Survival Outcomes Associated with the Location of BRCA Mutations in Ovarian Cancer: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
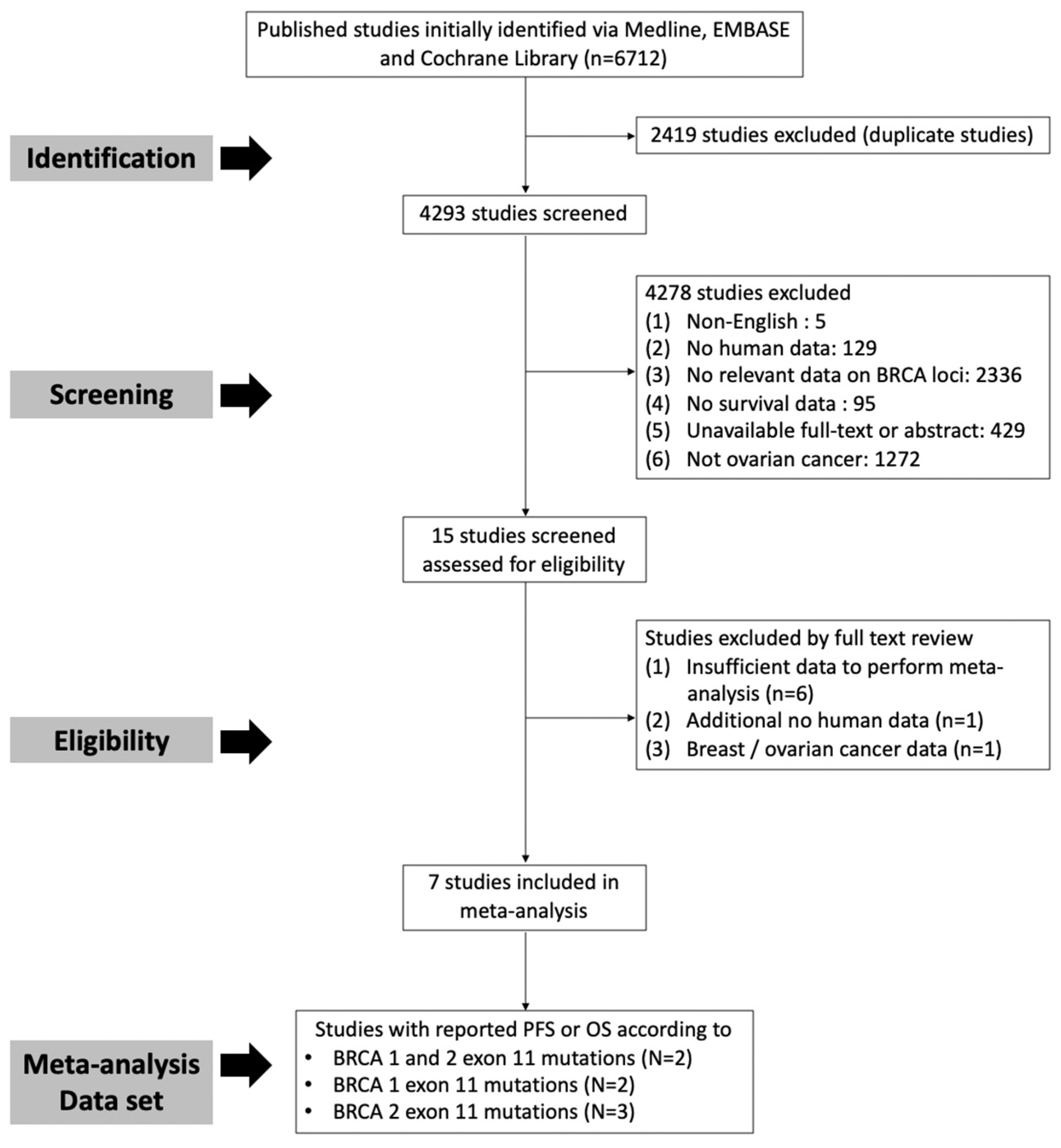
2.1. Subsection Literature Search and Selection Criteria
2.2. Study Selection, Data Extraction
2.3. Statistical Analysis
- Hazard rate (h): h = log(2)/M or −log(P)/T, where M is the median survival time, P is the survival probability, and T is time (years);
- Hazard ratio (HR) = h1/h;
- Standard error (SE) for log(HR): (log(upper CI) − log(lower CI))/(2 × 1.96) or sqrt(1/expected events1 + 1/expected events2), where expected events = 1/hazards.
