Simple Summary
Breast cancer is a leading cause of death among women worldwide, but survival rates vary significantly depending on race and socioeconomic status. Women from lower-income backgrounds and minority populations often face barriers to early diagnosis and timely treatment, leading to worse clinical outcomes. This study examines racial and socioeconomic disparities in breast cancer mortality and survival, aiming to quantify the impact of these factors through a systematic review and meta-analysis. By analyzing data from multiple studies, we assess how access to healthcare, screening programs, and treatment availability influence survival rates. Our findings highlight the urgent need for policies that improve access to early detection and equitable treatment, particularly for underserved communities. Understanding these disparities can help guide healthcare interventions and research efforts to reduce inequalities in breast cancer outcomes and improve survival rates for vulnerable populations.
Abstract
Background/Objectives: Breast cancer is one of the leading causes of female mortality worldwide, but significant racial and socioeconomic disparities persist in disease outcomes. This review aimed to analyze racial and socioeconomic inequalities in mortality and survival from breast cancer, identifying the impact of social risk factors on access to diagnosis and treatment. Methods: A systematic literature review and meta-analysis was performed following PRISMA guidelines. Eighteen studies published between 2014 and 2024 were included, with 11 contributing to the meta-analysis. Random-effect models were used to assess correlations between socioeconomic status, race, and clinical outcomes, including heterogeneity and publication bias analyses. Results: The strongest associations were observed between income, race, and breast cancer survival, with survival significantly worse among Black women and low-income populations (p < 0.001). Income also showed a strong positive correlation with clinical outcomes. In contrast, the overall effect on mortality was not statistically significant (p = 0.290), likely due to high heterogeneity across studies (I2 = 100%). These findings suggest that structural disparities in access to healthcare and early detection substantially affect survival rates. Conclusions: Racial and socioeconomic disparities in breast cancer outcomes remain critical public health challenges. Targeted policies to expand early diagnosis and ensure equitable access to effective treatment are essential to reduce these disparities and improve survival in underserved populations.
1. Introduction
With over 2.3 million new cases registered in 2022 [1] breast cancer is the most diagnosed cancer worldwide, 99% of which occur in women [2,3]. The World Health Organization (WHO) stated that breast cancer caused 670,000 deaths globally in 2022 [4]. Over 3 million cases and 1 million deaths from breast cancer are projected to occur yearly by 2040, which represents a global public health concern [2].
Several biological, environmental and social variables contribute to the development and progression of breast cancer [5,6]. Genetic factors (specifically family history), unhealthy lifestyle choices (e.g., diet, physical inactivity, smoking, drinking), environmental hazards (e.g., ionizing radiation), and social and psychological issues contribute to its prevalence [7,8]. Research has shown that hereditary factors and mutations account for 5–10% of breast cancer cases, while modifiable risk factors account for 20–30% [9].
Breast cancer mortality is intrinsically linked to racial and social inequities. Unfortunately, there is a significant disparity in mortality rates between different ethnic and socioeconomic groups, which reflects a clear inequality in access to health care and treatment outcomes. These racial inequities play a crucial role in breast cancer mortality. Studies have shown that women belonging to minority ethnic groups, such as Afro-descendants, Hispanics, and indigenous women have higher mortality rates compared to white women [10,11]. For example, in the United States, the five-year survival rate for non-Hispanic white women is approximately 91%, compared to 82% for Black women and 83% for Native American women [12]. Furthermore, Black women are 40% more likely to die from breast cancer than white women, despite having lower incidence rates [13]. These disparities reflect long-standing structural barriers in access to screening and timely treatment.
These inequalities can be attributed to a combination of factors, including differences in access to health services, lower quality of care, lower adherence to treatment, and diagnosis at more advanced stages of the disease. Women from low socioeconomic backgrounds, with lower educational levels and incomes, are more likely to face challenges in accessing health services and receive late diagnosis. These inequalities can be exacerbated by the lack of adequate health insurance, lack of transportation, language barriers, and lower awareness of the importance of early detection and regular follow-up [14].
Reducing disparities in breast cancer outcomes, early detection, and timely treatment depends on making relevant services available to everyone. The programs for reaching the underserved, however, must eliminate existing structural barriers, including but not limited to distance, costs, and poor health information. Available data support that structural factors, i.e., access to education, employment, and the health system, are not equal and have a significant effect on breast cancer outcomes, particularly in low- and middle-income countries [15,16]. Results that have been contrasted in studies across regions depict the ability of women in high-income countries to benefit from better coverage of screenings and access to treatment on time, women in resource-constrained settings often present with the disease at a later stage and few treatment options [16,17].
For instance, the WHO suggests that women in well-resourced areas who are 50 to 69 years old and at average risk for breast cancer undergo organized, population-based mammography screening every two years [18]. When resources are scarce—as they often are in low-resource areas where mammography screening is impractical or too expensive –, attention should instead be directed towards early detection by ensuring that women experiencing symptoms can get a proper diagnosis and treatment for their breast cancer as soon as possible [2].
