Prediction of Glomerular Filtration Rate Following Partial Nephrectomy for Localized Renal Cell Carcinoma with Different Machine Learning Techniques
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Ethics Statement
2.3. Statistical Analysis
3. Results
3.1. Baseline Perioperative Characteristics
3.2. Renal Function
3.3. Pathological Outcomes
3.4. Univariable Linear Regression Analyses
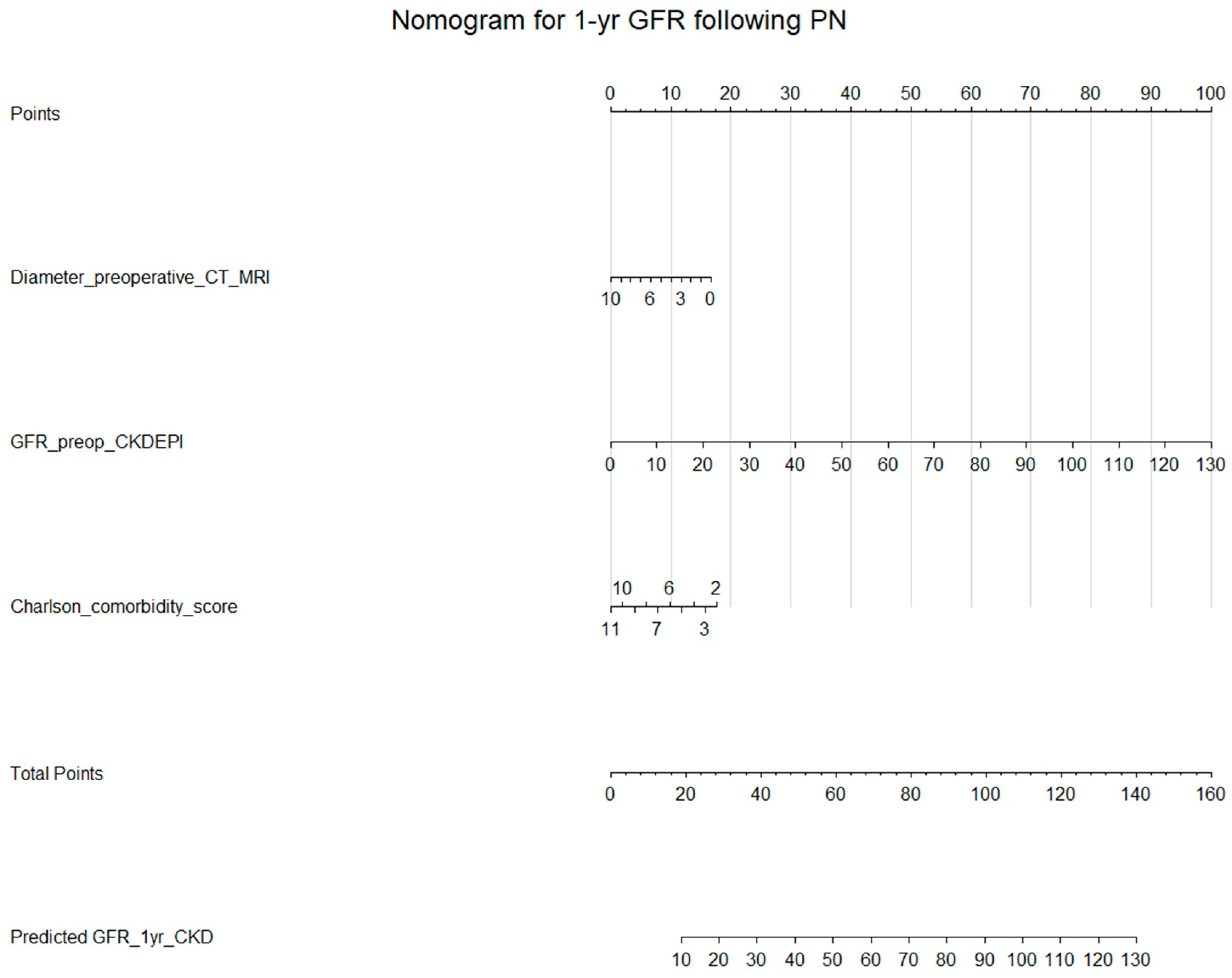
3.5. Multivariable Linear Regression with Preoperative Variables
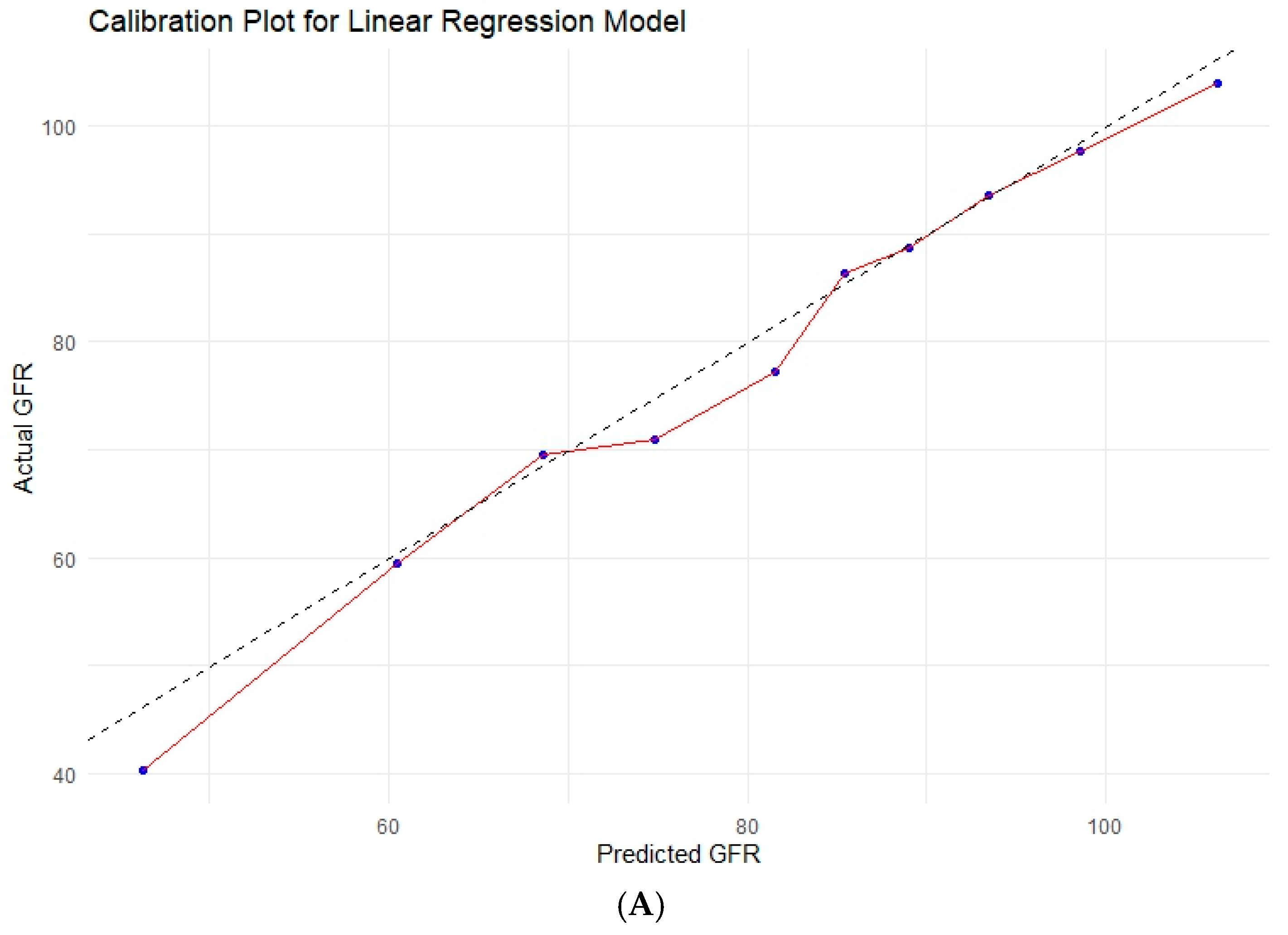
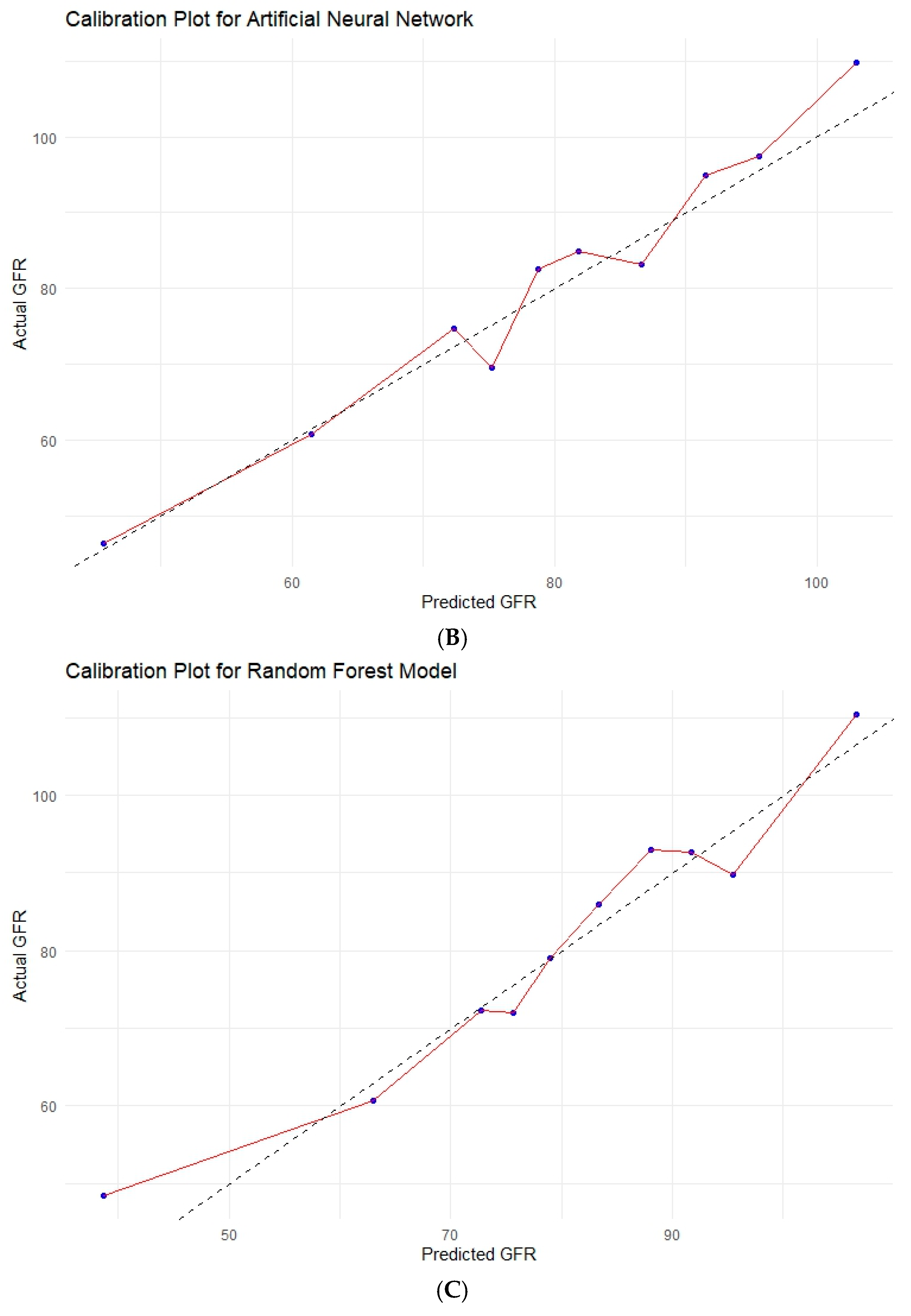
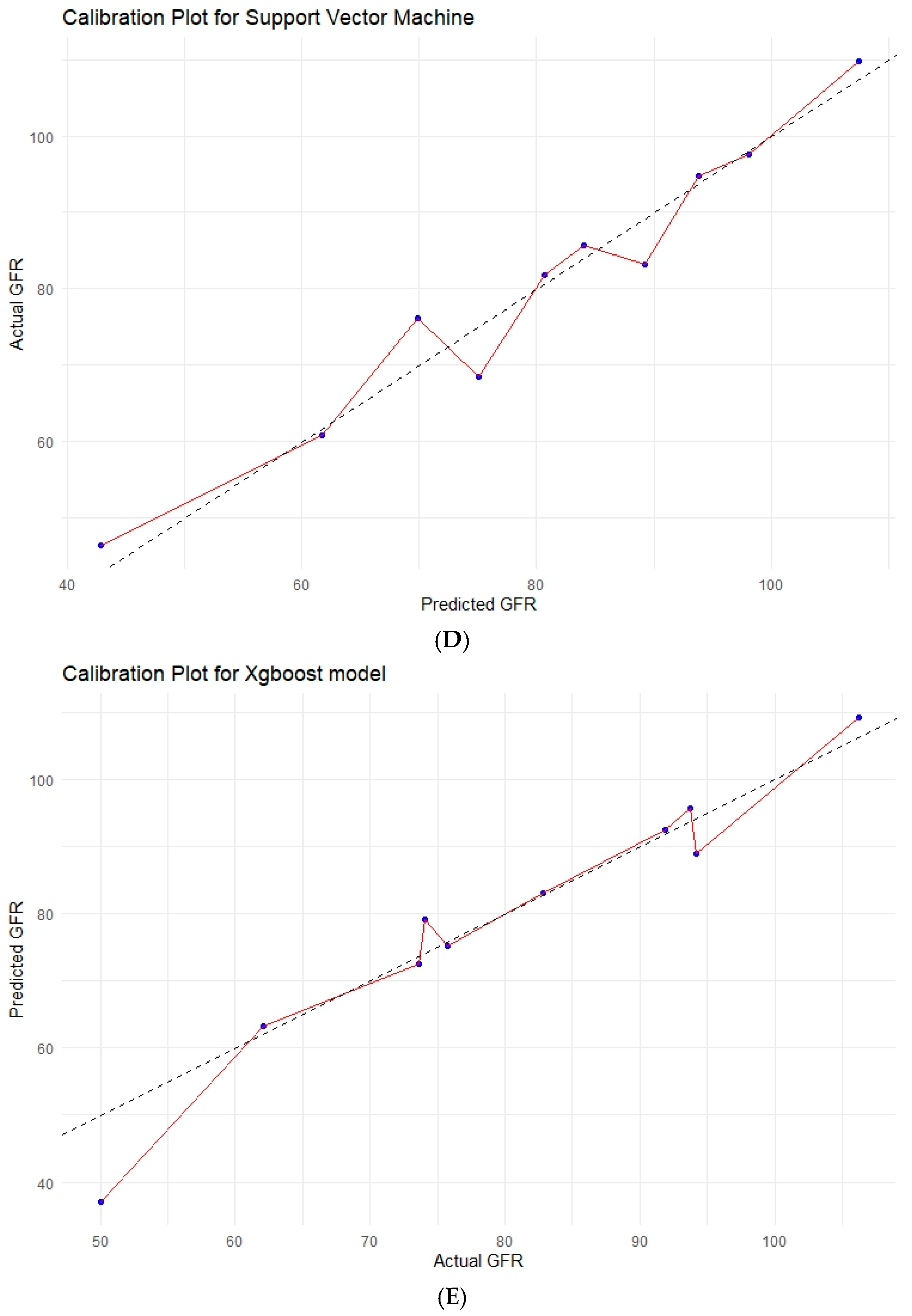
3.6. Various Machine Learning Techniques
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Kriegmair, M.C.; Mandel, P.; Moses, A.; Lenk, J.; Rothamel, M.; Budjan, J.; Michel, M.S.; Wagener, N.; Pfalzgraf, D. Defining Renal Masses: Comprehensive Comparison of RENAL, PADUA, NePhRO, and C-Index Score. Clin. Genitourin. Cancer 2017, 15, 248–255.e1. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, U.; Terrone, C.; Antonelli, A.; Minervini, A.; Volpe, A.; Furlan, M.; Matloob, R.; Regis, F.; Fiori, C.; Porpiglia, F.; et al. Nephron-Sparing Techniques Independently Decrease the Risk of Cardiovascular Events Relative to Radical Nephrectomy in Patients with a T1a-T1b Renal Mass and Normal Preoperative Renal Function. Eur. Urol. 2015, 67, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Arvizo, M.; García-Campa, M.; Santos-Santillana, K.M.; Klatte, T.; García-Chairez, L.R.; González-Colmenero, A.D.; Pallares-Méndez, R.; Cervantes-Miranda, D.E.; Plata-Huerta, H.H.; Rodriguez-Gutierrez, R.; et al. Renal Functional and Cardiovascular Outcomes of Partial Nephrectomy versus Radical Nephrectomy for Renal Tumors: A Systematic Review and Meta-Analysis. Urol. Oncol. 2023, 41, 113–124. [Google Scholar] [CrossRef]
- Van Poppel, H.; Da Pozzo, L.; Albrecht, W.; Matveev, V.; Bono, A.; Borkowski, A.; Colombel, M.; Klotz, L.; Skinner, E.; Keane, T.; et al. A Prospective, Randomised EORTC Intergroup Phase 3 Study Comparing the Oncologic Outcome of Elective Nephron-Sparing Surgery and Radical Nephrectomy for Low-Stage Renal Cell Carcinoma. Eur. Urol. 2011, 59, 543–552. [Google Scholar] [CrossRef]
