Disease Burden at the Time of Transplantation Is a Primary Predictor of Outcomes in Pediatric MDS: A Single-Center Experience
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Donor Selection
2.3. Supportive Care
2.4. Statistical Methods
3. Results
3.1. Patient and Disease Characteristics
3.2. HCT Characteristics and Engraftment
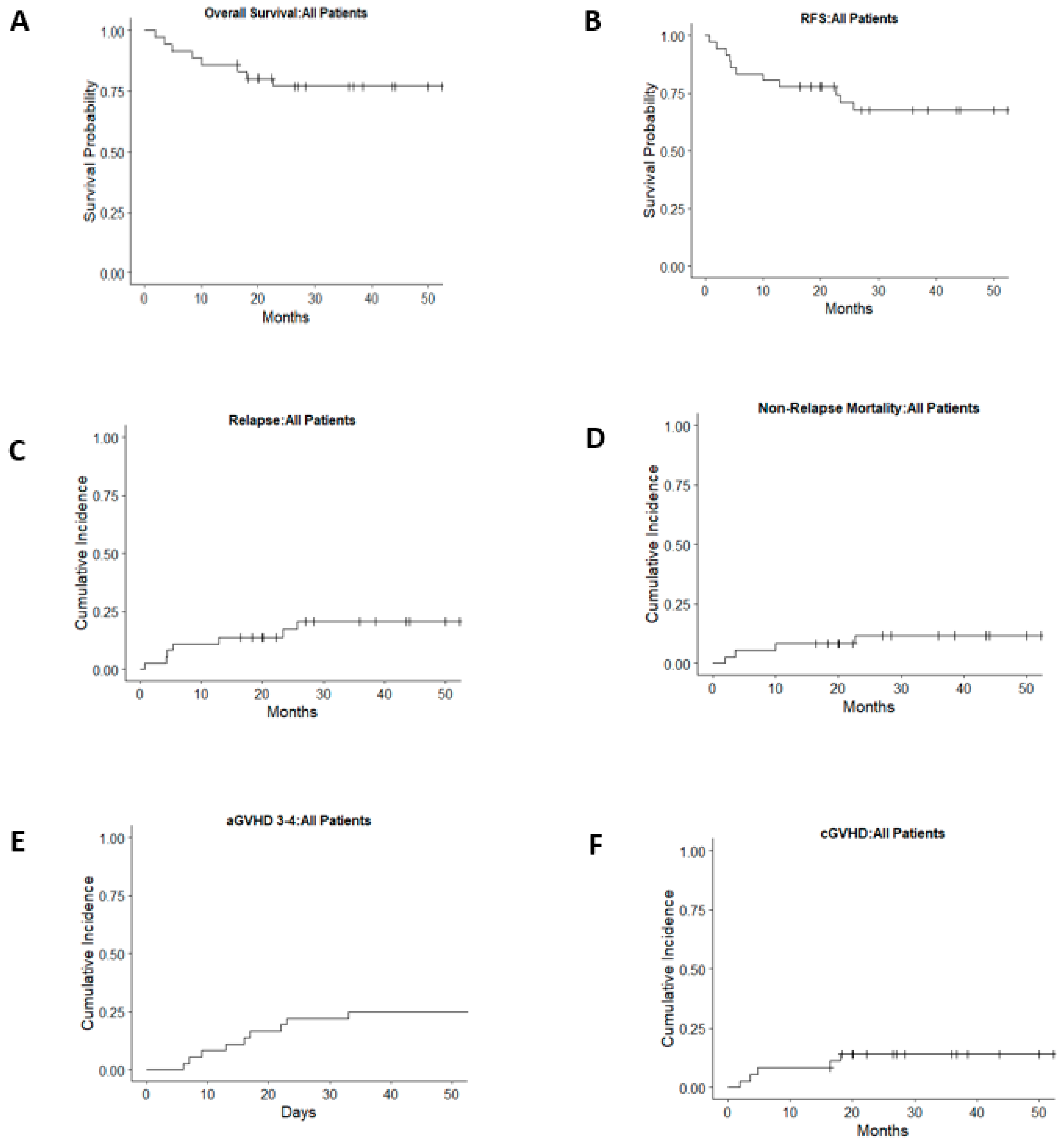
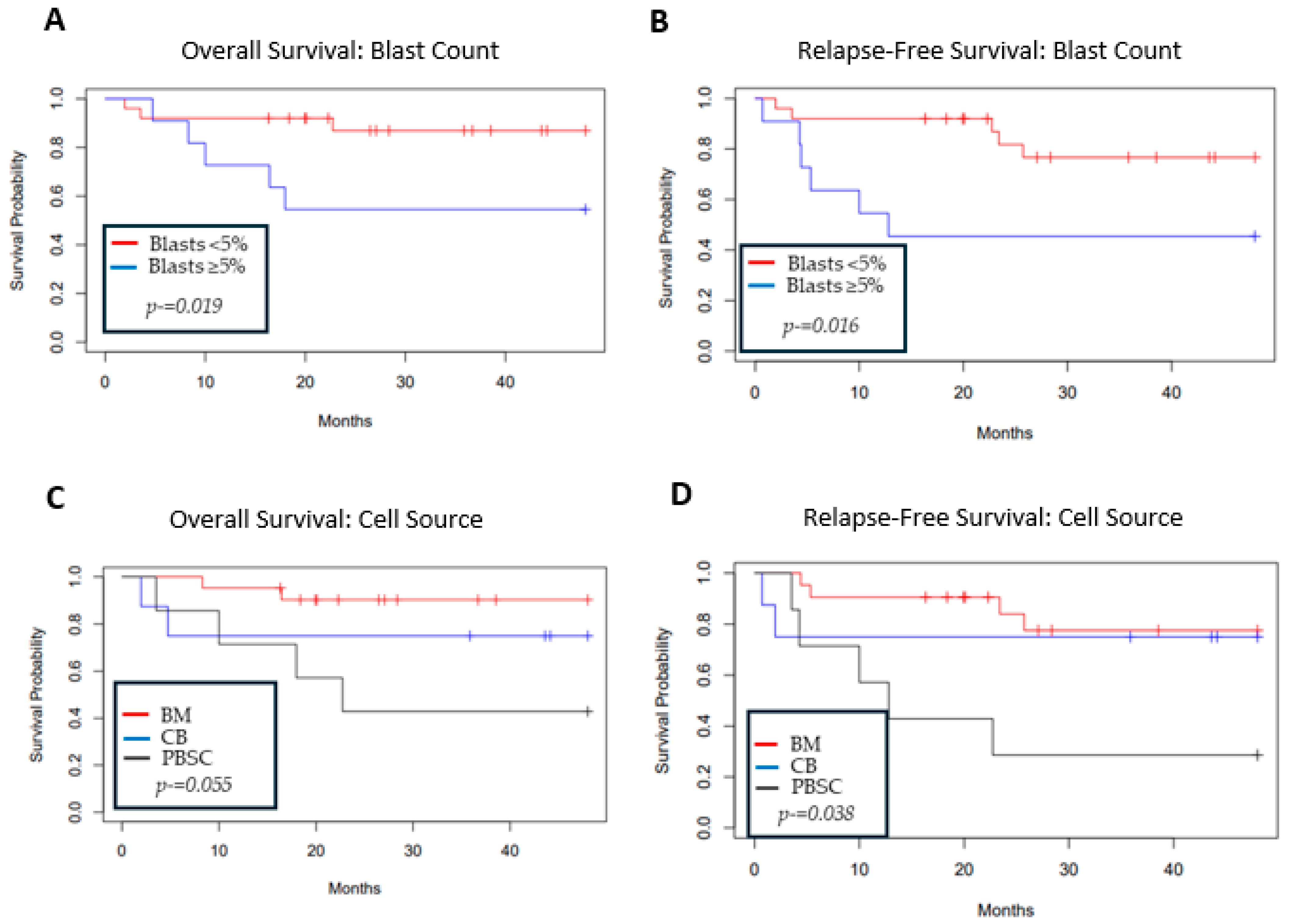
3.3. Overall and Relapse-Free Survival
3.4. Relapse
3.5. GVHD
3.6. Non-Relapse Morbidity and Mortality
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| HCT | Hematopoietic cell transplantation |
| MDS | Myelodysplastic syndrome |
| OS | Overall survival |
| RFS | Relapse-free survival |
| HR | Hazard ratio |
| NRM | Non-relapse mortality |
| GVHD | Graft-versus-host disease |
| AML | Acute myelogenous leukemia |
| IBMFS | Idiopathic bone marrow failure syndrome |
| MMUD | Mismatched unrelated donor |
| MSD | Matched sibling donor |
| PBSC | Peripheral blood stem cell |
| MUD | Matched unrelated donor |
| CB | Unrelated cord blood |
| CMV | Cytomegalovirus |
| TBI | Total body irradiation |
| ANC | Absolute neutrophil count |
| ICU | Intensive care unit |
| SOS | Sinusoidal obstructive syndrome |
| EBV | Epstein–Barr virus |
| RSV | Respiratory syncytial virus |
| HMA | Hypomethylating agent |
| DLI | Donor lymphocyte infusion |
References
- Hasle, H.; Niemeyer, C.M. Advances in the prognostication and management of advanced MDS in children. Br. J. Haematol. 2011, 154, 185–195. [Google Scholar] [CrossRef]
- Galaverna, F.; Ruggeri, A.; Locatelli, F. Myelodysplastic syndromes in children. Curr. Opin. Oncol. 2018, 30, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hasle, H.; Niemeyer, C.M.; Chessells, J.M.; Baumann, I.; Bennett, J.M.; Kerndrup, G.; Head, D.R. A pediatric approach to the WHO classification of myelodysplastic and myeloproliferative diseases. Leukemia 2003, 17, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Niemeyer, C.M.; Baumann, I. Myelodysplastic syndrome in children and adolescents. Semin. Hematol. 2008, 45, 60–70. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.-M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Pastor, V.B.; Goodings, C.; Voss, R.K.; Kozyra, E.J.; Szvetnik, A.; Noellke, P.; Dworzak, M.; Starý, J.; Locatelli, F.; et al. Publisher Correction: Clinical evolution, genetic landscape and trajectories of clonal hematopoiesis in SAMD9/SAMD9L syndromes. Nat. Med. 2021, 27, 2248. [Google Scholar] [CrossRef]
- McCall, D.; Abuasab, T.; Rodriguez-Sevilla, J.J.; Mohamed, S.F.; Patnaik, A.; Devireddy, K.; Arani, N.; Sheikh, I.; Jamshidi, R.; Gibson, A.; et al. Characteristics and outcomes in children, adolescent, and young adult patients with myelodysplastic neoplasms: A single-center retrospective analysis. Leuk. Res. 2024, 144, 107563. [Google Scholar] [CrossRef]