Researchers have shown the need for breast cancer studies that address variables such as environmental and lifestyle factors; barriers preventing women from being diagnosed and treated, such as cultural taboos, geographic location, socioeconomic issues, and more effective methods of cancer prevention at the populational level, evaluating screening methods and techniques that meet women’s needs. In a study carried out by Yedjou et al. (2019), the authors report that racial and economic disparities persist, and one can identify and reduce these disparities. Although several other studies have shown the importance of knowing and verifying the impact of disparities on breast cancer mortality, to our knowledge, no studies have systematically reviewed the scientific literature to identify how these disparities affect the morbidity and mortality of women with breast cancer and, therefore, how to develop interventions to improve quality of life [19].
A solid grasp of the global patterns and variability in disease burden is essential for the success of these endeavors. Yedjou et al. (2019) highlighted that new strategies and approaches are needed to promote prevention, improve survival rates, reduce breast cancer mortality, and improve health outcomes. Understanding whether there is a relationship between breast cancer mortality and socioeconomic and geographical location variables is important to help build preventive strategies to reduce breast cancer mortality rates. Therefore, the primary aims of this systematic literature review and meta-analysis were to (a) summarize the findings of studies examining racial and socioeconomic inequalities in mortality and survival from breast cancer, and (b) determine the magnitude of the overall associations between breast cancer mortality and social risk factors on access to diagnosis and treatment through a meta-analysis. This information may offer direction to women aiming to mitigate their heightened risk of breast cancer.
2. Materials and Methods
2.1. Protocol and Registration
This study is a systematic review conducted according to the methodology of the Joanna Briggs Institute (JBI) [20], recognized for its rigorous and practical approach to reviews in the health area. The review was also conducted using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and PRISMA-NMA guidelines [21]. This review is registered in PROSPERO (International Prospective Register of Systematic Reviews) under number CRD42024599149.
2.2. PICO(S) Criteria
The research design was structured based on the PICO(S) model, as follows: Population (P)—Women with breast cancer; Exposure/Indicator (I)—Analysis of mortality associated with breast cancer; Comparison (C)—Racial and social disparities in mortality; Outcome (O)—Identification of risk factors and impacts of screening strategies; and Type of Study (S)—Cross-sectional and longitudinal observational studies [22].
2.3. Search Strategy
In June 2024, a systematic literature search was conducted with the assistance of a research librarian. The search was conducted in the scientific databases PubMed, Embase, Scopus, Web of Science, Science Direct, SciELO, Biblioteca Virtual em Saúde (BVS), Cochrane Library and LILACS. Grey literature was also explored to broaden the scope of the results. The Medical Subject Heading (MeSH) terms “breast cancer”, “mortality”, “social health disparity” and “screening” were combined with Boolean operators and the search strategy was adapted to the specificities of each database. The search was conducted independently by two researchers and validated by the main author (Table 1).

Table 1.
Search Strategies Conducted in the Databases.
2.4. Eligibility Criteria
The target was cross-sectional and longitudinal population-based observational studies with random sampling, published between 2014 to 2024. The year 2014 marks the publication of the World Health Organization’s position paper on mammography screening [23], which provided global guidance for breast cancer early detection strategies. Limiting the inclusion of studies published after this milestone ensures that the findings reflect contemporary practices, technologies, and health policy environments. Only studies with adult participants (≥18 years) and published in English, Portuguese or Spanish were considered. We included cross-sectional and longitudinal population-based studies because these observational designs are the most appropriate for investigating associations between social determinants and cancer outcomes at a population level. Randomized trials on racial and socioeconomic disparities are extremely limited, and other study designs (e.g., qualitative or case studies) do not allow for generalizable, quantitative comparisons of mortality or survival. Cross-sectional studies offered insight into prevalence and disparities, while longitudinal studies allowed evaluation of trends over time and survival estimates.
Studies conducted in specific populations, such as pregnant women and indigenous people, as well as systematic reviews, meta-analyses, dissertations, theses, technical reports, editorials and qualitative studies, were excluded. This decision was made because Indigenous populations often present unique sociocultural, geographic, and health system-related characteristics that distinguish them significantly from the general population. Including such studies in this meta-analysis could introduce substantial heterogeneity, as these groups face specific barriers to healthcare access and have different health outcomes that require a tailored analytical framework. Therefore, we opted to exclude them in order to preserve methodological consistency and comparability across studies.
2.5. Study Selection
The study selection occurred in three stages. Initially, six researchers (Group 1) screened the articles based on title and abstract. In case of disagreements, there was a discussion among the members or a final decision was made by the project coordinator. Subsequently, the selected articles were randomly distributed among the researchers for full reading and data extraction. The final stage involved an independent review by three experts (Group 2), who certified the final selection of studies.
2.6. Data Extraction and Analysis
The search results from each database were imported into Rayyan® software (https://www.rayyan.ai, accessed on 14 August 2024) [24], a web-based tool designed to assist in the systematic review process, to streamline the review process. The extracted data included title, first author, year of publication, language, study objective, methodological design, collection site, sample size, mean age, socioeconomic variables and frequency of mammographic screening by age group and race/color.