3. Results
3.1. Study Characteristics
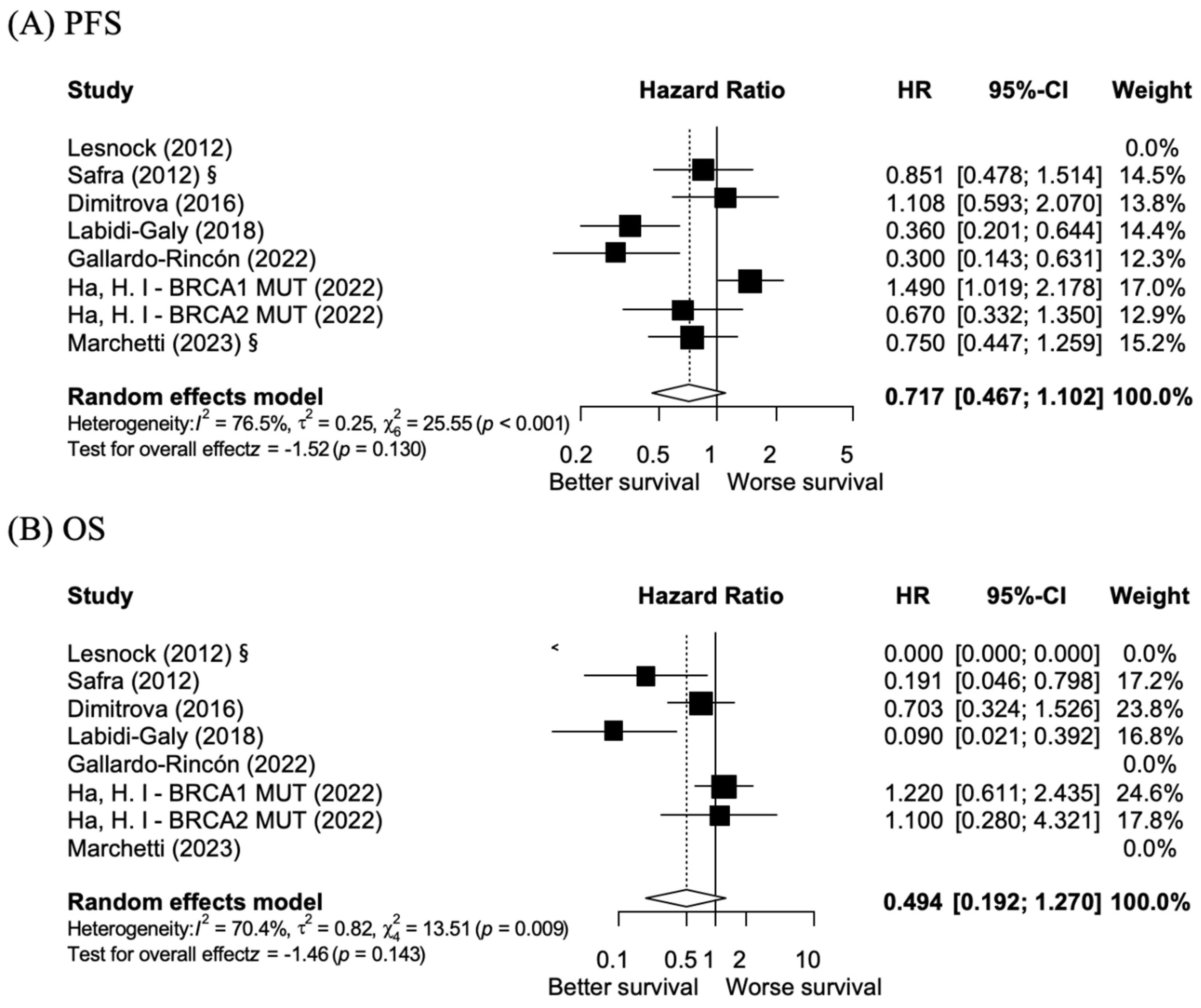
3.2. BRCA 1/2 Exon 11 Mutations vs. Non-BRCA 1/2 Exon 11 Mutations: PFS and OS
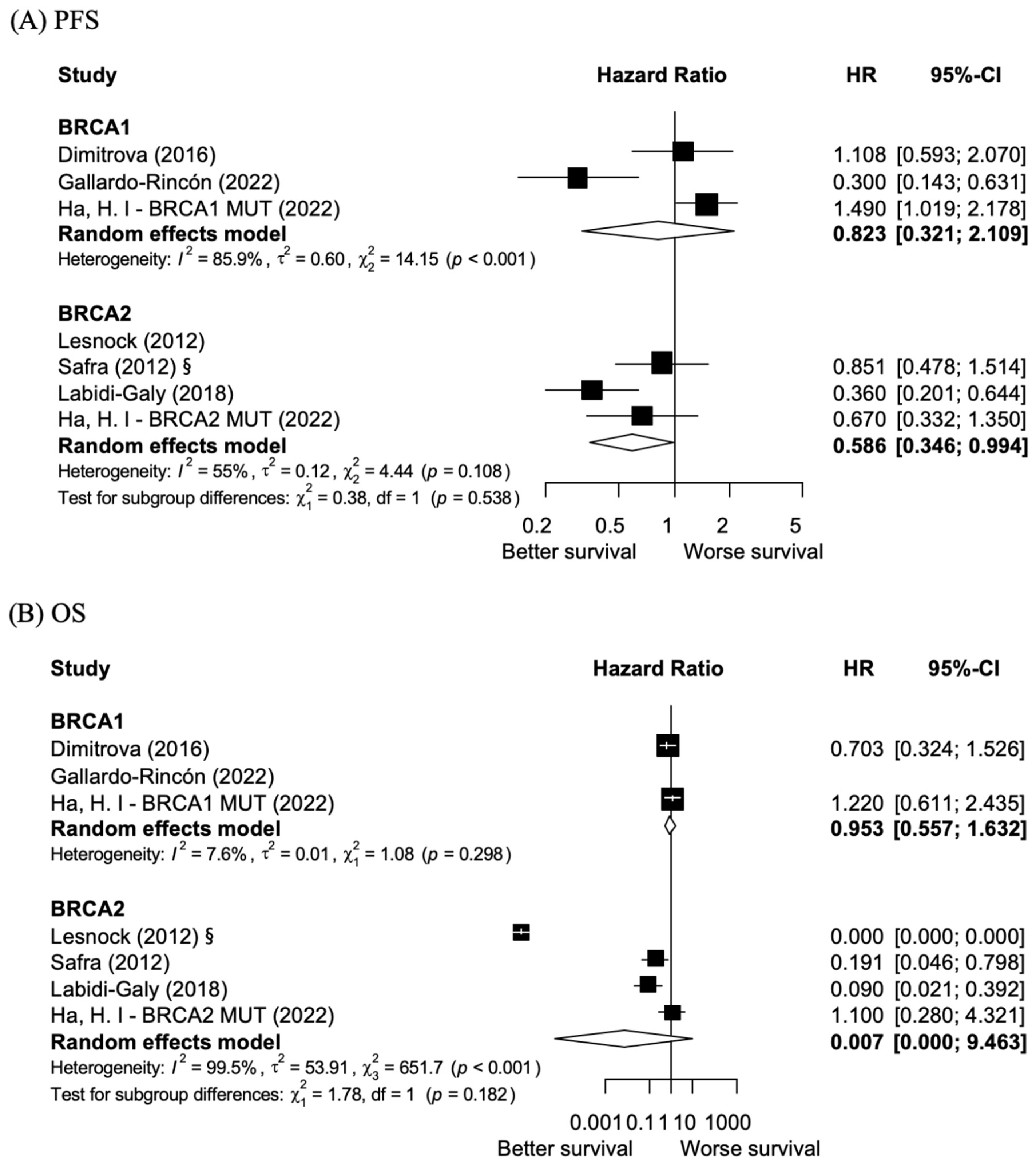
3.3. BRCA 1 Exon 11 Mutations vs. Non-BRCA 1 Exon 11 Mutations: PFS and OS
3.4. BRCA 2 Exon 11 Mutations vs. Non-BRCA 2 Exon 11 Mutations: PFS and OS
3.5. Publication Bias Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PFS | Progression-Free Survival |
| OS | Overall survival |
| HRD | Homologous recombination deficiency |
| PARPi | Poly ADP-ribose polymerase inhibitor |
| NMD | Nonsense-mediated mRNA decay |
| PTCs | Premature termination codons |
| HR | Hazard ratio |
| NOS | Newcastle–Ottawa scale |
| CI | Confidence interval |
| FIGO | International Federation of Gynecology and Obstetrics |
| SE | Standard error |
| PV | Pathogenic variant |
References
- McLaughlin, J.R.; Rosen, B.; Moody, J.; Pal, T.; Fan, I.; Shaw, P.A.; Risch, H.A.; Sellers, T.A.; Sun, P.; Narod, S.A. Long-term ovarian cancer survival associated with mutation in BRCA1 or BRCA2. J. Natl. Cancer Inst. 2013, 105, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Royer, R.; Li, S.; McLaughlin, J.R.; Rosen, B.; Risch, H.A.; Fan, I.; Bradley, L.; Shaw, P.A.; Narod, S.A. Frequencies of BRCA1 and BRCA2 mutations among 1342 unselected patients with invasive ovarian cancer. Gynecol. Oncol. 2011, 121, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef]
- Tan, D.S.; Kaye, S.B. Chemotherapy for Patients with BRCA1 and BRCA2-Mutated Ovarian Cancer: Same or Different? Am. Soc. Clin. Oncol. Educ. Book 2015, 35, 1. [Google Scholar] [CrossRef] [PubMed]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Gonzalez-Martin, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Perol, D.; Gonzalez-Martin, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Maenpaa, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- Gayther, S.A.; Warren, W.; Mazoyer, S.; Russell, P.A.; Harrington, P.A.; Chiano, M.; Seal, S.; Hamoudi, R.; van Rensburg, E.J.; Dunning, A.M.; et al. Germline mutations of the BRCA1 gene in breast and ovarian cancer families provide evidence for a genotype-phenotype correlation. Nat. Genet. 1995, 11, 428–433. [Google Scholar] [CrossRef]
