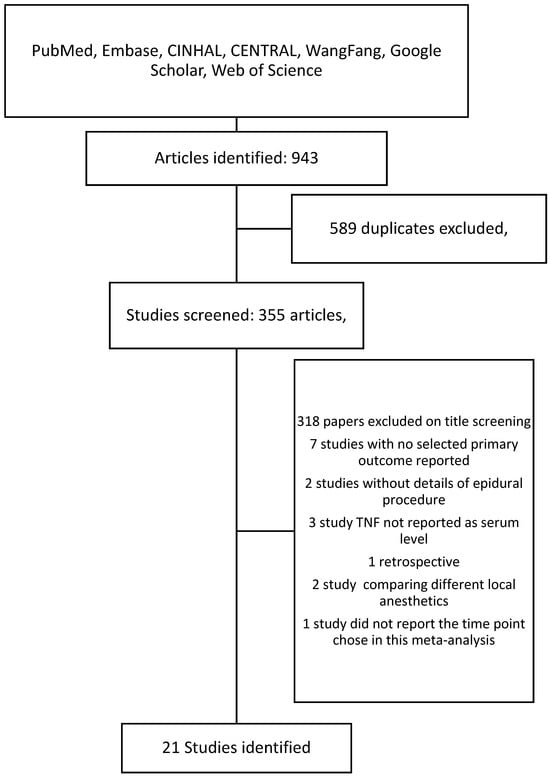
Figure 1.
Search flow chart.

Table 1.
Characteristics of the included studies.
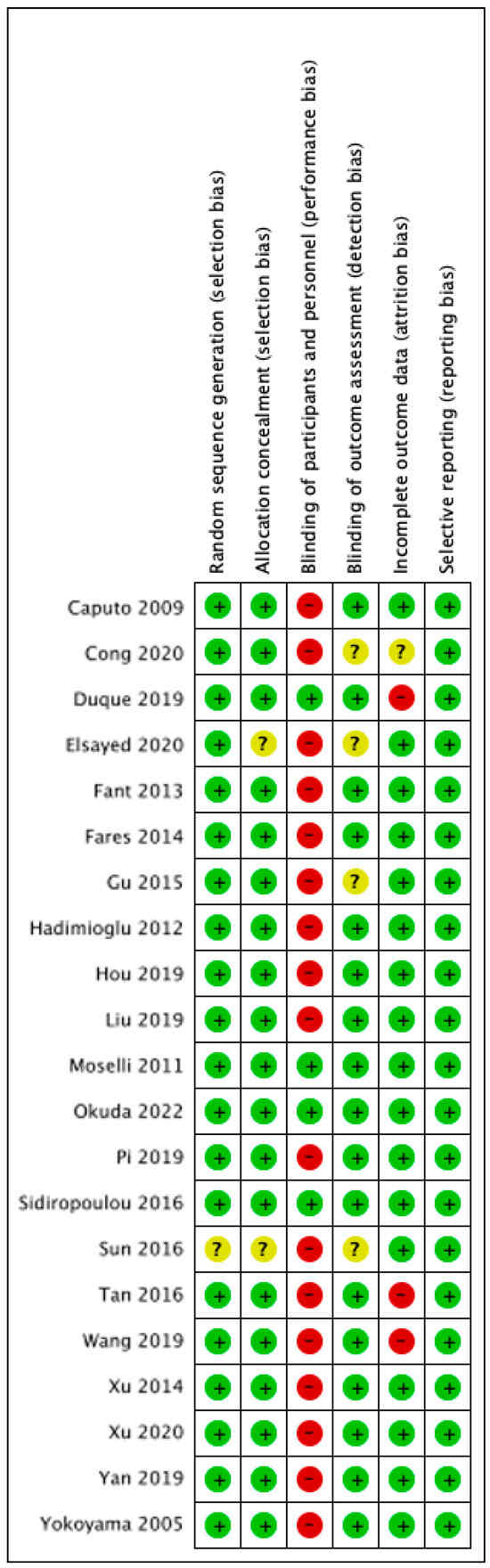
Figure 2.
Risk of bias assessment. Green: low risk, amber: some concerns, red: high risk [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42].
3.2. IL-6 24 Hours After Surgery
There were 16 studies which reported plasma levels of IL-6 24 after surgery [22,23,24,25,27,28,29,30,32,35,36,37,38,39,40,41]. This included a total of 480 patients who received epidural analgesia and 485 patients who only received general anesthesia. The pooled results showed statistically lower IL-6 levels in the EA + GA cohort in comparison to the GA cohort [standardized mean difference (SMD) = −1.99, 95% confidence interval (CI) = −2.67 to −1.31, I2 = 97%, Egger’s regression p < 0.01, trim and fill reported five missing studies, Figure 3, Supplementary Table S1]. Sensitivity analysis did not alter the finding when each of the studies was removed. Quality of evidence is very low due to significant data heterogeneity, as well as high risk of publication bias.
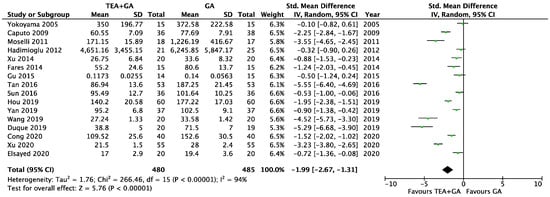
Figure 3.