The meta-analysis was conducted using a random-effects model, weighting the effects of each study by the inverse of its variance [25]. Heterogeneity was assessed using the I2 index and Cochran’s Q test, with values greater than 50% being considered indicative of moderate to high heterogeneity. Sensitivity analyses were performed using the robust Huber-White estimator and robust weighted variance meta-analysis. Statistical analyses were conducted using RStudio software, version 4.3.0.
Although the included studies differed in design (cross-sectional vs. longitudinal), all provided quantitative estimates of the association between sociodemographic variables and breast cancer mortality or survival. Given the small number of eligible studies and the use of a random-effects model, we chose to analyze the data jointly. Heterogeneity and publication bias were thoroughly assessed to ensure the robustness of the findings.
2.7. Assessment of Methodological Quality and Risk of Bias
Two researchers using the JBI checklist for prevalence studies and Crombie’s criteria for cross-sectional studies independently assessed the methodological quality of the studies. Nine items were considered, including sample adequacy, a detailed description of the setting and methodology, and measurement reliability. The risk of bias was analyzed using a funnel plot and Egger’s test. The assessment table is provided as Supplementary Materials (Table S1).
3. Results
3.1. General Presentation
The initial search identified 239 articles, of which 54 were duplicates. After screening titles and abstracts, 38 articles were considered relevant for a full-text review. Of these, 16 articles were excluded because they did not meet the inclusion criteria. Therefore, 18 articles were included in the final sample and 11 participated in the meta-analysis (Figure 1).
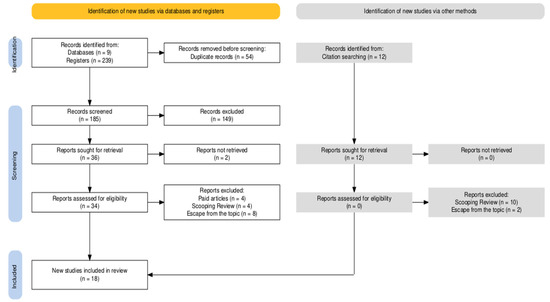
Figure 1.
Flow diagram of the systematic review steps.
After analyzing the 18 studies selected for this systematic review, the data were extracted and synthesized according to the research objectives. Regarding the geographical distribution of the studies, the majority (16) were carried out in the United States of America (USA), using large national databases such as SEER and the National Cancer Database [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. Two studies were conducted in Brazil, investigating regional and socioeconomic inequalities in breast cancer outcomes [42,43]. No studies conducted exclusively in Europe or Asia were identified, although some multicenter articles included diverse populations [39,40].
Data for the studies were collected from the early 1990s to the late 2020s. Most reported annual data, while others reported aggregate trends over a decade [29,35]. The studies were cross-sectional analyses for retrospective cohorts and population-based registries based on national health systems [37,38]. One study assessed racial disparities exclusively, and the others assessed an interaction of differences in socioeconomic status, health insurance coverage, and regional disparities in breast cancer outcomes [31].
Methodological approaches varied, with eight studies using large national databases and others employing smaller-scale data from academic hospitals and regional health systems. The studies uniformly observed large heterogeneity in breast cancer mortality rates, diagnostic predictions, and treatment outcomes, highlighting the very complex interplay of biological, socioeconomic, and systemic factors that drive disparities.
Overall, the results of these studies emphasized that there is an urgent need for targeted public health and policy interventions to address the identified inequalities, especially among racial minorities and low-income populations. Table 2 demonstrates the individual characteristics of the studies included in the review.

Table 2.
General characteristics of the articles included in the review.
3.2. Meta-Analysis
The data presented in Table 3 and Figure 2 indicate that mortality did not exhibit a significant combined effect (p = 0.290, coefficient = −0.192), despite high heterogeneity (I2 = 100%, p < 0.001), suggesting substantial variations across studies. These differences may be influenced by factors such as regional disparities, healthcare policies, and population characteristics. Additionally, Egger’s test (p = 0.003) suggests potential publication bias, supported by the asymmetry in the funnel plot (Figure 2B).

Table 3.
Meta-Analysis Results for Mortality, Survival, and Income.
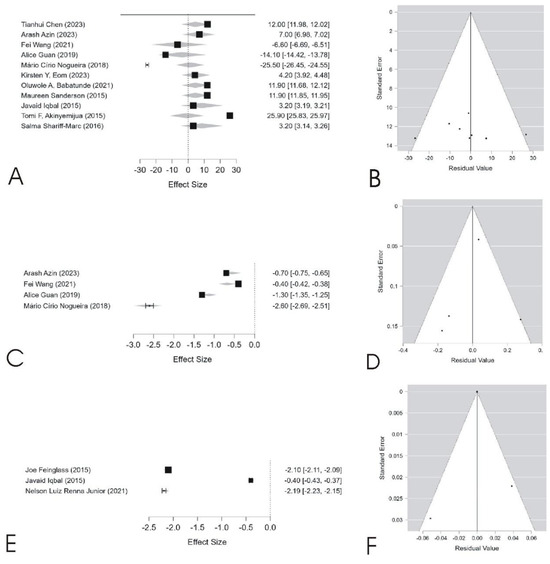
Figure 2.