- Woodard, P.; Carpenter, P.A.; Davies, S.M.; Gross, T.G.; He, W.; Zhang, M.-J.; Horn, B.N.; Margolis, D.A.; Perentesis, J.P.; Sanders, J.E.; et al. Unrelated donor bone marrow transplantation for myelodysplastic syndrome in children. Biol. Blood Marrow Transpl. 2011, 17, 723–728. [Google Scholar] [CrossRef]
- Strahm, B.; Nollke, P.; Zecca, M.; Korthof, E.T.; Bierings, M.; Furlan, I.; Sedlacek, P.; Chybicka, A.; Schmugge, M.; Bordon, V.; et al. Hematopoietic stem cell transplantation for advanced myelodysplastic syndrome in children: Results of the EWOG-MDS 98 study. Leukemia 2011, 25, 455–462. [Google Scholar] [CrossRef]
- Parikh, S.H.; Mendizabal, A.; Martin, P.L.; Prasad, V.K.; Szabolcs, P.; Driscoll, T.A.; Kurtzberg, J. Unrelated donor umbilical cord blood transplantation in pediatric myelodysplastic syndrome: A single-center experience. Biol. Blood Marrow Transpl. 2009, 15, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Merli, P.; Pagliara, D.; Mina, T.; Bertaina, V.; Pira, G.L.; Lazzaro, S.; Biagini, S.; Galaverna, F.; Strocchio, L.; Carta, R.; et al. alphabetaT- and B-cell-depleted HLA-haploidentical hematopoietic stem cell transplantation in children with myelodysplastic syndromes. Haematologica 2022, 107, 2966–2971. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.W.; Im, H.J.; Kim, H.; Koh, K.-N.; Kang, S.H.; Min, S.Y.; Choi, E.S.; Jang, S.; Park, C.-J.; Seo, J.J. Improved outcomes of allogeneic hematopoietic stem cell transplantation including haploidentical transplantation for childhood myelodysplastic syndrome. Bone Marrow Transpl. 2020, 55, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Woodard, P.; Barfield, R.; Hale, G.; Horwitz, E.; Leung, W.; Ribeiro, R.; Rubnitz, J.; Srivistava, D.K.; Tong, X.; Yusuf, U.; et al. Outcome of hematopoietic stem cell transplantation for pediatric patients with therapy-related acute myeloid leukemia or myelodysplastic syndrome. Pediatr. Blood Cancer 2006, 47, 931–935. [Google Scholar] [CrossRef]
- Smith, A.R.; Christiansen, E.C.; Wagner, J.E.; Cao, Q.; MacMillan, M.L.; Stefanski, H.E.; Trotz, B.A.; Burke, M.J.; Verneris, M.R. Early hematopoietic stem cell transplant is associated with favorable outcomes in children with MDS. Pediatr. Blood Cancer 2013, 60, 705–710. [Google Scholar] [CrossRef]
- Przepiorka, D.; Weisdorf, D.; Martin, P.; Klingemann, H.G.; Beatty, P.; Hows, J.; Thomas, E.D. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transpl. 1995, 15, 825–828. [Google Scholar]