Forest plots of IL-6 between GA and GA plus EA cohorts [22,23,24,25,27,28,29,30,32,35,36,37,38,39,40,41].
To address the heterogeneity, we conducted subgroup analyses based on surgery type and based on the length of surgery. We found that thoracic and upper gastrointestinal surgery groups were not significantly different from abdominal/pelvic procedures, and surgeries longer than 180 min were comparable to shorter surgeries. Neither subgroup analyses significantly reduced the heterogeneity (Supplementary Figure S1A,B).
We also conducted a subgroup analysis, including only the studies involving cancer surgery. This included twelve studies [23,24,27,28,30,32,35,37,38,39,40,41], and the IL-6 level was significantly lower in EA + GA cohort (SMD = −1.91, 95% CI = −2.60 to −1.21, I2 = 93%, Egger’s regression p < 0.01, trim and fill reported three missing studies, Supplementary Figure S1C). Sensitivity analysis did not alter the finding when each of the studies was removed. Quality of evidence is very low due to significant data heterogeneity, as well as high risk of publication bias.
3.3. TNF-α 24 Hours After Surgery
There were nine studies which reported serum TNF-α levels 24 h after surgery, this included a total of 355 patients who received epidural analgesia and 357 patients who only received general anesthesia [23,24,29,30,31,32,33,36,41]. The pooled results showed significantly lower TNF-α levels in the EA cohort (SMD = −1.13, 95% CI = −1.74 to −0.51, I2 = 93%, Egger’s regression p = 0.11, trim and fill reported one missing study, Figure 4). Sensitivity analysis did not alter the finding when each of the studies was removed. Quality of evidence is low due to significant data heterogeneity.
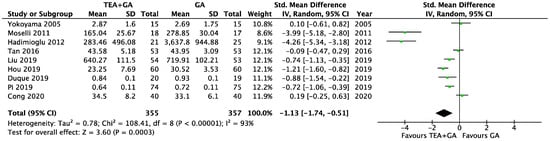
Figure 4.
Forest plots of TNF-α between GA and GA plus EA cohorts [23,24,29,30,31,32,33,36,41].
The cancer surgery only subgroup analysis included seven studies [23,24,30,31,32,33,41], and the TNF-α levels was significantly lower in EA cohort (SMD = −0.89, 95% CI = −1.47 to −0.32, I2 = 89%, Egger’s regression p = 0.05, trim and fill reported no missing studies, Supplementary Figure S2). Sensitivity analysis did not alter the finding when each of the studies was removed. Quality of evidence is low due to significant data heterogeneity.
3.4. CRP 24 Hours After Surgery
There were seven studies which reported serum CRP levels 24 h after surgery, this included a total of 239 patients who received epidural analgesia and 242 patients who only received general anesthesia [24,26,31,33,34,40,41]. The pooled results showed significantly lower CRP levels in the EA cohort (SMD = −0.61, 95% CI = −0.97 to −0.24, I2 = 71%, Egger’s regression p = 0.11, trim and fill reported no missing studies, Figure 5). Sensitivity analysis did not alter the finding when each of the studies was removed. Quality of evidence is low due to significant data heterogeneity.
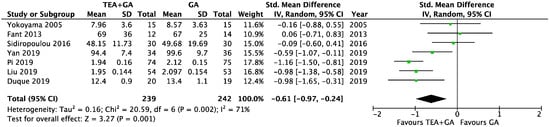
Figure 5.
Forest plots of CRP between GA and GA plus EA cohorts [24,26,31,33,34,40,41].
The cancer surgery only subgroup analysis included six studies [24,26,31,33,40,41], and the CRP level was significantly lower in EA cohort (SMD = −0.72, 95% CI = −1.07 to −0.36, I2 = 63%, Egger’s regression p = 0.06, trim and fill reported no missing studies, Supplementary Figure S3). Sensitivity analysis did not alter the finding when each of the studies was removed. Quality of evidence is medium due to data heterogeneity.
3.5. IL-6 at the End of Surgery
There were 11 studies which reported plasma levels of IL-6 immediately after surgery [22,23,24,27,28,29,35,36,39,41,42]. The pooled results showed statistically lower IL-6 levels in the EA + GA cohort in comparison to the GA cohort (SMD = −1.26, 95% CI = −2.22 to −0.30, I2 = 96%, Egger’s regression p < 0.01, trim and fill reported no missing studies, Supplementary Figure S4A). Sensitivity analysis reported non-significant pooled SMD when the study by Fares et al. was removed [27]. Quality of evidence is very low due to significant data heterogeneity and sensitivity analysis finding.
The cancer surgery only subgroup analysis included seven studies, and the IL-6 level was significantly lower in EA cohort (SMD = −1.75, 95% CI = −2.61 to −0.9, I2 = 93%, Egger’s regression p = 0.01, trim and fill reported no missing studies, Supplementary Figure S4B) [23,24,27,28,35,39,41,42]. Sensitivity analysis did not alter the finding when each of the studies was removed. Quality of evidence is low due to significant data heterogeneity.