- Pecoraro, A.; Campi, R.; Bertolo, R.; Mir, M.C.; Marchioni, M.; Serni, S.; Joniau, S.; Van Poppel, H.; Albersen, M.; Roussel, E. Estimating Postoperative Renal Function After Surgery for Nonmetastatic Renal Masses: A Systematic Review of Available Prediction Models. Eur. Urol. Oncol. 2023, 6, 137–147. [Google Scholar] [CrossRef]
- Scilipoti, P.; Rosiello, G.; Belladelli, F.; Pellegrino, F.; Trevisani, F.; Bettiga, A.; Re, C.; Musso, G.; Cei, F.; Salerno, L.; et al. Exploring the Effect of Patient Characteristics on the Association between Warm Ischemia Time and the Risk of Postoperative Acute Kidney Injury after Partial Nephrectomy. Urol. Oncol. 2025, 43, 333.e1–333.e8. [Google Scholar] [CrossRef]
- Kim, H.L.; Shah, S.K.; Tan, W.; Shikanov, S.A.; Zorn, K.C.; Shalhav, A.L.; Wilding, G.E. Estimation and Prediction of Renal Function in Patients with Renal Tumor. J. Urol. 2009, 181, 2451–2460; discussion 2460–2461. [Google Scholar] [CrossRef]
- Shum, C.F.; Bahler, C.D.; Cary, C.; Masterson, T.A.; Boris, R.S.; Gardner, T.A.; Kaimakliotis, H.Z.; Foster, R.S.; Bihrle, R.; Koch, M.O.; et al. Preoperative Nomograms for Predicting Renal Function at 1 Year After Partial Nephrectomy. J. Endourol. 2017, 31, 711–718. [Google Scholar] [CrossRef]
- Palacios, D.A.; Wilson, B.; Ascha, M.; Campbell, R.A.; Song, S.; DeWitt-Foy, M.E.; Campbell, S.C.; Abouassaly, R. New Baseline Renal Function after Radical or Partial Nephrectomy: A Simple and Accurate Predictive Model. J. Urol. 2021, 205, 1310–1320. [Google Scholar] [CrossRef]
- Martini, A.; Cumarasamy, S.; Beksac, A.T.; Abaza, R.; Eun, D.D.; Bhandari, A.; Hemal, A.K.; Porter, J.R.; Badani, K.K. A Nomogram to Predict Significant Estimated Glomerular Filtration Rate Reduction After Robotic Partial Nephrectomy. Eur. Urol. 2018, 74, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Turin, T.C.; Matsushita, K.; Sang, Y.; Ballew, S.H.; Appel, L.J.; Arima, H.; Chadban, S.J.; Cirillo, M.; Djurdjev, O.; et al. Decline in Estimated Glomerular Filtration Rate and Subsequent Risk of End-Stage Renal Disease and Mortality. JAMA 2014, 311, 2518–2531. [Google Scholar] [CrossRef] [PubMed]