- Jagasia, M.H.; Greinix, H.T.; Arora, M.; Williams, K.M.; Wolff, D.; Cowen, E.W.; Palmer, J.; Weisdorf, D.; Treister, N.S.; Cheng, G.-S.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol. Blood Marrow Transpl. 2015, 21, 389–401.e1. [Google Scholar] [CrossRef]
- Deeg, H.J.; Stevens, E.A.; Salit, R.B.; Ermoian, R.P.; Fang, M.; Gyurkocza, B.; Sorror, M.L.; Fatobene, G.; Baumgart, J.; Burroughs, L.M.; et al. Transplant Conditioning with Treosulfan/Fludarabine with or without Total Body Irradiation: A Randomized Phase II Trial in Patients with Myelodysplastic Syndrome and Acute Myeloid Leukemia. Biol. Blood Marrow Transpl. 2018, 24, 956–963. [Google Scholar] [CrossRef]
- Milano, F.; Gutman, J.A.; Deeg, H.J.; Nemecek, E.R.; Baumgart, J.; Thur, L.; Dahlberg, A.; Salit, R.B.; Summers, C.; Appelbaum, F.R.; et al. Treosulfan-based conditioning is feasible and effective for cord blood recipients: A phase 2 multicenter study. Blood Adv. 2020, 4, 3302–3310. [Google Scholar] [CrossRef]
- Yusuf, U.; Frangoul, H.A.; Gooley, T.A.; Woolfrey, A.E.; Carpenter, P.A.; Andrews, R.G.; Deeg, H.J.; Appelbaum, F.R.; Anasetti, C.; Storb, R.; et al. Allogeneic bone marrow transplantation in children with myelodysplastic syndrome or juvenile myelomonocytic leukemia: The Seattle experience. Bone Marrow Transpl. 2004, 33, 805–814. [Google Scholar] [CrossRef][Green Version]
- Kardos, G.; Baumann, I.; Passmore, S.J.; Locatelli, F.; Hasle, H.; Schultz, K.R.; Starý, J.; Schmitt-Graeff, A.; Fischer, A.; Harbott, J.; et al. Refractory anemia in childhood: A retrospective analysis of 67 patients with particular reference to monosomy 7. Blood 2003, 102, 1997–2003. [Google Scholar] [CrossRef] [PubMed]
- Göhring, G.; Michalova, K.; Beverloo, H.B.; Betts, D.; Harbott, J.; Haas, O.A.; Kerndrup, G.; Sainati, L.; Bergstraesser, E.; Hasle, H.; et al. Complex karyotype newly defined: The strongest prognostic factor in advanced childhood myelodysplastic syndrome. Blood 2010, 116, 3766–3769. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.A.; Lau, B.W.; Dickerson, K.E.; Wlodarski, M.; Pollard, J.; Shimamura, A.; Hofmann, I.; Sasa, G.; Elghetany, T.; Cada, M.; et al. Diagnosis and treatment of pediatric myelodysplastic syndromes: A survey of the North American Pediatric Aplastic Anemia Consortium. Pediatr. Blood Cancer 2020, 67, e28652. [Google Scholar] [CrossRef]
- Myers, K.C.; Furutani, E.; Weller, E.; Siegele, B.; Galvin, A.; Arsenault, V.; Alter, B.P.; Boulad, F.; Bueso-Ramos, C.; Burroughs, L.; et al. Clinical features and outcomes of patients with Shwachman-Diamond syndrome and myelodysplastic syndrome or acute myeloid leukaemia: A multicentre, retrospective, cohort study. Lancet Haematol. 2020, 7, e238–e246. [Google Scholar] [CrossRef]