3.6. TNF-α at the End of Surgery
There were seven studies which reported serum TNF-α levels immediately after surgery [23,24,29,31,33,36,41]. The pooled results showed significantly lower TNF-α levels in the EA cohort (SMD = −0.80, 95% CI = −1.53 to −0.08, I2 = 93%, Egger’s regression p < 0.01, trim and fill predicted no missing studies, Supplementary Figure S5A). The difference appears to be driven primarily by one study by Hadimioglu et al. [29], and exclusion of the said study in the sensitivity analysis resulted in non-significant pooled SMD. Quality of evidence is very low due to significant data heterogeneity and sensitivity analysis finding.
Five of the included studies were conducted on cancer patients, the pooled data did not demonstrate significant difference between EA and GA cohorts (SMD = −0.08, 95% CI = −0.39 to 0.23, I2 = 54%, Supplementary Figure S5B) [23,24,31,33,41].
3.7. CRP at the End of Surgery
There were five studies which reported serum CRP levels at the end of surgery [24,31,33,34,41]. The pooled results showed significantly lower CRP levels in the EA cohort (SMD = −1.60, 95% CI = −2.78 to −0.42, I2 = 96%, Egger’s regression p = 0.15, trim and fill predicted no missing studies, Supplementary Figure S6A). The difference appears to be driven primarily by one study by Duque et al. [24], and exclusion of the said study in the sensitivity analysis resulted in non-significant findings. Quality of evidence is low due to significant data heterogeneity.
Four of them were conducted on cancer surgery [24,31,33,41], and the lower CRP levels in the EA cohort again appear to be driven primarily by the study Duque et al. [24]. (Supplementary Figure S6B).
3.8. Cortisol Levels After Surgery
There were eight studies which reported serum cortisol levels 24 h after surgery [23,24,28,30,34,35,39,41], this included a total of 270 patients in each cohort. The pooled results showed significantly lower cortisol levels in the EA cohort (SMD = −0.82, 95% CI = −1.22 to −0.43, I2 = 78%, Egger’s regression p = 0.86, trim and fill predicted no missing studies, Supplementary Figure S7A). Sensitivity analysis did not alter the findings when each of the studies was removed. Quality of evidence is low due to significant data heterogeneity.
There were seven studies which reported serum cortisol levels at the end of surgery [23,24,28,34,35,39,41]. The pooled results showed significantly lower cortisol levels in the EA cohort (SMD = −1.94, 95% CI = −3.19 to −0.68, I2 = 96%, Egger’s regression p < 0.01, trim and fill predicted no missing studies, Supplementary Figure S7B). Sensitivity analysis did not alter the finding when each of the studies was removed. Quality of evidence is low due to significant data heterogeneity.
3.9. Other Interleukin Levels After Surgery
There were five studies which reported serum IL-1β levels 24 h after surgery, this included a total of 187 patients who received epidural analgesia and 179 patients who only received general anesthesia [24,31,32,33,41]. The pooled results showed significantly lower IL-1β levels in the EA cohort (SMD = −1.70, 95% CI = −2.66 to −0.74, I2 = 93%, Egger’s regression p = 0.47, trim and fill predicted no missing studies, Supplementary Figure S8A). Sensitivity analysis did not alter the finding when each of the studies was removed. Quality of evidence is medium due to data heterogeneity.
There were five studies which reported serum IL-4 levels 24 h after surgery, this included a total of 112 patients who received epidural analgesia and 111 patients who only received general anesthesia [23,24,28,32,37]. The pooled results showed no difference between the two cohorts (SMD = 0.83, 95% CI = −1.26 to 2.91, I2 = 93%, Egger’s regression p = 0.13, trim and fill predicted no missing studies, Supplementary Figure S8B). Sensitivity analysis did not alter the finding when each of the studies was removed. Quality of evidence is low due to significant data heterogeneity.
There were six studies which reported serum IL-8 levels 24 h after surgery, this included a total of 219 patients who received epidural analgesia and 220 patients who only received general anesthesia [22,24,25,27,31,33]. The pooled results showed significantly lower IL-8 levels in the EA cohort (SMD = −1.53, 95% CI = −2.04 to −1.03, I2 = 79%, Egger’s regression p = 0.84, trim and fill predicted no missing studies, Supplementary Figure S8C). Sensitivity analysis did not alter the finding when each of the studies was removed. Quality of evidence is low due to significant data heterogeneity.
There were five studies which reported serum IL-10 levels 24 h after surgery, this included a total of 149 patients who received epidural analgesia and 148 patients who only received general anesthesia [30,32,35,38,41]. The pooled results showed no difference between the two cohorts (SMD = 1.06, 95% CI = −0.52 to 2.64, I2 = 79%, Egger’s regression p < 0.01, trim and fill predicted no missing studies, Supplementary Figure S8D). Sensitivity analysis did not alter the finding when each of the studies was removed. Quality of evidence is low due to significant data heterogeneity.