- Guglielmetti, G.B.; Dos Anjos, G.C.; Sawczyn, G.; Rodrigues, G.; Cardili, L.; Cordeiro, M.D.; Neves, L.C.O.; Pontes Junior, J.; Fazoli, A.; Coelho, R.F.; et al. A Prospective, Randomized Trial Comparing the Outcomes of Open vs Laparoscopic Partial Nephrectomy. J. Urol. 2022, 208, 259–267. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Liu, B.; Cai, C.; Ye, H.; Lv, C.; Yang, Q.; Sheng, J.; Song, S.; Qu, L.; et al. Robotic versus Open Partial Nephrectomy: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e94878. [Google Scholar] [CrossRef]
- Flammia, R.S.; Anceschi, U.; Tuderti, G.; Di Maida, F.; Grosso, A.A.; Lambertini, L.; Mari, A.; Mastroianni, R.; Bove, A.; Capitanio, U.; et al. Development and Internal Validation of a Nomogram Predicting 3-Year Chronic Kidney Disease Upstaging Following Robot-Assisted Partial Nephrectomy. Int. Urol. Nephrol. 2024, 56, 913–921. [Google Scholar] [CrossRef]
- Anceschi, U.; Brassetti, A.; Tuderti, G.; Consiglia Ferriero, M.; Minervini, A.; Mari, A.; Grosso, A.A.; Carini, M.; Capitanio, U.; Larcher, A.; et al. Risk Factors for Progression of Chronic Kidney Disease after Robotic Partial Nephrectomy in Elderly Patients: Results from a Multi-Institutional Collaborative Series. Minerva Urol. Nephrol. 2022, 74, 452–460. [Google Scholar] [CrossRef]
- Volpe, A.; Blute, M.L.; Ficarra, V.; Gill, I.S.; Kutikov, A.; Porpiglia, F.; Rogers, C.; Touijer, K.A.; Van Poppel, H.; Thompson, R.H. Renal Ischemia and Function After Partial Nephrectomy: A Collaborative Review of the Literature. Eur. Urol. 2015, 68, 61–74. [Google Scholar] [CrossRef]
- Katsimperis, S.; Tzelves, L.; Bellos, T.; Manolitsis, I.; Mourmouris, P.; Kostakopoulos, N.; Pyrgidis, N.; Somani, B.; Papatsoris, A.; Skolarikos, A. The Use of Indocyanine Green in Partial Nephrectomy: A Systematic Review. Cent. Eur. J. Urol. 2024, 77, 15–21. [Google Scholar] [CrossRef]