- Waespe, N.; Akker, M.V.D.; Klaassen, R.J.; Lieberman, L.; Irwin, M.S.; Ali, S.S.; Abdelhaleem, M.; Zlateska, B.; Liebman, M.; Cada, M.; et al. Response to treatment with azacitidine in children with advanced myelodysplastic syndrome prior to hematopoietic stem cell transplantation. Haematologica 2016, 101, 1508–1515. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Zhou, W.; Wang, R.; Li, Y.; Yu, L. Pre-transplant therapy for patients with myelodysplastic syndromes: A systematic review and meta-analysis. Leuk. Res. 2021, 110, 106645. [Google Scholar] [CrossRef]
- Schroeder, T.; Wegener, N.; Lauseker, M.; Rautenberg, C.; Nachtkamp, K.; Schuler, E.; Kondakci, M.; Haas, R.; Germing, U.; Kobbe, G. Comparison between Upfront Transplantation and different Pretransplant Cytoreductive Treatment Approaches in Patients with High-Risk Myelodysplastic Syndrome and Secondary Acute Myelogenous Leukemia. Biol. Blood Marrow Transpl. 2019, 25, 1550–1559. [Google Scholar] [CrossRef]
- Modi, D.; Kim, S.; Singh, V.; Ayash, L.; Alavi, A.; Ratanatharathorn, V.; Uberti, J.P.; Deol, A. Pre-transplant hypomethylating agents do not influence post-transplant survival in myelodysplastic syndrome. Leuk. Lymphoma 2019, 60, 2762–2770. [Google Scholar] [CrossRef]
- Ball, B.J.; Famulare, C.A.; Stein, E.M.; Tallman, M.S.; Derkach, A.; Roshal, M.; Gill, S.I.; Manning, B.M.; Koprivnikar, J.; McCloskey, J.; et al. Venetoclax and hypomethylating agens (HMAs) induce high response rates in MDS, including patients after HMA therapy failure. Blood Adv. 2020, 4, 2866–2870. [Google Scholar] [CrossRef]
- Masetti, R.; Baccelli, F.; Leardini, D.; Gottardi, F.; Vendemini, F.; Di Gangi, A.; Becilli, M.; Lodi, M.; Tumino, M.; Vinci, L.; et al. Venetoclax-based therapies in pediatric advanced MDS and relapsed/refractory AML: A multicenter retrospective analysis. Blood Adv. 2023, 7, 4366–4370. [Google Scholar] [CrossRef]
- Khanam, R.; Shahzad, M.; Chaudhary, S.G.; Ali, F.; Shah, Z.; Pachika, P.S.; Ahmed, Z.; Chattaraj, A.; Masood, A.; Ahmed, N.; et al. Outcomes after venetoclax with hypomethylating agents in myelodysplastic syndromes: A systematic review and meta-analysis. Leuk. Lymphoma 2022, 63, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Huschart, E.; Miller, H.; Salzberg, D.; Campbell, C.; Beebe, K.; Schwalbach, C.; Magee, K.; Adams, R.H.; Ngwube, A. Azacitidine and prophylactic donor lymphocyte infusions after hematopoietic stem cell transplantation for pediatric high-risk acute myeloid leukemia. Pediatr. Hematol. Oncol. 2021, 38, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Oshrine, B.R.; Shyr, D.; Hale, G.; Petrovic, A. Low-dose azacitidine for relapse prevention after allogeneic hematopoietic cell transplantation in children with myeloid malignancies. Pediatr. Transpl. 