4. Discussion
Epidural anesthesia is one of the first regional anesthesia techniques conceived and was historically the ‘gold standard’ for postoperative analgesia after major truncal surgeries [43]. Despite the development of peripheral nerve blocks in recent years, epidural analgesia remains in widespread use [44]. Epidural infusion of local anesthetics offers excellent relief for postoperative pain, reduces postoperative pain and the need for systemic opioid analgesia, which is associated with a myriad of complications, including immune response alteration [44]. In addition to its effect on the somatic sensory (and to a lesser extent, motor nerves), the spread of local anesthetics also affects the sympathetic trunk near the infusion site [14]. Sympathetic blockade is a known effect of epidurals and can be associated with intraoperative hypotension. However, they also blunt the extent of sympathetic response to surgery, and have been shown to reduce postoperative cardiac events [45]. As the immune response is intricately linked to the neuroendocrine system, it is physiologically feasible that blunted perioperative stress response through the use of epidural could alter postoperative immune response.
In this meta-analysis, we found that EA + GA was associated with lower levels of IL-6, TNF-α, and CRP, as well as other pro-inflammatory cytokines, postoperatively. This supports our hypothesis that in addition to alleviating postoperative pain and sympathetic activation, the use of EA may also reduce the pro-inflammatory response after surgery. Tissue damage from surgical manipulation results in local inflammation, which then induces systemic acute-phase response. IL-6 and TNF-α are two cytokines that initiate the early inflammatory responding to tissue injury. TNF-α has a short half-life (4.6 min) and the peak concentration is usually seen within two hours after trauma [5,46]. TNF-α also activates macrophages and is potent chemokine for neutrophil. In addition, the release of TNF-α also stimulates the production of IL-6, which subsequently enters the systemic circulation [47]. As a pleiotropic cytokine, IL-6 (serum half-life of 15.5 h) plays an important role in various physiological and pathological processes, especially in regulating the proliferation and differentiation of T and B lymphocytes and natural killer cells [48,49]. The activity of both TNF-α and IL-6 also stimulates the production of acute phase proteins including CRP and the production of corticotrophin releasing hormones, which upregulate the hypothalamo–pituitary–adrenal axis.
Pro-inflammatory cytokines such as IL-6, TNF-α, and IL-8 are believed to be important mediators in systemic response to surgery and have a high predictive value for the development of postoperative complications such as acute respiratory distress syndrome, systemic inflammatory response syndrome, sepsis, multiple organ failure, and multiple organ dysfunction syndrome. Szczepanik et al. analyzed 99 consecutive patients who underwent gastrectomy, and reported that elevated IL-6 on postoperative day 1 is an independent risk factor for postoperative complications [50]. Similarly, Straatman et al. found that CRP on postoperative day 3 is independently associate with the development of postoperative complications [51].
Cortisol (serum half-life is 60 to 120 min) is a steroid hormone which is elevated by physiological stress [52]. Elevated serum cortisol is commonly observed after surgery, and possible mechanisms include the upregulated synthesis secondary to IL-6 and TNF-α stimulation [53]. Excessive circulating of corticosteroids is associated with impaired wound healing, insulin resistance, as well as immunosuppression, all of which will adversely affect postoperative recovery [54].
IL-4 (19 min after intravenous administration, over 24 h by combing with anti-IL-4 antibody) is a regulatory cytokine that promotes the differentiation of B cells and T cells but downregulates macrophage activity and promotes wound healing [55]. In addition, IL-4 also upregulates the production of IL-10 by T helper cells [56]. IL-10 is also a regulatory cytokine which downregulates macrophage and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activities. It also reduces the production of TNF-α, Interferon γ, and other proinflammatory cytokines [57]. IL-4 and IL-10 are also the main cytokines involved in promoting wound healing [58,59]. While the pooled data suggest that EA cohort had slightly higher serum IL-4 and IL-10 levels, neither were not statistically significant. Considering the small number of studies and the heterogeneous surgical procedures [57], it is not clear if epidural analgesia has a significant effect on the expression of regulatory cytokines.
There is increasing evidence that perioperative events may affect the outcome of cancer surgery. While the exact mechanism is not clear, this may be attributable to the direct effect of surgical manipulation, perioperative medications (including anesthetic agents), postoperative inflammation and immune function changes [60]. It is thought that IL-6/JAK/STAT pathways could affect the proliferation, survival, and metastasis of cancer cells, while blocking the pathway in animal studies reduced the tumor burden [61]. In addition, TNF-α, IL-1β, and IL-6 have been demonstrated to suppress cell mediated immunity and promote intravascular tumor cell adhesion [62,63].
We therefore conducted subgroup analyses, including only the cancer surgery trials. We found that at 24 h after surgery, the EA cohort had significantly lower IL-6, TNF-α, and CRP. The release of proinflammatory cytokines can alter the immune functions and lead to impaired response to malignancy [64]. In cancer surgery, the immunosuppressive effect of general anesthesia and systemic opioids is problematic, considering that the suppressed CMI may affect the recognition of circulating tumor cells and thereby promote the formation of metastatic niche [65]. In such cases, blunted pro-inflammatory response after surgery afforded by EA may subsequently translate to better postoperative outcomes [60].