- Zanoni, M.; Grizzi, F.; Vota, P.; Toia, G.; Mazzieri, C.; Clementi, M.C.; Beatrici, E.; Taverna, G. Off-Clamp Robotic-Assisted Partial Nephrectomy: Surgical Experience from a Single Centre. Cent. Eur. J. Urol. 2023, 76, 123–127. [Google Scholar] [CrossRef]
- Kazama, A.; Attawettayanon, W.; Munoz-Lopez, C.; Rathi, N.; Lewis, K.; Maina, E.; Campbell, R.A.; Lone, Z.; Boumitri, M.; Kaouk, J.; et al. Parenchymal Volume Preservation during Partial Nephrectomy: Improved Methodology to Assess Impact and Predictive Factors. BJU Int. 2024, 134, 219–228. [Google Scholar] [CrossRef]
- Bhindi, B.; Lohse, C.M.; Schulte, P.J.; Mason, R.J.; Cheville, J.C.; Boorjian, S.A.; Leibovich, B.C.; Thompson, R.H. Predicting Renal Function Outcomes After Partial and Radical Nephrectomy. Eur. Urol. 2019, 75, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Ślusarczyk, A.; Garbas, K.; Zapała, P.; Zapała, Ł.; Radziszewski, P. Oncological Outcomes of High-Grade T1 Non-Muscle-Invasive Bladder Cancer Treatment in Octogenarians. Int. Urol. Nephrol. 2021, 53, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Ślusarczyk, A.; Wolański, R.; Miłow, J.; Piekarczyk, H.; Lipiński, P.; Zapała, P.; Niemczyk, G.; Kurzyna, P.; Wróbel, A.; Różański, W.; et al. Can We Go beyond Pathology? The Prognostic Role of Risk Scoring Tools for Cancer-Specific Survival of Patients with Bladder Cancer Undergoing Radical Cystectomy. Biomedicines 2024, 12, 1541. [Google Scholar] [CrossRef] [PubMed]
- Zapała, Ł.; Ślusarczyk, A.; Wolański, R.; Kurzyna, P.; Garbas, K.; Zapała, P.; Radziszewski, P. The Four-Feature Prognostic Models for Cancer-Specific and Overall Survival after Surgery for Localized Clear Cell Renal Cancer: Is There a Place for Inflammatory Markers? Biomedicines 2022, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- Mari, A.; Tellini, R.; Antonelli, A.; Porpiglia, F.; Schiavina, R.; Amparore, D.; Bertini, R.; Brunocilla, E.; Capitanio, U.; Checcucci, E.; et al. A Nomogram for the Prediction of Intermediate Significant Renal Function Loss After Robot-Assisted Partial Nephrectomy for Localized Renal Tumors: A Prospective Multicenter Observational Study (RECORd2 Project). Eur. Urol. Focus 2022, 8, 980–987. [Google Scholar] [CrossRef]