The figure presents the meta-analysis results for mortality (A,B), survival (C,D), and income (E,F). Forest plots (A,C,E) display the effect sizes and confidence intervals for each study, illustrating variability in estimates across different populations. Funnel plots (B,D,F) assess publication bias, where asymmetry suggests potential bias in mortality (B) and income (F), as confirmed by Egger’s test. High heterogeneity is observed in all outcomes, indicating significant variations among studies, which may be influenced by regional, socioeconomic, and methodological differences. Original figure created by the authors based on data included in the present study.
In contrast, the survival analysis revealed a significant positive effect (p < 0.001, coefficient = 5.596, 95% CI: 4.377 to 6.815), with high heterogeneity (I2 = 98.6%, p < 0.001). Although the corresponding funnel plot (Figure 2D) appears relatively symmetrical, Egger’s test (p = 0.006) indicates mild publication bias. Similarly, income showed a significant association with outcomes (p < 0.001, coefficient = 5.010, 95% CI: 4.757 to 5.263), with notable heterogeneity (I2 = 84.7%). The funnel plot (Figure 2F) suggests substantial asymmetry, confirmed by Egger’s test (p < 0.001). These findings highlight the influence of socioeconomic and demographic factors on the analyzed outcomes and reinforce the need for careful interpretation, particularly in the presence of high heterogeneity and potential bias.
The high heterogeneity observed in the meta-analysis (I2 = 100% for mortality, I2 = 98.6% for survival) suggests that racial and socioeconomic disparities in breast cancer outcomes vary across different contexts. These variations may be attributed to differences in healthcare systems, screening policies, and population characteristics. Although some studies report survival improvements among Black women, heterogeneity remains high, indicating persistent structural challenges that hinder full equity.
While subgroup analyses could help identify sources of heterogeneity, the limited number of eligible studies (n = 11) reduced the feasibility of stratified meta-analyses. We acknowledge this as a limitation and suggest that future reviews explore larger datasets or focus on specific study designs to enable meta-regression or subgroup comparisons.
In addition to the aggregated effects presented above, Table 4 provides a study-by-study summary of the 11 articles included in the meta-analysis, specifying the outcome assessed, the main sociodemographic variables evaluated, and whether the associations were statistically significant.

Table 4.
Summary of key data extracted from the 11 studies included in the Meta-Analysis.
For each study included in the meta-analysis, key information was systematically extracted, including sample size, outcomes assessed, sociodemographic variables analyzed, effect size (ES), standard error (SE), and statistical significance. Effect sizes and corresponding standard errors were directly collected from the results reported by the original authors, considering adjusted estimates (e.g., Odds Ratio, Hazard Ratio, or regression coefficients) whenever available. When the standard error was not explicitly provided, it was calculated based on the confidence intervals reported, following standard statistical procedures. Statistical significance was recorded according to the criteria adopted by each study, with p-values < 0.05 considered significant.
3.3. Methodological Quality Assessment
The studies included in this review were classified according to their methodological design, using the Joanna Briggs Institute’s (JBI) criteria applied to prevalence studies and Crombie’s criteria used for cross-sectional studies. The methodological assessment indicated that, in most prevalence studies, quality criteria were adequately met, particularly regarding sample representativeness, appropriate recruitment, and the use of validated measures. However, some limitations were identified, especially concerning the detailed description of the study setting and methodology, as well as the lack of control for confounding factors, which may compromise the reliability of the findings.
Overall, the methodological quality of the included studies was classified as moderate to high, with some specific limitations. Nevertheless, the presence of studies with low sample representativeness and insufficient control of confounding variables should be considered when interpreting the findings of this systematic review
3.4. Bias Risk Assessment
To assess the risk of bias, an inverted funnel plot and Egger’s test were used [44,45]. The funnel plot (Figure 3) visually suggests a relatively balanced distribution of studies, indicating no strong publication bias. Egger’s test (p = 0.832) further confirmed the absence of publication bias (p > 0.05), suggesting that the findings of this review are robust and reliable.
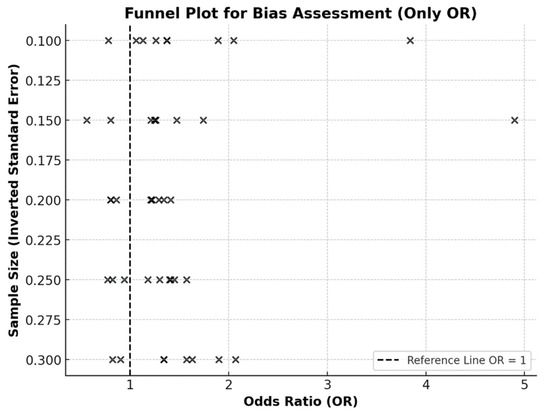
Figure 3.
Funnel plot (inverted, or “Christmas tree” shape) assessing potential bias in studies reporting odds ratios (OR). Each “×” denotes a single study. The dashed vertical line represents the null effect (OR = 1).