This meta-analysis has several limitations. There was considerable inter-study heterogeneity across all the outcomes, which reduces the reliability of the evidence. This could be due to the heterogeneity of surgical procedures and postoperative care from the different studies or may represent differences in the assays used in the different studies. Different surgeries involves various levels of surgical trauma and subsequently, the magnitude of cytokine release. For example, the renal transplant surgery (Hadimioglu 2012 [29]) showed significantly higher absolute levels of inflammatory cytokines than other studies since the surgery of renal transplantation is typically a lengthy and more invasive procedure. Immunosuppressants are typically in the real transplant patients to prevent rejection but do not seem to abolish the release of inflammatory cytokines. Another factor to be considered is the usage of opioids. Of the 21 included studies, 4 studies (Elsayed 2020, Fares 2014, Tan 2016, Wang 2019 [25,27,36,37]) combined local anesthetics with opioid during surgery an 5 studies (Gu 2015, Liu 2019, Xu 2014, Xu 2020, Yokoyama 2005 [28,31,38,39,41]) added opioids in the postoperative analgesia. Only one study, Tan 2016 [36], reported a significantly higher increase in IL-6 levels in the GA group compared to the TEA+ GA group, with a difference greater than those observed in other studies. Therefore, opioid use during perioperative and postoperative periods does not appear to be the primary factor contributing to the difference in proinflammatory cytokine levels between TEA + GA and GA-only groups. As most studies only reported cytokines up to 24 h after surgery, there are also insufficient data to determine the duration of effect from epidural analgesia. Another limitation is that while we have demonstrated statistically significant cytokine changes, it is not clear if the immune response changes translate to improved clinical outcomes. While there are clinical evidence linking perioperative inflammatory mediators with postoperative complication, there are limited data on whether altering the inflammatory response leads to better outcomes. Larger studies with longer postoperative follow up periods are required to reliably assess the effect of EA on cytokines on postoperative outcomes. This is especially important for cancer surgery, as recurrences often take years to become apparent.
5. Conclusions
In conclusion, this meta-analysis found that epidural analgesia is associated with significantly lower levels of pro-inflammatory cytokines, CRP, and cortisol 24 h after surgery. A similar pattern was also seen in the cancer surgery sub-cohort. The evidence on epidural analgesia and the expression of regulatory cytokines (including IL-4 and IL-10) is insufficient for drawing conclusions. Studies with longer postoperative follow up periods are needed to assess of the change in cytokine profiles are associated with better clinical outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17101667/s1, Figure S1: Subgroup analysis of IL-6 at 24 h after surgery. Forest plot of subgroup analysis based on (A) surgery type, (B) surgery length, and (C) cancer or non-cancer surgery; Figure S2: Subgroup analysis of TNF- α at 24 h after surgery; Figure S3: Subgroup analysis of CRP at 24 h after surgery; Figure S4: Serum level of IL-6 at the end of surgery. (A) Overall survival analysis of IL-6 level at the end of surgery. (B) Subgroup analysis of IL-6 level at the end of surgery; Figure S5: Serum level of TNF-α at the end of surgery. (A) Overall analysis of TNF-α level at the end of surgery. (B) Subgroup analysis of TNF-α level at the end of surgery; Figure S6: Serum level of CRP at the end of surgery. (A) Overall analysis of CRP level at the end of surgery. (B) Subgroup analysis of CRP level at the end of surgery; Figure S7: Serum level of cortisol after surgery. Forest plot of cortisol at (A) 24 h after surgery and (B) the end of surgery; Figure S8: Forest plot of IL-1β (A), IL-4 (B), IL-8 (C), and IL-10 (D) level at 24 h after surgery; Table S1: summary of the meta-analysis outcomes.
Author Contributions
E.J.L., H.J.L. and R.L. helped with literature search, statistical analysis, and data extraction. S.P. and S.S. helped with the reviewing and editing of the manuscript. R.L. and E.J.L. helped with writing and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to thank Zhaosheng Jin for his help in data analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GA | general anesthesia |
| EA | epidural anesthesia |
| CRP | C-reactive protein |
| IL-6 | interleukin-6 |
| TNF-α | tumor necrosis factor-α |
| SMD | standardized mean differences |
References