- Suarez-Ibarrola, R.; Hein, S.; Reis, G.; Gratzke, C.; Miernik, A. Current and Future Applications of Machine and Deep Learning in Urology: A Review of the Literature on Urolithiasis, Renal Cell Carcinoma, and Bladder and Prostate Cancer. World J. Urol. 2020, 38, 2329–2347. [Google Scholar] [CrossRef]
- Ślusarczyk, A.; Zapała, P.; Olszewska-Ślusarczyk, Z.; Radziszewski, P. The Prediction of Cancer-Specific Mortality in T1 Non-Muscle-Invasive Bladder Cancer: Comparison of Logistic Regression and Artificial Neural Network: A SEER Population-Based Study. Int. Urol. Nephrol. 2023, 55, 2205–2213. [Google Scholar] [CrossRef]
- Nedbal, C.; Bres-Niewada, E.; Dybowski, B.; Somani, B.K. The Impact of Artificial Intelligence in Revolutionizing All Aspects of Urological Care: A Glimpse in the Future. Cent. Eur. J. Urol. 2024, 77, 12–14. [Google Scholar] [CrossRef]
- Uleri, A.; Baboudjian, M.; Gallioli, A.; Territo, A.; Gaya, J.M.; Sanz, I.; Robalino, J.; Casadevall, M.; Diana, P.; Verri, P.; et al. A New Machine-Learning Model to Predict Long-Term Renal Function Impairment after Minimally Invasive Partial Nephrectomy: The Fundació Puigvert Predictive Model. World J. Urol. 2023, 41, 2985–2990. [Google Scholar] [CrossRef]
- Oh, S.W.; Byun, S.-S.; Kim, J.K.; Jeong, C.W.; Kwak, C.; Hwang, E.C.; Kang, S.H.; Chung, J.; Kim, Y.-J.; Ha, Y.-S.; et al. Machine Learning Models for Predicting the Onset of Chronic Kidney Disease after Surgery in Patients with Renal Cell Carcinoma. BMC Med. Inf. Decis. Mak. 2024, 24, 85. [Google Scholar] [CrossRef]
- Abdallah, N.; Benidir, T.; Heller, N.; Wood, A.; Isensee, F.; Tejpaul, R.; Suk-ouichai, C.; Rathi, N.; Aguilar Palacios, D.; You, A.; et al. Accuracy of Fully Automated, AI-Generated Models Compared with Validated Clinical Model to Predict Post-Operative Glomerular Filtration Rate after Renal Surgery. J. Clin. Oncol. 2023, 41, 693. [Google Scholar] [CrossRef]