3.5. Summarized Results
Differences in access to healthcare services and mammographic screening significantly impact clinical outcomes among socioeconomic groups. Patients with private health insurance are more likely to undergo regular mammograms and receive an early diagnosis compared to those with public insurance [39]. However, paradoxically, privately insured patients also exhibit a higher risk of diagnostic delays compared to those in the public healthcare system [35], potentially due to differences in referral protocols, waiting times, and treatment coverage.
Regular mammographic screening is associated with a significant reduction in breast cancer mortality, with studies indicating up to a 45% mortality reduction [40]. Moreover, shorter screening intervals have been linked to reduced overall mortality (adjusted OR = 0.57; 95% CI: 0.36–0.89; p = 0.013) [27]. However, low-income women continue to have reduced access to regular screenings, contributing to delayed diagnoses and increased mortality rates.
Socioeconomic factors such as income, education, and housing conditions strongly influence breast cancer outcomes [43]. Women with lower educational attainment exhibit higher mortality rates even after adjusting for clinical variables (HR = 1.27; 95% CI: 1.24–1.31) [42]. Patients in socioeconomically disadvantaged communities also face barriers such as limited access to advanced medical technologies and reduced social support, directly impacting survival [41]. These findings highlight the need for targeted public policies to ensure equitable access to screening and treatment, minimizing socioeconomic disparities.
Finally, the methodological quality assessment revealed that while most studies employed appropriate statistical methods, approximately 40% did not adequately justify sample size, and 35% had methodological deficiencies that may have influenced results. However, the absence of publication bias, as confirmed by Egger’s test (p = 0.832), suggests that the findings of this review are reliable and robust.
4. Discussion
Although breast cancer mortality has been extensively studied worldwide, gaps remain regarding racial and socioeconomic disparities associated with the disease. Our meta-analysis reinforces that socioeconomic and racial factors remain decisive in determining breast cancer outcomes, particularly in relation to survival rates. The high degree of heterogeneity found among the studies analyzed indicates significant variations in demographic characteristics, methodological differences, and access to healthcare systems.
According to Ahmed [46], barriers to accessing mammography screening and early treatment are closely linked to socioeconomic and racial factors. Similarly, a meta-analysis by Silva et al. [15] found that women in low- and middle-income countries experience higher mortality rates due to late diagnosis and a lack of screening infrastructure. These findings suggest that access to healthcare services significantly influences disparities in breast cancer prognosis.
The discrepancies in survival rates among women with breast cancer reflect not only biological tumor behavior but also the impact of social determinants such as access to healthcare services and socioeconomic status. Newman et al. [13] emphasize the importance of public policies aimed at eliminating barriers faced by low-income African-American and Hispanic women, ensuring early screening, appropriate treatments, and strategies tailored to vulnerable populations. Additionally, educational programs, free screenings funded by non-commercial sources, and initiatives promoting equitable distribution of modern evidence-based treatments have been proposed to reduce these disparities.
Targeted intervention programs have demonstrated positive effects on survival among vulnerable groups. According to Grant et al. [47], while such programs improve outcomes, uneven coverage of screening services sustains gaps in late-stage diagnoses. Yedjou et al. [19] further suggest that awareness-raising and social support programs can help reduce inequalities in disadvantaged populations, highlighting the need for more comprehensive interventions.
There is a strong correlation between low income and more advanced clinical stages of breast cancer. Women with lower socioeconomic status have limited access to preventive screenings, early diagnosis, and guideline-recommended drug treatments, which contributes to disease progression and increased mortality. Our meta-analysis confirms these associations, adding pooled statistical support to the literature. Opia [48] observed that economic interventions aimed at expanding access to screening and treatment are associated with reduced inequality. This is supported by Pearson [49], who emphasizes that financial and geographic barriers continue to limit timely access to screening, particularly for women relying on underfunded public health systems.
Study Limitations
The included studies were assessed using different methodological criteria: the JBI Checklist for prevalence studies and Crombie’s Criteria for cross-sectional studies. While this approach ensured a systematic and structured analysis, differences in methodological designs may have influenced the findings. Notably, variations in sample size justification and inadequate control of confounding factors in some studies contributed to the observed heterogeneity. One limitation is the combination of different study designs (cross-sectional and longitudinal), which may introduce heterogeneity. However, this was mitigated by the use of a random-effects model and sensitivity analyses. Additionally, the lack of standardization in analyzed variables impacted the consolidated interpretation of findings. Differences in statistical methods, inclusion and exclusion criteria, and outcome measurement approaches pose challenges for generalizing conclusions. Approximately 45% of the studies did not provide clear information on response rates or sample attrition management, potentially introducing bias.
Despite these limitations, the rigorous selection and methodological assessment process—conducted independently and repeatedly—enhances the reliability of this meta-analysis. This comprehensive and methodologically robust approach provides valuable insights into the impact of socioeconomic and racial disparities on breast cancer mortality.