2019, 23, e13423. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Ishida, T.; Saito, A.; Yamamoto, N.; Yokoi, T.; Uemura, S.; Nino, N.; Fujiwara, T.; Tahara, T.; Nakamura, S.; et al. Low-dose azacitidine maintenance therapy after allogeneic stem cell transplantation for high-risk pediatric acute myeloid leukemia. Pediatr. Blood Cancer 2018, 65, e27284. [Google Scholar] [CrossRef]
- Nemecek, E.R.; Hilger, R.A.; Adams, A.; Shaw, B.E.; Kiefer, D.; Le-Rademacher, J.; Levine, J.E.; Yanik, G.; Leung, W.; Talano, J.-A.; et al. Treosulfan, Fludarabine, and Low-Dose Total Body Irradiation for Children and Young Adults with Acute Myeloid Leukemia or Myelodysplastic Syndrome Undergoing Allogeneic Hematopoietic Cell Transplantation: Prospective Phase II Trial of the Pediatric Blood and Marrow Transplant Consortium. Biol. Blood Marrow Transpl. 2018, 24, 1651–1656. [Google Scholar]
| Characteristic | n |
|---|---|
| Gender | |
| Male | 17 |
| Female | 19 |
| Median Age | 11.7 years (1–18) |
| Type MDS | |
| Primary, no underlying cause | 15 |
| Evolved from SAA | 6 |
| Secondary (from malignancy or prior intensive chemotherapy) | 7 (3 ALL, 1 Burkitt, 1 sarcoma, 1 NB, 1 SCID s/p first transplant) (20%) |
| Underlying predisposition * | 8 |
| Classification by the WHO 2016 criteria | |
| RCC | 17 |
| MDS-EB1 | 8 |
| MDS-EB2 | 4 |
| TA-MDS | 7 |
| Cytogenetics | |
| Monosomy 7/Del 7q | 18 |
| Trisomy 8 | 7 |
| Complex | 4 |
| Del 5 | 1 |
| Normal | 6 |
| Pre-HCT Therapy | |
| Chemotherapy for MDS (not prior malignancy) | 5 |
| IST | 6 |
| None | 25 |
| Time from diagnosis to HCT | |
| <4 months | 15 (median 89 days) |
| ≥4 months | 21 (median 162 days) |
| Year of HCT | |
| 2000–2008 | 19 |
| 2009–2019 | 17 |
| Pre-HCT blast count | |
| <5% | 25 |
| ≥5% | 11 |
| Graft Source | |
| MSD (BM) | 8 |
| MSD (PBSC) | 1 |
| MUD (BM) | 10 |
| MUD (PBSC) | 1 |
| MMUD (BM) | 3 |
| MMUD (PBSC) | 5 |
| CBT | 8 |
| Conditioning Regimen | |
| Busulfan/Cyclophosphamide +/− ATG | 21 |
| TBI/Cyclophosphamide | 6 |
| Reduced toxicity | 8 |
| Other | 1 |
| Graft failure | |
| Yes | 1 (2nd HCT early TRM) |
| Age | Measurable Disease at Transplant (% Blasts) | WHO-2016 Classification at Time of Diagnosis | Donor Source | Conditioning | Time to Transplant (Days) | Pre-tx Chemo | Relapse (Days) | Cause of Death | Disease Detail | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10.8 | 7 | RCC | CBT | HD-TBI/Cy | 134 | Infection (CMV) | Prior history of SAA (received IST); Monosomy 7 | ||
| 2 | 16.1 | 0 | RCC | MUD BMT | Bu/Cy | 366 | Prior history of SAA (received IST); Monosomy 7 | |||