- Shah, P.H.; Moreira, D.M.; Okhunov, Z.; Patel, V.R.; Chopra, S.; Razmaria, A.A.; Alom, M.; George, A.K.; Yaskiv, O.; Schwartz, M.J.; et al. Positive Surgical Margins Increase Risk of Recurrence after Partial Nephrectomy for High Risk Renal Tumors. J. Urol. 2016, 196, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Tramanzoli, P.; Castellani, D.; De Stefano, V.; Brocca, C.; Nedbal, C.; Chiacchio, G.; Galosi, A.B.; Da Silva, R.D.; Teoh, J.Y.-C.; Tiong, H.Y.; et al. Radiomics vs Radiologist in Bladder and Renal Cancer. Results from a Systematic Review. Cent. Eur. J. Urol. 2023, 76, 12–19. [Google Scholar] [CrossRef]
| General Characteristics | No. of Pts./Median | %/IQR | |
|---|---|---|---|
| Gender | Female | 141 | 33.98 |
| Male | 274 | 60.02 | |
| Age | 63 | 54–69 | |
| Body mass index | kg/m2 | 25.2 | 23.0–28.7 |
| Preoperative characteristics | |||
| Clinical T stage | cT1a | 348 | 83.86 |
| cT1b | 62 | 14.94 | |
| cT2a | 3 | 0.72 | |
| cT2b | 2 | 0.48 | |
| cT3 | 0 | 0 | |
| Tumor diameter on CT/MRI | cm | 3.07 | 2.0–3.8 |
| Charlson Comorbidity Index | median | 4.0 | 3.0–5.0 |
| Diabetes | no | 345 | 83.13 |
| yes | 54 | 13.01 | |
| unknown | 16 | 3.86 | |
| Hypertension | no | 183 | 44.10 |
| yes | 231 | 55.66 | |
| unknown | 1 | 0.24 | |
| Coronary Artery Disease | no | 344 | 82.89 |
| yes | 55 | 13.25 | |
| unknown | 16 | 3.86 | |
| Myocardial infarction | no | 377 | 90.84 |
| yes | 22 | 5.30 | |
| unknown | 16 | 3.86 | |
| Stroke | no | 350 | 84.34 |
| yes | 16 | 3.86 | |
| unknown | 49 | 11.80 | |
| Chronic kidney disease | no | 303 | 73.01 |
| yes | 94 | 22.65 | |
| unknown | 18 | 4.34 | |
| Frailty index | <18.2 | 284 | 71.36 |
| ≥18.2 | 114 | 28.64 | |
| Postoperative characteristics | |||
| pT stage | T1a | 355 | 85.54 |
| T1b | 50 | 12.05 | |
| T2a | 6 | 1.45 | |
| T2b | 0 | 0 | |
| T3a | 4 | 0.96 | |
| pN stage | N0 | 370 | 89.16 |
| N1 | 8 | 1.93 | |
| Nx | 37 | 8.92 | |
| Grade | 1 | 145 | 34.94 |
| 2 | 206 | 49.64 | |
| 3 | 56 | 13.49 | |
| 4 | 8 | 1.93 | |
| Surgical margin | negative | 367 | 88.43 |
| positive | 48 | 11.57 | |
| Tumor diameter | cm | 3.0 | 2.0–3.9 |
| Histology type | clear cell | 323 | 77.83 |
| papillary | 64 | 15.42 | |
| chromophobe | 28 | 6.75 | |
| Surgery and hospitalization | |||
| Surgical treatment modality | Open | 215 | 51.81 |