- Gayther, S.A.; Mangion, J.; Russell, P.; Seal, S.; Barfoot, R.; Ponder, B.A.; Stratton, M.R.; Easton, D. Variation of risks of breast and ovarian cancer associated with different germline mutations of the BRCA2 gene. Nat. Genet. 1997, 15, 103–105. [Google Scholar] [CrossRef]
- Chen, Y.; Farmer, A.A.; Chen, C.F.; Jones, D.C.; Chen, P.L.; Lee, W.H. BRCA1 is a 220-kDa nuclear phosphoprotein that is expressed and phosphorylated in a cell cycle-dependent manner. Cancer Res. 1996, 56, 3168–3172. [Google Scholar] [PubMed]
- Marquis, S.T.; Rajan, J.V.; Wynshaw-Boris, A.; Xu, J.; Yin, G.Y.; Abel, K.J.; Weber, B.L.; Chodosh, L.A. The developmental pattern of Brca1 expression implies a role in differentiation of the breast and other tissues. Nat. Genet. 1995, 11, 17–26. [Google Scholar] [CrossRef]
- Raponi, M.; Smith, L.D.; Silipo, M.; Stuani, C.; Buratti, E.; Baralle, D. BRCA1 exon 11 a model of long exon splicing regulation. RNA Biol. 2014, 11, 351–359. [Google Scholar] [CrossRef]
- Ware, M.D.; DeSilva, D.; Sinilnikova, O.M.; Stoppa-Lyonnet, D.; Tavtigian, S.V.; Mazoyer, S. Does nonsense-mediated mRNA decay explain the ovarian cancer cluster region of the BRCA2 gene? Oncogene 2006, 25, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Perrin-Vidoz, L.; Sinilnikova, O.M.; Stoppa-Lyonnet, D.; Lenoir, G.M.; Mazoyer, S. The nonsense-mediated mRNA decay pathway triggers degradation of most BRCA1 mRNAs bearing premature termination codons. Hum. Mol. Genet. 2002, 11, 2805–2814. [Google Scholar] [CrossRef]
- Bolton, K.L.; Chenevix-Trench, G.; Goh, C.; Sadetzki, S.; Ramus, S.J.; Karlan, B.Y.; Lambrechts, D.; Despierre, E.; Barrowdale, D.; McGuffog, L.; et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA 2012, 307, 382–390. [Google Scholar] [CrossRef]