| 3 | 15.7 | 4 | TA-MDS | MSD BMT | Bu/Cy | 104 | TA-MDS (sarcoma) Inversion 6, t(11;19) | |||
| 4 | 3 | 0 | RCC | CBT | HD-TBI/Cy/ATG | 60 | GVHD | Prior history of SAA (received IST); Normal karyotype | ||
| 5 | 3.5 | 4.6 | TA-MDS | MSD BMT | Bu/Cy | 110 | TA-MDS (neuroblastoma) Trisomy 8 | |||
| 6 | 11.7 | 5 | RCC | MUD BMT | Bu/Cy | 165 | Familial amegakaryocytic dysplasia Monosomy 7 | |||
| 7 | 13 | 3 | RCC | MUD PBSC | Bu/Cy | 28 | GVHD/infection | Prior history of SAA (received IST); Del 7q- on one marrow | ||
| 8 | 15.6 | >5% | MDS-EB1 | MUD PBSC | Bu/Cy | 336 | Chronic GVHD | Prior history of SAA (received IST); Normal karyotype | ||
| 9 | 14.1 | 5 | MDS-EB1 | MSD BMT | Bu/Cy/ATG | 114 | ADE × 2 | 132 | Relapse * | Normal karyotype |
| 10 | 12.8 | 7 | TA-MDS | MSD BMT | Bu/Cy/ATG | 63 | 132 | Relapse ** | TA-MDS (ALL) Complex karyotype | |
| 11 | 14.3 | 13 | MDS-EB2 | MSD PBSC | Bu/Cy/ATG | 134 | 384 | Post-relapse reemission after IST taper Del-7q (1; 7) | ||
| 12 | 1.8 | 3 | TA-MDS | CBT | HD-TBI/Cy/ATG | 162 | TA-MDS (Burkitt) | |||
| 13 | 1 | 1.5 | RCC | MUD PBSC | HD-TBI/Cy | 126 | Monosomy 7 | |||
| 14 | 11.1 | 0 | RCC | MUD BMT | Bu/Cy/ATG | 971 | Familial amegakaryocytic dysplasia Normal karyotype | |||
| 15 | 13.6 | 2.8 | RCC | MUD PBSC | Bu/Cy | 121 | Prior history of SAA (received IST); Monosomy 7 | |||
| 16 | 12.1 | 5 | MDS-EB1 | MUD PBSC | Bu/Cy | 131 | 128 | Relapse ** | Trisomy 8 | |
| 17 | 17.9 | 1 | RCC | MUD PBSC | Flu, TBI (3 Gy) | 1981 | Etop/Dex (Received for secondary HLH and not MDS) | Respiratory failure/infection *** | Graft failure after 1st transplant; early TRM after 2nd transplant; Trisomy 8 | |
| 18 | 6.8 | 10 | MDS-EB2 | MUD BMT | HD-TBI/Cy | 153 | Monosomy 7 | |||
| 19 | 10.4 | 1 | RCC | MUD BMT | Bu/Cy | 142 | Familial marrow failure; Complex karyotype | |||
| 20 | 11.6 | 0 | TA-MDS | MUD BMT | Bu/Cy | 84 | TA-MDS (ALL); Del (18) | |||
| 21 | 16.8 | 5 | MDS-EB2 | CBT | Treo/Flu/TBI (2 Gy) | 168 | Trisomy 8 | |||
| 22 | 6.8 | 1.6 | RCC | MSD BMT | Treo/Flu/TBI (2 Gy) | 77 | Monosomy 7 | |||
| 23 | 2.2 | 1 | RCC | MUD BMT | Bu/Cy | 186 | SAMD9, familial monosomy 7 | |||
| 24 | 15.2 | 1 | RCC | CBT | Treo/Flu/TBI (2 Gy) | 614 | Familial thrombocytopenia; RUNX1 heterozygous; Del 5q | |||
| 25 | 12.1 | 6 | MDS-EB1 | CBT | Treo/Flu/TBI (2 Gy) | 119 | ELA-2 mutation (received chronic G-CSF) Monosomy 7 | |||
| 26 | 2.8 | 2 | RCC | MSD BMT | Treo/Flu/TBI (2 Gy) | 84 | Monosomy 7 | |||
| 27 | 17.6 | 4 | MDS-EB1 | CBT | Treo/Flu/TBI (2 Gy) | 94 | NGS-positive GATA2 (confirmed germline); Monosomy 7 | |||
| 28 | 8 | 0 | MDS-EB2 | MSD BMT | Treo/Flu/TBI (2 Gy) | 133 | ADE | 772 **** | Monosomy 7, NGS-positive CSF3R, NF1, PTPN11 | |