| Laparoscopic | 160 | 38.55 | |
| Robotic | 40 | 9.64 | |
| Warm ischemia time | ≥20 min | 129 | 31.08 |
| <20 min | 286 | 68.92 | |
| Length of stay | median | 7 | 5–9 |
| Complications Clavien–Dindo scale | 0 | 194 | 48.74 |
| 1 | 164 | 39.52 | |
| 2 | 26 | 6.27 | |
| 3 | 10 | 2.41 | |
| 4 | 4 | 0.96 | |
| unknown | 17 | 4.10 |
| Renal Function | No. of Pts./Median | %/IQR | |
|---|---|---|---|
| Preoperative GFR CKD-EPI | mL/min | 88.33 | 70.98–100.80 |
| Discharge GFR CKD-EPI | mL/min | 81.47 | 62.47–96.89 |
| 1-year GFR CKD-EPI | mL/min | 81.0 | 66.0–96.89 |
| Significant GFR loss at discharge | no | 371 | 89.40 |
| yes | 44 | 10.60 | |
| Significant * GFR loss at 1 yr | no | 379 | 91.33 |
| yes | 36 | 8.67 | |
| CKD upstage ** at 1 yr | no | 292 | 70.36 |
| yes | 123 | 29.64 | |
| Oncological outcomes | |||
| Recurrence | no | 331 | 79.76 |
| yes | 82 | 19.76 | |
| unknown | 2 | 0.48 | |
| All-cause death | no | 364 | 87.71 |
| yes | 49 | 11.81 | |
| unknown | 2 | 0.48 | |
| Cancer-specific death | no | 385 | 92.77 |
| yes | 28 | 6.75 | |
| unknown | 2 | 0.48 |
| A. Univariable Analysis | ||||
| Variable | Class | Estimate | Std. Error | p-value |
| Age | −0.93 | 0.10 | <0.001 | |
| Gender | male | ref | ||
| female | 1.47 | 2.87 | 0.608 | |
| Diabetes | no | ref | ||
| yes | −9.93 | 4.07 | 0.015 | |
| Myocardial Infarction | no | ref | ||
| yes | −12.31 | 5.70 | 0.032 | |
| Coronary Artery Disease | no | ref | ||
| yes | −9.60 | 4.08 | 0.019 | |
| Surgical Treatment Modality | open | ref | ||
| laparoscopy | 11.19 | 2.68 | <0.001 | |
| Frailty Index | <18.2 | ref | ||
| ≥18.2 | −10.59 | 2.07 | <0.001 | |
| Preoperative Tumor Diameter On CT/MRI | cm | −2.49 | 1.00 | 0.014 |
| Preoperative Chronic Kidney Disease | no | ref | ||
| yes | −24.26 | 2.96 | <0.001 | |
| Charlson Comorbidity Index | continuous | −7.29 | 0.68 | <0.001 |
| Preoperative GFR CKD-EPI | mL/min | 0.85 | 0.04 | <0.001 |
| Preoperative Creatinine | mg/dL | −26.07 | 1.92 | <0.001 |
| Preoperative Hemoglobin | g/dL | 0.12 | 0.08 | 0.147 |
| B. Multivariable analysis | ||||
| Variable | Class | Estimate | Std. Error | p-value |