Future research should address these limitations through methodological standardization, more detailed sample size justification, stricter control of confounding factors, and inclusion of grey literature. These efforts will ensure more precise and comparable analyses, further elucidating the impact of inequalities on breast cancer outcomes. Further studies should also consider using mixed-methods approaches, including qualitative analyses, to explore how social, cultural, and psychological dimensions shape access to cancer care and influence outcomes. Such approaches can offer a deeper understanding of the lived experiences behind the statistical disparities. Additionally, there is a need to include evidence from underrepresented regions, particularly in Asia and Africa, where socioeconomic and racial dynamics may differ substantially. Expanding geographic representation will enhance the generalizability of findings and support the development of more globally inclusive health policies.
5. Conclusions
This systematic review and meta-analysis highlighted that racial and socioeconomic disparities continue to play a major role in breast cancer outcomes, especially for African American, Hispanic populations, and those belonging to low-income groups. While such inequalities have been reported in previous studies, few have synthesised comparable quantitative data on death and survival, which indicate high heterogeneity at the population and regional levels. The findings bring out the need for public policies to ensure access and equal rights, to have screening done as early as possible, and to diagnose and treat breast cancer. Actions should include having government-funded programs for screening on a population basis, educating vulnerable communities, and ensuring that there is a fair allocation of evidence-based interventions. On top of this, the current disparities in cancer care show the need for an international collaborative network to cut gaps in equitable access and early detection. Health policies must reinforce health systems with financial support for cancer control programs and ensure that every patient has access to high-quality cancer services. Only through shared world plans can we truly achieve fair results in breast cancer prevention and care.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17101641/s1, Table S1: Methodological Quality of Prevalence Studies (JBI Checklist).
Author Contributions
Conceptualization, H.F.R. and S.M.P.; methodology, R.B.P., C.P. and H.F.R.; software, C.P.; validation, H.F.R., B.S.d.F. and C.W.C.; formal analysis, R.B.P., C.P. and H.F.R.; investigation, H.F.R., B.S.d.F. and C.W.C.; resources, H.F.R.; data curation, H.F.R., B.S.d.F. and C.W.C.; writing—original draft preparation, H.F.R., F.C.P., B.S.d.F., C.W.C., M.D.d.B.C., V.D.M., M.R.B., K.P.S., P.B.B., D.H.P.B., P.A.E., A.C.J.A., R.K.N.C., I.M.T.H., M.E.L.C., C.P., C.L., R.B.P. and S.M.P.; writing—review and editing, H.F.R., R.B.P., C.L. and S.M.P.; visualization, H.F.R., R.B.P., S.M.P. and C.L.; supervision, S.M.P.; project administration, H.F.R. and S.M.P.; funding acquisition, H.F.R. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Coordination for the Improvement of Higher Education Personnel (CAPES) (financial code 001). This work is also supported by national funds through FCT—Fundação para a Ciência e a Tecnologia, I.P. (UIDB/05704/2020 and UIDP/05704/2020) and under the Scientific Employment Stimulus-Institutional Call (https://doi.org/10.54499/CEECINST/00051/2018/CP1566/CT0012, accessed on 19 April 2025).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This study is based on a systematic review and meta-analysis of previously published research. All data used were extracted from peer-reviewed articles indexed in databases such as PubMed, Scopus, and Web of Science. The list of included studies is provided in Table 2 and in the References section.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- WHO. Breast Cancer Cases and Deaths Are Projected to Rise Globally. 2025. Available online: https://www.iarc.who.int/wp-content/uploads/2025/02/pr361_E.pdf (accessed on 25 February 2025).
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- WHO. Breast Cancer. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 20 February 2025).
- Aizaz, M.; Khan, M.; Khan, F.I.; Ahmad, S.; Obeagu, E.I. Burden of breast cancer: Developing countries perspective. Int. J. Innov. Appl. Res. 2023, 11, 31–37. [Google Scholar]
- Obeagu, E.I.; Babar, Q.; Vincent, C.C.; Udenze, C.L.; Eze, R.; Okafor, C.J.; Ifionu, B.I.; Amaeze, A.A.; Amaeze, F.N. Therapeutic targets in breast cancer signaling: A review. J. Pharm. Res. Int. 2021, 33, 82–99. [Google Scholar] [CrossRef]