- Ha, H.I.; Park, E.Y.; Eoh, K.J.; Lee, Y.J.; Seo, S.S.; Kang, S.; Park, S.Y.; Lim, M.C. Clinical outcomes of BRCA1/2 pathogenic variants in ovarian cancer cluster region in patients with primary peritoneal, epithelial ovarian, and fallopian tube cancer. Gynecol. Oncol. 2022, 164, 415–420. [Google Scholar] [CrossRef]
- Tobalina, L.; Armenia, J.; Irving, E.; O’Connor, M.J.; Forment, J.V. A meta-analysis of reversion mutations in BRCA genes identifies signatures of DNA end-joining repair mechanisms driving therapy resistance. Ann. Oncol. 2021, 32, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Labidi-Galy, S.I.; Rodrigues, M.; Sandoval, J.L.; Kurtz, J.E.; Heitz, F.; Mosconi, A.M.; Romero, I.; Denison, U.; Nagao, S.; Vergote, I.; et al. Association of location of BRCA1 and BRCA2 mutations with benefit from olaparib and bevacizumab maintenance in high-grade ovarian cancer: Phase III PAOLA-1/ENGOT-ov25 trial subgroup exploratory analysis. Ann. Oncol. 2023, 34, 152–162. [Google Scholar] [CrossRef]
- Labidi-Galy, S.I.; Olivier, T.; Rodrigues, M.; Ferraioli, D.; Derbel, O.; Bodmer, A.; Petignat, P.; Rak, B.; Chopin, N.; Tredan, O.; et al. Location of Mutation in BRCA2 Gene and Survival in Patients with Ovarian Cancer. Clin. Cancer Res. 2018, 24, 326–333. [Google Scholar] [CrossRef]
- Yang, D.; Khan, S.; Sun, Y.; Hess, K.; Shmulevich, I.; Sood, A.K.; Zhang, W. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA 2011, 306, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.; Ashworth, A. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol. Med. 2002, 8, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.; Alzneika, S.; Riguene, E.; Al-Maraghi, S.; Alabdulrazzak, A.; Al-Khal, N.; Fetais, S.; Thanassoulas, A.; AlFarsi, H.; Nomikos, M. BRCA1 and Its Vulnerable C-Terminal BRCT Domain: Structure, Function, Genetic Mutations and Links to Diagnosis and Treatment of Breast and Ovarian Cancer. Pharmaceuticals 2024, 17, 333. [Google Scholar] [CrossRef]