| 29 | 6.9 | 0 | RCC | MSD BMT | Bu/Cy | 124 | Germline GATA-2 Monosomy 7 | |||
| 30 | 3.2 | 2 | TA-MDS | MUD BMT | Bu/Cy | 115 | TA-MDS (SCID/Omenn syndrome with MDS s/p 1st transplant) Complex karyotype | |||
| 31 | 1.8 | 0 | RCC | MUD BMT | Bu/Cy | 191 | SAMD9, familial monosomy 7, NGS otherwise negative | |||
| 32 | 1.5 | 5 | MDS-EB1 | CBT | Treo/Flu/TBI (2 Gy) | 94 | 21 | Relapse/MOF | Monosomy 7, NGS-positive NRAS | |
| 33 | 13.7 | 1 | RCC | MUD BMT | Bu/Cy | 98 | Monosomy 7, NGS-negative | |||
| 34 | 13 | 1 | MDS-EB2 | MUD BMT | HD-TBI/Cy | 184 | Decitabine followed by mito/Ara-C | NGS-positive PTPN11, TET2 Trisomy 8 | ||
| 35 | 9.5 | 1 | MDS-EB1 | MUD BMT | Bu/Cy | 82 | Decitabine and ruxolitinib | 702 **** | NGS-positive for JAK2, TP53 Trisomy 8 | |
| 36 | 12 | 0 | TA-MDS | MUD BMT | Bu/Cy | 126 | TA-MDS (ALL) 2nd transplant (1st transplant for ALL) Complex karyotype |
| Hazard Ratio * | 95% CI | p-Value | |
|---|---|---|---|
| Overall Survival | |||
| Age (≤12 years vs. >12 years) | 1.63 | 0.44–6.1 | 0.47 |
| Donor source (MSD vs. MUD/MMUD/CBT) | 1 | 0–∞ | 1 |
| Stem cell source (BM vs. CB; BM vs. PBSC) | 4.39; 6.29 | 0.7–26.3; 1.14–34.57 | 0.1; 0.03 |
| Pre-transplant chemotherapy (No therapy vs. therapy) | 2.43 | 0.49–12.13 | 0.28 |
| Monosomy 7 (No vs. Yes) | 0.46 | 0.12–1.85 | 0.27 |
| Complex karyotype (<3 abnormalities vs. ≥3) | 0.51 | 0.06–4.07 | 0.52 |
| Blast percent at transplant (<5% vs. ≥5%) | 4.62 | 1.14–18.7 | 0.03 |
| Time from dx to transplant (<120 days vs. ≥120 days) | 0.47 | 0.12–1.77 | 0.26 |
| Conditioning intensity (MAC vs. RIC) | 1 | 0.2–4.87 | 0.99 |
| Year of transplant (2000–2008 vs. 2009–2019) | 0.14 | 0.02–1.18 | 0.07 |
| Relapse | |||
| Age (≤12 years vs. >12 years) | 2.03 | 0.45–9.16 | 0.36 |
| Donor source (MSD vs. MUD/MMUD/CBT) | 0.79 | 0.08–7.22 | 0.83 |
| Stem cell source (BM vs. CB; BM vs. PBSC) | 0.71; 2.2 | 0.07–6.38; 0.4–12.19 | 0.76; 0.36 |
| Pre-transplant chemotherapy (No therapy vs. therapy) | 6.72 | 1.47–30.7 | 0.014 |
| Monosomy 7 (No vs. Yes) | 0.64 | 0.14–2.88 | 0.56 |
| Complex karyotype (<3 abnormalities vs. ≥3) | 1.7 | 0.38–8.84 | 0.53 |
| Blast percent at transplant (<5% vs. ≥5%) | 7.23 | 1.39–37.46 | 0.02 |
| Time from dx to transplant (<120 days vs. ≥120 days) | 0.46 | 0.1–2.08 | 0.32 |
| Conditioning intensity (MAC vs. RTC) | 1.24 | 0.24–6.42 | 0.8 |
| Year of transplant (2000–2008 vs. 2009–2019) | 0.75 | 0.17–3.35 | 0.7 |
| Non-Relapse Mortality | |||
| Age (≤12 years vs. >12 years) | 0.94 | 0.15–5.69 | 0.94 |
| Donor source (MSD vs. MUD/MMUD/CBT) | 1.03 | 0–∞ | 1 |
| Stem cell source (BM vs. CB; BM vs. PBSC) | Too few events to calculate | ||