- Løyland, B.; Sandbekken, I.H.; Grov, E.K.; Utne, I. Causes and Risk Factors of Breast Cancer, What Do We Know for Sure? An Evidence Synthesis of Systematic Reviews and Meta-Analyses. Cancers 2024, 16, 1583. [Google Scholar] [CrossRef]
- Cohen, S.Y.; Stoll, C.R.; Anandarajah, A.; Doering, M.; Colditz, G.A. Modifiable risk factors in women at high risk of breast cancer: A systematic review. Breast Cancer Res. 2023, 25, 45. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk factors and preventions of breast cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef]
- Hines, R.B.; Johnson, A.M.; Lee, E.; Erickson, S.; Rahman, S.M.M. Trends in breast cancer survival by race-ethnicity in Florida, 1990–2015. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1408–1415. [Google Scholar] [CrossRef]
- Miller, B.C.; Bowers, J.M.; Payne, J.B.; Moyer, A. Barriers to mammography screening among racial and ethnic minority women. Soc. Sci. Med. 2019, 239, 112494. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Sauer, A.G.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef]
- Newman, L.A. Disparities in breast cancer and African ancestry: A global perspective. Breast J. 2015, 21, 133–139. [Google Scholar] [CrossRef]
- Ribeiro, H.F.; Carvalho, M.D.d.B.; Pelloso, F.C.; Santos, L.d.; Silva, M.d.A.P.; Stevanato, K.P.; Borghesan, D.H.P.; Romani, I.; Marques, V.D.; de Freitas, K.M.S.; et al. Maternal Risk Factors Associated with Negative COVID-19 Outcomes and Their Relation to Socioeconomic Indicators in Brazil. Healthcare 2023, 11, 2072. [Google Scholar] [CrossRef]
- E Silva, J.D.D.; Pedroso, R.B.; Pelloso, F.C.; Carvalho, M.D.B.; Santos, T.D.S.; Dutra, A.C.; Stevanato, K.P.; Marques, V.D.; De Andrade, L.; Araújo, D.C.M.; et al. Mortality of Young Women due to Breast Cancer in Low, Middle and High-Income Countries: Systematic Literature Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2024, 25, 2219–2227. [Google Scholar] [CrossRef]
- Anampa-Guzmán, A.; Acevedo, F.; Partridge, A.H.; Alfano, C.M.; Nekhlyudov, L. Cancer Survivorship in Latin America: Current Status and Opportunities. JCO Glob. Oncol. 2021, 7, 1472–1479. [Google Scholar] [CrossRef]
- Unger-Saldaña, K. Challenges to the early diagnosis and treatment of breast cancer in developing countries. World J. Clin. Oncol. 2014, 5, 465–477. [Google Scholar] [CrossRef]
- WHO. Position Paper on Mammography Screening. 2014. Available online: https://www.who.int/publications/i/item/9789241507936 (accessed on 25 February 2025).
- Yedjou, C.G.; Sims, J.N.; Miele, L.; Abusamaan, M.S.; Payton, M.; Tchounwou, P.B. Health and racial disparity in breast cancer. Adv. Exp. Med. Biol. 2019, 1152, 31–49. [Google Scholar] [CrossRef]
- Joanna Briggs Institute. Annual Report 2018; Joanna Briggs Institute: Adelaide, Australia, 2018; Available online: https://jbi.global/sites/default/files/2019-10/31a_Annual_Report_2018.pdf (accessed on 5 March 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- World Health Organization. WHO Position Paper on Mammography Screening; World Health Organization: Geneva, Switzerland, 2014; Available online: https://iris.who.int/handle/10665/137339 (accessed on 17 April 2025).
- Hammady, M.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; Wiley: Chichester, UK, 2009; Available online: https://www.agropustaka.id/wp-content/uploads/2020/04/agropustaka.id_buku_Introduction-to-Meta-Analysis.pdf (accessed on 17 April 2025).
- Anderson, T.; Herrera, D.; Mireku, F.; Barner, K.; Kokkinakis, A.; Dao, H.; Webber, A.; Merida, A.D.; Gallo, T.; Pierobon, M. Geographical variation in social determinants of female breast cancer mortality across US counties. JAMA Netw. Open 2023, 6, e2333618. [Google Scholar] [CrossRef]
- Akinyemiju, T.; Sakhuja, S.; Vin Raviv, N. Racial and socio-economic disparities in breast cancer hospitalization outcomes by insurance status. Ethn. Health 2018, 23, 499–511. [Google Scholar] [CrossRef]
- Feinglass, J.; Rydzewski, N.; Yang, A. The socioeconomic gradient in all-cause mortality for women with breast cancer: Findings from the 1998 to 2006 National Cancer Data Base with follow-up through 2011. Ann. Epidemiol. 2015, 25, 574–579. [Google Scholar] [CrossRef]
- John, E.M.; McGuire, V.; Kurian, A.W.; Koo, J.; Shariff-Marco, S.; Gomez, S.L.; Cheng, I.; Keegan, T.H.M.; Kwan, M.L.; Bernstein, L.; et al. Racial/ethnic disparities in survival after breast cancer diagnosis by estrogen and progesterone receptor status: A pooled analysis. Cancer Epidemiol. Biomark. Prev. 2021, 30, 531–540. [Google Scholar] [CrossRef]
- Boyko, A.; Qureshi, M.M.; Fishman, M.D.C.; Slanetz, P.J. Predictors of breast cancer outcome in a cohort of women seeking care at a safety net hospital. Acad. Radiol. 2024, 31, 663–670. [Google Scholar] [CrossRef]