- Wang, B. BRCA1 tumor suppressor network: Focusing on its tail. Cell Biosci. 2012, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bernhardy, A.J.; Cruz, C.; Krais, J.J.; Nacson, J.; Nicolas, E.; Peri, S.; van der Gulden, H.; van der Heijden, I.; O’Brien, S.W.; et al. The BRCA1-Δ11q Alternative Splice Isoform Bypasses Germline Mutations and Promotes Therapeutic Resistance to PARP Inhibition and Cisplatin. Cancer Res. 2016, 76, 2778–2790. [Google Scholar] [CrossRef]
- Nesic, K.; Krais, J.J.; Wang, Y.; Vandenberg, C.J.; Patel, P.; Cai, K.Q.; Kwan, T.; Lieschke, E.; Ho, G.Y.; Barker, H.E.; et al. BRCA1 secondary splice-site mutations drive exon-skipping and PARP inhibitor resistance. Mol. Cancer 2024, 23, 158. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Mitra, N.; Wan, F.; Sinilnikova, O.M.; Healey, S.; McGuffog, L.; Mazoyer, S.; Chenevix-Trench, G.; Easton, D.F.; Antoniou, A.C.; et al. Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. JAMA 2015, 313, 1347–1361. [Google Scholar] [CrossRef]
- Gentile, D.; Losurdo, A.; Sagona, A.; Zuradelli, M.; Gatzemeier, W.; Barbieri, E.; Testori, A.; Errico, V.; Bianchi, P.; Biondi, E.; et al. Surgical management of BRCA-mutation carriers: A single institution experience. Eur. J. Surg. Oncol. 2022, 48, 1706–1712. [Google Scholar] [CrossRef]
| No. | Author | Year | Total (N) | Mutation (N) | Control (N) | BRCA1/2 Mutation | Median Follow-Up (Months) | PFS HR | CI | p-Value | OS HR | CI | p-Value | Median Age | FIGO Stage III–IV | Histology (Serous) | Chemotherapy | PARPis | NOS | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lesnock, J. et al. [20] | 2012 | 315 | 5 | 56 | BRCA2 | 35.4 | 8/9 | poster | |||||||||||
| 2 | Safra, T. et al. [21] | 2012 | 190 | 12 | 100 | BRCA2 | 56 | 0.851 | 0.478–1.514 | 0.583 | 0.191 | 0.05–0.798 | 0.023 | 55.5 | 169 (88.9%) | 132 (69.5%) | (+) | (−) | 8/9 | |
| 3 | Dimitrova, D. et al. [22] | 2016 | 263 | 18 | 235 | BRCA1 | 51 | 1.108 | 0.593–2.070 | 0.748 | 0.703 | 0.33–1.53 | 0.373 | 56 | 188 (71.5%) | 211 (80.2%) | (+) | (−) | 8/9 | |