- Desborough, J.P. The stress response to trauma and surgery. Br. J. Anaesth. 2000, 85, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Sheeran, P.; Hall, G.M. Cytokines in anaesthesia. Br. J. Anaesth. 1997, 78, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Hsing, C.; Wang, J. Clinical implication of perioperative inflammatory cytokine alteration. Acta Anaesthesiol. Taiwanica Off. J. Taiwan Soc. Anesthesiol. 2015, 53, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Angele, M.K.; Faist, E. Clinical review: Immunodepression in the surgical patient and increased susceptibility to infection. Crit. Care 2002, 6, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Shimazui, T.; Oami, T.; Shimada, T.; Tomita, K.; Nakada, T.A. Age-dependent differences in the association between blood interleukin-6 levels and mortality in patients with sepsis: A retrospective observational study. J. Intensive Care 2025, 13, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watt, D.G.; McSorley, S.T.; Park, J.H.; Horgan, P.G.; McMillan, D.C. A Postoperative Systemic Inflammation Score Predicts Short- and Long-Term Outcomes in Patients Undergoing Surgery for Colorectal Cancer. Ann. Surg. Oncol. 2017, 24, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- McSorley, S.T.; Watt, D.G.; Horgan, P.G.; McMillan, D.C. Postoperative Systemic Inflammatory Response, Complication Severity, and Survival Following Surgery for Colorectal Cancer. Ann. Surg. Oncol. 2016, 23, 2832–2840. [Google Scholar] [CrossRef] [PubMed]
- Desmond, F.; McCormack, J.; Mulligan, N.; Stokes, M.; Buggy, D.J. Effect of anaesthetic technique on immune cell infiltration in breast cancer: A follow-up pilot analysis of a prospective, randomised, investigator-masked study. Anticancer Res. 2015, 35, 1311–1319. [Google Scholar] [PubMed]
- Liang, X.; Liu, R.; Chen, C.; Ji, F.; Li, T. Opioid System Modulates the Immune Function: A Review. Transl. Perioper. Pain Med. 2016, 1, 5. [Google Scholar] [PubMed]
- Stenger, M.; Fabrin, A.; Schmidt, H.; Greisen, J.; Erik, M.P.; Jakobsen, C. High thoracic epidural analgesia as an adjunct to general anesthesia is associated with better outcome in low-to-moderate risk cardiac surgery patients. J. Cardiothorac. Vasc. Anesth. 2013, 27, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Wijeysundera, D.; Beattie, W.; Austin, P.; Hux, J.; Laupacis, A. Epidural anaesthesia and survival after intermediate-to-high risk non-cardiac surgery: A population-based cohort study. Lancet 2008, 372, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xing, H.; Xu, X. Comparison of midazolam and dexmedetomidine combined with thoracic paravertebral block in hemodynamics, inflammation and stress response, and cognitive function in elderly lung cancer patients. Int. Immunopharmacol. 2025, 147, 113961. [Google Scholar] [CrossRef] [PubMed]
- Veering, B.; Cousins, M. Cardiovascular and pulmonary effects of epidural anaesthesia. Anaesth. Intensive Care 2000, 28, 620–635. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Ogata, M.; Kawasaki, C.; Okamot, K.; Sata, T. Effects of epidural anaesthesia on surgical stress-induced immunosuppression during upper abdominal surgery. Br. J. Anaesth. 2007, 98, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Grandhi, R.K.; Lee, S.; Abd-Elsayed, A. The Relationship Between Regional Anesthesia and Cancer: A Metaanalysis. Ochsner J. 2017, 17, 345–361. [Google Scholar] [PubMed] [PubMed Central]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- WebPlotDigitizer. 2011. Available online: https://automeris.io (accessed on 12 May 2025).
- Sharma, D.; Ulaganathan, S.P.; Sharma, V.; Piplani, S.; Niraj, R.R.K. Deep Meta Tool: GUI tool to obtain Mean and Standard Deviation (SD) from Median and Interquartile range (IQR). Research Square 2021. [Google Scholar] [CrossRef]
- Sterne, J.; Savović, J.; Page, M.; Elbers, R.; Blencowe, N.; Boutron, I.; Cates, C.; Cheng, H.; MS, C.; SM, E.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ (Clin. Res. Ed.) 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Suurmond, R.; van Rhee, H.; Hak, T. Introduction, comparison, and validation of Meta-Essentials: A free and simple tool for meta-analysis. Res. Synth. Methods 2017, 8, 537–553. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caputo, M.; Alwair, H.; Rogers, C.A.; Ginty, M.; Monk, C.; Tomkins, S.; Mokhtari, A.; Angelini, G.D. Myocardial, inflammatory, and stress responses in off-pump coronary artery bypass graft surgery with thoracic epidural anesthesia. Ann. Thorac. Surg. 2009, 87, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Zhang, W.; Huang, Z.; Zhang, L.; Sun, M.; Chang, E.; Zhang, J. Effects of Different Anaesthesia Methods on Perioperative Cellular Immune Function and Longterm Outcomes in Patients Undergoing Radical Resection of Esophageal Cancer: A Prospective Cohort Study. Anesthesiol. Pain Med. 2020; epub ahead of print. [Google Scholar] [CrossRef]
- Duque, P.; Garutti, I.; Terradillos, E.; Ledesma, B.; Rancan, L.; Simon, C.; Vara, E. Modulation of CCL2 Expression by Laparoscopic Versus Open Surgery for Colorectal Cancer Surgery. Surg. Laparosc. Endosc. Percutan. Tech. 2019, 29, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, A.A.; Ei-deen, N.M.G.; Shams, G.H.R.; Aly, A.E.A.-e.; Mohammed, W.S. Comparative Study between General Anesthesia versus General Anesthesia Combined with Thoracic Epidural Analgesia on Cytokine Response in Laparoscopic Cholecystectomy Patients. Open J. Anesthesiol. 2020, 10, 247–262. [Google Scholar] [CrossRef]