- Guan, A.; Lichtensztajn, D.; Oh, D.; Jain, J.; Tao, L.; Hiatt, R.A.; Gomez, S.L.; Fejerman, L. Breast cancer in San Francisco: Disentangling disparities at the neighborhood level. Cancer Epidemiol. Biomark. Prev. 2020, 29, 56–65. [Google Scholar] [CrossRef]
- Azin, A.; Tahmasebi, H.; Brar, A.; Azin, S.; Ko, G.; Covelli, A.; Cil, T. Racial, ethnic and socioeconomic disparities in diagnosis, treatment, and survival of patients with breast cancer. Am. J. Surg. 2023, 225, 123–130. [Google Scholar] [CrossRef]
- Tao, L.; Gomez, S.L.; Keegan, T.H.M.; Kurian, A.W.; Clarke, C.A. Breast cancer mortality in African-American and non-Hispanic white women by molecular subtype and stage at diagnosis: A population-based study. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1039–1045. [Google Scholar] [CrossRef]
- Shariff-Marco, S.; Yang, J.; John, E.M.; Kurian, A.W.; Cheng, I.; Leung, R.; Koo, J.; Monroe, K.R.; Henderson, B.E.; Bernstein, L.; et al. Intersection of race/ethnicity and socioeconomic status in mortality after breast cancer. J. Community Health 2015, 40, 1287–1299. [Google Scholar] [CrossRef]
- Babatunde, O.A.; Eberth, J.M.; Felder, T.; Moran, R.; Truman, S.; Hebert, J.R.; Zhang, J.; Adams, S.A. Social determinants of racial disparities in breast cancer mortality among Black and White women. J. Racial Ethn. Health Disparities 2021, 8, 147–156. [Google Scholar] [CrossRef]
- Wang, F.; Zheng, W.; Bailey, C.E.; Mayer, I.A.; Pietenpol, J.A.; Shu, X.O. Racial/ethnic disparities in all-cause mortality among patients diagnosed with triple-negative breast cancer. Cancer Res. 2021, 81, 1163–1170. [Google Scholar] [CrossRef]
- Iqbal, J.; Ginsburg, O.; Rochon, P.A.; Sun, P.; Narod, S.A. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 2015, 313, 165–173. [Google Scholar] [CrossRef]
- Sanderson, M.; Levine, R.S.; Fadden, M.K.; Kilbourne, B.; Pisu, M.; Cain, V.; Husaini, B.A.; Langston, M.; Gittner, L.; Zoorob, R.; et al. Mammography screening among the elderly: A research challenge. Am. J. Med. 2015, 128, 1362.e7–1362.e14. [Google Scholar] [CrossRef]
- Chen, T.; Kharazmi, E.; Fallah, M. Race and ethnicity-adjusted age recommendation for initiating breast cancer screening. JAMA Netw. Open 2023, 6, e238893. [Google Scholar] [CrossRef]
- Eom, K.Y.; Berg, K.A.; Joseph, N.E.; Runner, K.; Tarabichi, Y.; Khiyami, A.; Perzynski, A.T.; Sossey-Alaoui, K. Neighborhood and racial influences on triple negative breast cancer: Evidence from Northeast Ohio. Breast Cancer Res. Treat. 2023, 198, 369–381. [Google Scholar] [CrossRef]
- Akinyemiju, T.F.; Vin-Raviv, N.; Chavez-Yenter, D.; Zhao, X.; Budhwani, H. Race/ethnicity and socio-economic differences in breast cancer surgery outcomes. Cancer Epidemiol. 2015, 39, 745–751. [Google Scholar] [CrossRef]
- Nogueira, M.C.; Guerra, M.R.; Cintra, J.R.D.; Corrêa, C.S.L.; Fayer, V.A.; Bustamante-Teixeira, M.T. Disparidade racial na sobrevida de 10 anos no câncer de mama: Uma análise de mediação usando abordagem de respostas potenciais. Cad. Saúde Pública. 2018, 34, e00211717. [Google Scholar] [CrossRef]
- Junior, N.L.R.; Lima, C.A.; Laporte, C.A.; Coleman, M.P.; Silva, G.A.E. Ethnic, racial and socioeconomic disparities in breast cancer survival in two Brazilian capitals between 1996 and 2012. Cancer Epidemiol. 2021, 75, 102048. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, A.; Hattori, S. A likelihood-based sensitivity analysis for publication bias on the summary receiver operating characteristic in meta-analysis of diagnostic test accuracy. Stat. Med. 2023, 42, 781–798. [Google Scholar] [CrossRef]
- Ahmed, A.T.; Welch, B.T.; Brinjikji, W.; Farah, W.H.; Henrichsen, T.L.; Murad, M.H.; Knudsen, J.M. Racial disparities in screening mammography in the United States: A systematic review and meta-analysis. J. Am. Coll. Radiol. 2017, 14, 157–165.e9. [Google Scholar] [CrossRef]
- Grant, S.J.; Yanguela, J.; Odebunmi, O.; Grimshaw, A.A.; Giri, S.; Wheeler, S.B. Systematic review of interventions addressing racial and ethnic disparities in cancer care and health outcomes. J. Clin. Oncol. 2024, 42, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Opia, F.N.; Matthew, K.A. Socioeconomic disparities in breast cancer care: Addressing global challenges in oncology outcomes. Int. J. Comput. Appl. Technol. Res. 2025, 14, 1003. [Google Scholar] [CrossRef]
- Pearson, S.A.; Taylor, S.; Marsden, A.; Zdenkowski, N.; Bates, N.; McNeil, C.; Nair, B.; Wyld, D.; Stockler, M.R.; Kiely, B.E.; et al. Geographic and sociodemographic access to systemic anticancer therapies for secondary breast cancer: A systematic review. Syst. Rev. 2024, 13, 35. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).