| Intercept | 29.37 | 5.69 | <0.001 | |
| Preoperative Tumor Diameter on CT/MRI | cm | −1.65 | 0.58 | 0.005 |
| Preoperative GFR CKD-EPI | mL/min | 0.76 | 0.04 | <0.001 |
| Charlson Comorbidity Index | continuous | −1.95 | 0.54 | 0.0004 |
| Model | R2 | RMSE | MAE | Brier Score (MSE) | Calibration Slope | Calibration-in-the-Large |
|---|---|---|---|---|---|---|
| Linear Regression | 0.668 | 13.13 | 10.07 | 172.44 | 1.038 | −4.700 |
| ANN | 0.679 | 12.92 | 10.15 | 166.88 | 1.076 | −7.588 |
| XGBoost | 0.554 | 15.23 | 11.76 | 231.85 | 0.905 | 5.974 |
| Random Forest | 0.643 | 13.63 | 10.58 | 185.69 | 1.025 | −3.736 |
| SNV | 0.663 | 13.23 | 10.32 | 175.15 | 1.049 | −6.587 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ślusarczyk, A.; Sharma, S.; Garbas, K.; Piekarczyk, H.; Zapała, P.; Shi, J.; Radziszewski, P.; Qu, L.; Zapała, Ł. Prediction of Glomerular Filtration Rate Following Partial Nephrectomy for Localized Renal Cell Carcinoma with Different Machine Learning Techniques. Cancers 2025, 17, 1647. https://doi.org/10.3390/cancers17101647
Ślusarczyk A, Sharma S, Garbas K, Piekarczyk H, Zapała P, Shi J, Radziszewski P, Qu L, Zapała Ł. Prediction of Glomerular Filtration Rate Following Partial Nephrectomy for Localized Renal Cell Carcinoma with Different Machine Learning Techniques. Cancers. 2025; 17(10):1647. https://doi.org/10.3390/cancers17101647
Chicago/Turabian StyleŚlusarczyk, Aleksander, Sumit Sharma, Karolina Garbas, Hanna Piekarczyk, Piotr Zapała, Jinhao Shi, Piotr Radziszewski, Le Qu, and Łukasz Zapała. 2025. "Prediction of Glomerular Filtration Rate Following Partial Nephrectomy for Localized Renal Cell Carcinoma with Different Machine Learning Techniques" Cancers 17, no. 10: 1647. https://doi.org/10.3390/cancers17101647
APA StyleŚlusarczyk, A., Sharma, S., Garbas, K., Piekarczyk, H., Zapała, P., Shi, J., Radziszewski, P., Qu, L., & Zapała, Ł. (2025). Prediction of Glomerular Filtration Rate Following Partial Nephrectomy for Localized Renal Cell Carcinoma with Different Machine Learning Techniques. Cancers, 17(10), 1647. https://doi.org/10.3390/cancers17101647