| Pre-transplant chemotherapy (No therapy vs. therapy) | 2.48 | 0.25–24.2 | 0.43 |
| Monosomy 7 (No vs. Yes) | 0.21 | 0.02–1.87 | 0.16 |
| Complex karyotype (<3 abnormalities vs. ≥3) | Too few events to calculate | ||
| Blast percent at transplant (<5% vs. ≥5%) | 1.62 | 0.26–9.98 | 0.6 |
| Time from dx to transplant (<120 days vs. ≥120 days) | 0.81 | 0.13–4.96 | 0.82 |
| Conditioning intensity (MAC vs. RIC) | 0.92 | 0.1–8.35 | 0.94 |
| Year of transplant (2000–2008 vs. 2009–2019) | 1 | 1 | 0.99 |
| Relapse-Free Survival | |||
| Age (<12 vs. ≥12) | 1.47 | 0.47–4.61 | 0.5 |
| Donor source (MSD vs. MUD/MMUD/CBT) | 0.78 | 0.09–7.1 | 0.82 |
| Stem cell source (BM vs. CB; BM vs. PBSC) | 2.13; 4.89 | 0.48–9.55; 1.3–18.37 | 0.32; 0.02 |
| Pre-transplant chemotherapy (No therapy vs. therapy) | 4.77 | 1.38–16.5 | 0.013 |
| Monosomy 7 (No vs. Yes) | 0.42 | 0.13–1.42 | 0.16 |
| Complex karyotype (<3 abnormalities vs. ≥3) | 0.9 | 0.19–4.12 | 0.89 |
| Blast percent at transplant (<5% vs. ≥5%) | 3.8 | 1.19–12.09 | 0.02 |
| Time from dx to transplant (<120 days vs. ≥120 days) | 0.58 | 0.19–1.82 | 0.35 |
| Conditioning intensity (MAC vs. RIC) | 1.11 | 0.3–4.12 | 0.88 |
| Year of transplant (2000–2008 vs. 2009–2019) | 0.37 | 0.1–1.4 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahlberg, A.; Stevenson, P.; Bhatt, N.S.; Burroughs, L.; Carpenter, P.A.; Summers, C.; Tarlock, K.; Thakar, M.S.; Milano, F.; Deeg, H.J.; et al. Disease Burden at the Time of Transplantation Is a Primary Predictor of Outcomes in Pediatric MDS: A Single-Center Experience. Cancers 2025, 17, 1645. https://doi.org/10.3390/cancers17101645
Dahlberg A, Stevenson P, Bhatt NS, Burroughs L, Carpenter PA, Summers C, Tarlock K, Thakar MS, Milano F, Deeg HJ, et al. Disease Burden at the Time of Transplantation Is a Primary Predictor of Outcomes in Pediatric MDS: A Single-Center Experience. Cancers. 2025; 17(10):1645. https://doi.org/10.3390/cancers17101645
Chicago/Turabian StyleDahlberg, Ann, Phil Stevenson, Neel S. Bhatt, Lauri Burroughs, Paul A. Carpenter, Corinne Summers, Katherine Tarlock, Monica S. Thakar, Filippo Milano, H. Joachim Deeg, and et al. 2025. "Disease Burden at the Time of Transplantation Is a Primary Predictor of Outcomes in Pediatric MDS: A Single-Center Experience" Cancers 17, no. 10: 1645. https://doi.org/10.3390/cancers17101645
APA StyleDahlberg, A., Stevenson, P., Bhatt, N. S., Burroughs, L., Carpenter, P. A., Summers, C., Tarlock, K., Thakar, M. S., Milano, F., Deeg, H. J., & Bleakley, M. (2025). Disease Burden at the Time of Transplantation Is a Primary Predictor of Outcomes in Pediatric MDS: A Single-Center Experience. Cancers, 17(10), 1645. https://doi.org/10.3390/cancers17101645