- Fant, F.; Tina, E.; Sandblom, D.; Andersson, S.O.; Magnuson, A.; Hultgren-Hörnkvist, E.; Axelsson, K.; Gupta, A. Thoracic epidural analgesia inhibits the neuro-hormonal but not the acute inflammatory stress response after radical retropubic prostatectomy. Br. J. Anaesth. 2013, 110, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Fares, K.M.; Mohamed, S.A.; Hamza, H.M.; Sayed, D.M.; Hetta, D.F. Effect of Thoracic Epidural Analgesia on Proinflammatory Cytokines in Patients Subjected to Protective Lung Ventilation During Ivor Lewis Esophagectomy. Pain Physician 2014, 17, 305–315. [Google Scholar]
- Gu, C.Y.; Zhang, J.; Qian, Y.N.; Tang, Q.F. Effects of epidural anesthesia and postoperative epidural analgesia on immune function in esophageal carcinoma patients undergoing thoracic surgery. Mol. Clin. Oncol. 2015, 3, 190–196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hadimioglu, N.; Ulugol, H.; Akbas, H.; Coskunfirat, N.; Ertug, Z.; Dinckan, A. Combination of epidural anesthesia and general anesthesia attenuates stress response to renal transplantation surgery. Transpl. Proc 2012, 44, 2949–2954. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.J.; Du, Y.; Gu, S.X.; Fan, J.; Wang, R.; Deng, H.; Guo, D.X.; Wang, L.; Wang, Y.Y. General anesthesia combined with epidural anesthesia maintaining appropriate anesthesia depth may protect excessive production of inflammatory cytokines and stress hormones in colon cancer patients during and after surgery. Medicine 2019, 98, e16610. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, W.; Wu, L.; Zhang, M.; Zhao, L. Effects of general anesthesia with combined epidural anesthesia on inflammatory response in patients with early-stage gastric cancer undergoing tumor resection. Exp. Ther. Med. 2019, 17, 35–40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moselli, N.M.; Baricocchi, E.; Ribero, D.; Sottile, A.; Suita, L.; Debernardi, F. Intraoperative epidural analgesia prevents the early proinflammatory response to surgical trauma. Results from a prospective randomized clinical trial of intraoperative epidural versus general analgesia. Ann. Surg. Oncol. 2011, 18, 2722–2731. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Sun, Y.; Zhang, Z.; Wan, C. Combined anesthesia shows better curative effect and less perioperative neuroendocrine disorder than general anesthesia in early stage NSCLC patients. J. Int. Med. Res. 2019, 47, 4743–4752. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulou, I.; Tsaousi, G.G.; Pourzitaki, C.; Logotheti, H.; Tsantilas, D.; Vasilakos, D.G. Impact of anesthetic technique on the stress response elicited by laparoscopic cholecystectomy: A randomized trial. J. Anesth. 2016, 30, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Z.; Song, Y.L.; Wang, X.Y. Effects of Different Anesthetic Methods on Cellular Immune and Neuroendocrine Functions in Patients With Hepatocellular Carcinoma Before and After Surgery. J. Clin. Lab. Anal. 2016, 30, 1175–1182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, W.F.; Guo, B.; Ma, H.; Li, X.Q.; Fang, B.; Lv, H.W. Changes in postoperative night bispectral index of patients undergoing thoracic surgery with different types of anaesthesia management: A randomized controlled trial. Clin. Exp. Pharmacol. Physiol. 2016, 43, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liang, S.; Chen, H.; Xu, Y.; Wang, Y. The effects of epidural anaesthesia and analgesia on T lymphocytes differentiation markers and cytokines in patients after gastric cancer resection. BMC Anesth. 2019, 19, 102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, Y.J.; Chen, W.K.; Zhu, Y.; Wang, S.L.; Miao, C.H. Effect of thoracic epidural anaesthesia on serum vascular endothelial growth factor C and cytokines in patients undergoing anaesthesia and surgery for colon cancer. Br. J. Anaesth. 2014, 113, i49–i55. [Google Scholar] [CrossRef]
- Xu, Y.J.; Sun, X.; Jiang, H.; Yin, Y.H.; Weng, M.L.; Sun, Z.R.; Chen, W.K.; Miao, C.H. Randomized clinical trial of continuous transversus abdominis plane block, epidural or patient-controlled analgesia for patients undergoing laparoscopic colorectal cancer surgery. Br. J. Surg. 2020, 107, e133–e141. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Mao, H.; Qiu, P. Effects of different analgesia regimens on early post-operative cognitive dysfunction in elderly patients undergoing radical resection of cervical carcinoma. Exp. Ther. Med. 2019, 18, 1465–1469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yokoyama, M.; Itano, Y.; Katayama, H.; Morimatsu, H.; Takeda, Y.; Takahashi, T.; Nagano, O.; Morita, K. The effects of continuous epidural anesthesia and analgesia on stress response and immune function in patients undergoing radical esophagectomy. Anesth. Analg. 2005, 101, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Okuda, J.; Suzuki, T.; Wakaizumi, K.; Kato, J.; Yamada, T.; Morisaki, H. Effects of Thoracic Epidural Anesthesia on Systemic and Local Inflammatory Responses in Patients Undergoing Lung Cancer Surgery: A Randomized Controlled Trial. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Engelman, R.M.; Rousou, J.A.; Flack, J.E., 3rd; Deaton, D.W.; Humphrey, C.B.; Ellison, L.H.; Allmendinger, P.D.; Owen, S.G.; Pekow, P.S. Fast-track recovery of the coronary bypass patient. Ann. Thorac. Surg. 1994, 58, 1742–1746. [Google Scholar] [CrossRef] [PubMed]