| 4 | Labidi-Galy, S. I. et al. [23] | 2018 | 353 | 42 | 36 | BRCA2 | 48 | 0.360 | 0.201–0.644 | 0.001 | 0.090 | 0.02–0.39 | 0.001 | 59 | 222 (23.5%) | 180 (67.7%) | (+) | (−) | 8/9 | |
| 5 | Gallardo-Rincón, D. et al. [24] | 2022 | 35 | 9 | 26 | BRCA1 | 12.87 | 0.300 | 0.143–0.631 | 0.002 | 51 | 31 (88.6%) | 33 (94.2%) | (+) | (+) | 7/9 | ||||
| 6 | * Ha, H. I. et al.—BRCA1 [17] | 2022 | 238 | 79 | 83 | BRCA1 | 25.5 | 1.490 | 1.019–2.178 | 0.040 | 1.220 | 0.61–2.44 | 0.573 | 54 | 71 (91.1%) | 36 (45.6%) | (+) | (−) | 9/9 | |
| 7 | * Ha, H. I. et al.—BRCA2 [17] | 2022 | 238 | 27 | 49 | BRCA2 | 25.5 | 0.670 | 0.332–1.35 | 0.263 | 1.100 | 0.28–4.32 | 0.891 | 58 | 24 (88.9%) | 13 (48.1%) | (+) | (−) | 8/9 | |
| 8 | Marchetti, C. et al. [25] | 2023 | 141 | BRCA1/2 | 20 | 0.750 | 0.447–1.259 | 0.277 | (+) | 9/9 | poster |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Yoon, H.J.; Ha, H.I.; Kim, E.T.; Kim, D.E.; Kim, S.; Bae, J.K.; Lim, M.C. Survival Outcomes Associated with the Location of BRCA Mutations in Ovarian Cancer: A Systematic Review and Meta-Analysis. Cancers 2025, 17, 1661. https://doi.org/10.3390/cancers17101661
Kim JH, Yoon HJ, Ha HI, Kim ET, Kim DE, Kim S, Bae JK, Lim MC. Survival Outcomes Associated with the Location of BRCA Mutations in Ovarian Cancer: A Systematic Review and Meta-Analysis. Cancers. 2025; 17(10):1661. https://doi.org/10.3390/cancers17101661
Chicago/Turabian StyleKim, Ji Hyun, Hyung Joon Yoon, Hyeong In Ha, Eun Taeg Kim, Dongkyu Eugene Kim, Sangeon Kim, Jae Kyung Bae, and Myong Cheol Lim. 2025. "Survival Outcomes Associated with the Location of BRCA Mutations in Ovarian Cancer: A Systematic Review and Meta-Analysis" Cancers 17, no. 10: 1661. https://doi.org/10.3390/cancers17101661
APA StyleKim, J. H., Yoon, H. J., Ha, H. I., Kim, E. T., Kim, D. E., Kim, S., Bae, J. K., & Lim, M. C. (2025). Survival Outcomes Associated with the Location of BRCA Mutations in Ovarian Cancer: A Systematic Review and Meta-Analysis. Cancers, 17(10), 1661. https://doi.org/10.3390/cancers17101661