- Block, B.; Liu, S.; Rowlingson, A.; Cowan, A.; Cowan, J.; Wu, C. Efficacy of postoperative epidural analgesia: A meta-analysis. JAMA 2003, 290, 2455–2463. [Google Scholar] [CrossRef] [PubMed]
- Beattie, W.; Badner, N.; Choi, P. Epidural analgesia reduces postoperative myocardial infarction: A meta-analysis. Anesth. Analg. 2001, 93, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Diez-Ruiz, A.; Tilz, G.P.; Zangerle, R.; Baier-Bitterlich, G.; Wachter, H.; Fuchs, D. Soluble receptors for tumour necrosis factor in clinical laboratory diagnosis. Eur. J. Haematol. 1995, 54, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Matsushima-Nishiwaki, R.; Yamaguchi, S.; Iida, H.; Dohi, S.; Kozawa, O. Mechanisms of tumor necrosis factor-alpha-induced interleukin-6 synthesis in glioma cells. J. Neuroinflamm. 2010, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Kuribayashi, T. Elimination half-lives of interleukin-6 and cytokine-induced neutrophil chemoattractant-1 synthesized in response to inflammatory stimulation in rats. Lab. Anim. Res. 2018, 34, 80–83. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szczepanik, A.; Scislo, L.; Scully, T.; Walewska, E.; Siedlar, M.; Kolodziejczyk, P.; Lenart, M.; Rutkowska, M.; Galas, A.; Czupryna, A.; et al. IL-6 serum levels predict postoperative morbidity in gastric cancer patients. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2011, 14, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Straatman, J.; Cuesta, M.; Tuynman, J.; Veenhof, A.; Bemelman, W.; van der Peet, D. C-reactive protein in predicting major postoperative complications are there differences in open and minimally invasive colorectal surgery? Substudy from a randomized clinical trial. Surg. Endosc. 2018, 32, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, J.W.; Walker, E.A.; Bujalska, I.J.; Draper, N.; Lavery, G.G.; Cooper, M.S.; Hewison, M.; Stewart, P.M. 11beta-hydroxysteroid dehydrogenase type 1: A tissue-specific regulator of glucocorticoid response. Endocr. Rev. 2004, 25, 831–866. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.N.; Sternberg, E.M. Glucocorticoid regulation of inflammation and its functional correlates: From HPA axis to glucocorticoid receptor dysfunction. Ann. N. Y. Acad. Sci. 2012, 1261, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.L.; Li, L.H.; Wang, D.X.; Li, N.; Shan, G.J.; Li, J.; Yu, Q.J.; Shi, C.X. High postoperative serum cortisol level is associated with increased risk of cognitive dysfunction early after coronary artery bypass graft surgery: A prospective cohort study. PLoS ONE 2013, 8, e77637. [Google Scholar] [CrossRef] [PubMed]
- Shintani, Y.; Ito, T.; Fields, L.; Shiraishi, M.; Ichihara, Y.; Sato, N.; Podaru, M.; Kainuma, S.; Tanaka, H.; Suzuki, K. IL-4 as a Repurposed Biological Drug for Myocardial Infarction through Augmentation of Reparative Cardiac Macrophages: Proof-of-Concept Data in Mice. Sci. Rep. 2017, 7, 6877. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.; Hassan, M.; Burton, B.; Britton, G.; Hill, E.; Verhagen, J.; Wraith, D. IL-4 enhances IL-10 production in Th1 cells: Implications for Th1 and Th2 regulation. Sci. Rep. 2017, 7, 11315. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.; Zhang, X. Interleukin-10: New perspectives on an old cytokine. Immunol. Rev. 2008, 226, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Salmon-Eh, R.V.; Ramont, L.; Godeau, G.; Birembaut, P.; Guenounou, M.; Bernard, P.; Maquart, F. Implication of interleukin-4 in wound healing. Lab. Investig. A J. Tech. Methods Pathol. 2000, 80, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Balaji, S.; Le, L.; Crombleholme, T.; Keswani, S. Regenerative Wound Healing: The Role of Interleukin-10. Adv. Wound Care 2014, 3, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Yap, A.; Lopez-Olivo, M.A.; Dubowitz, J.; Hiller, J.; Riedel, B. Anesthetic technique and cancer outcomes: A meta-analysis of total intravenous versus volatile anesthesia. Can. J. Anaesth. = J. Can. D’anesth. 2019, 66, 546–561. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Huang, Y.; Lin, J. Distinct effects of general anesthetics on lung metastasis mediated by IL-6/JAK/STAT3 pathway in mouse models. Nat. Commun. 2020, 11, 642. [Google Scholar] [CrossRef]
- Yu, G.; Tang, B.; Yu, P.; Peng, Z.; Qian, F.; Sun, G. Systemic and peritoneal inflammatory response after laparoscopic-assisted gastrectomy and the effect of inflammatory cytokines on adhesion of gastric cancer cells to peritoneal mesothelial cells. Surg. Endosc. 2010, 24, 2860–2870. [Google Scholar] [CrossRef] [PubMed]
- Arimoto, A.; Yamashita, K.; Hasegawa, H.; Sugita, Y.; Fukuoka, E.; Tanaka, T.; Suzuki, S.; Kakeji, Y. Immunosuppression Induced by Perioperative Peritonitis Promotes Lung Metastasis. Anticancer Res. 2018, 38, 4333–4338. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Fujieda, K.; Senju, S.; Ikeda, T.; Oshiumi, H.; Nishimura, Y. Immune-suppressive effects of interleukin-6 on T-cell-mediated anti-tumor immunity. Cancer Sci. 2018, 109, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).