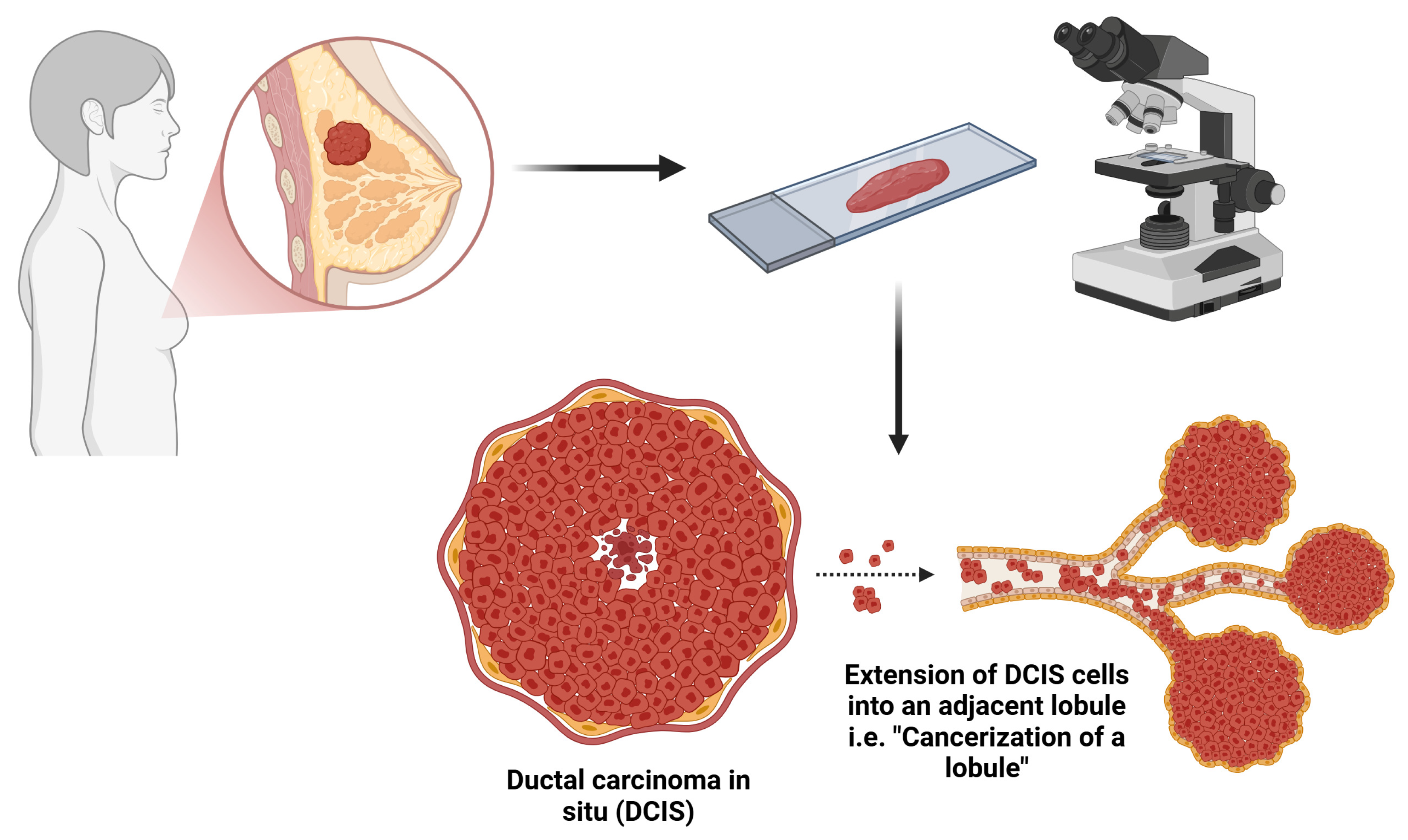
Influence of Cancerization of Lobules in Ductal Carcinoma In Situ of the Breast on the Pathological Outcomes in Mastectomy Specimens
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Ethical Considerations
2.3. Patients’ Selection
2.4. Clinicopathological Variables of Patients
2.5. Histopathologic and Immunohistochemical Staining and Evaluation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Druesne-Pecollo, N.; Touvier, M.; Barrandon, E.; Chan, D.S.; Norat, T.; Zelek, L.; Hercberg, S.; Latino-Martel, P. Excess body weight and second primary cancer risk after breast cancer: A systematic review and meta-analysis of prospective studies. Breast Cancer Res. Treat. 2012, 135, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef]
- Pfeiffer, R.M.; Webb-Vargas, Y.; Wheeler, W.; Gail, M.H. Proportion of U.S. Trends in Breast Cancer Incidence Attributable to Long-term Changes in Risk Factor Distributions. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Classification of Tumours: Breast Tumours, 5th ed. Available online: https://tumourclassification.iarc.who.int/welcome/ (accessed on 12 December 2024).
- Burstein, H.J.; Monica, M. Malignant Tumors of the Breast. In Cancer Principles & Practice of Oncology; Devita, V.T., Lawrence, T.S., Rosenberg, S.A., Eds.; Williams and Wilkins, Wolters Kluwer Lippincott: Philadelphia, PA, USA, 2008; Volume 2, pp. 1606–1654. [Google Scholar]
- Ellis, I.O.; Galea, M.; Broughton, N.; Locker, A.; Blamey, R.W.; Elston, C.W. Pathological prognostic factors in breast cancer. II. Histological type. Relationship with survival in a large study with long-term follow-up. Histopathology 1992, 20, 479–489. [Google Scholar] [CrossRef]
- Li, C.I.; Anderson, B.O.; Daling, J.R.; Moe, R.E. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA 2003, 289, 1421–1424. [Google Scholar] [CrossRef]
- Li, C.I.; Uribe, D.J.; Daling, J.R. Clinical characteristics of different histologic types of breast cancer. Br. J. Cancer 2005, 93, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Sastre-Garau, X.; Jouve, M.; Asselain, B.; Vincent-Salomon, A.; Beuzeboc, P.; Dorval, T.; Durand, J.C.; Fourquet, A.; Pouillart, P. Infiltrating lobular carcinoma of the breast. Clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer 1996, 77, 113–120. [Google Scholar] [CrossRef]
- Baxter, N.N.; Virnig, B.A.; Durham, S.B.; Tuttle, T.M. Trends in the treatment of ductal carcinoma in situ of the breast. J. Natl. Cancer Inst. 2004, 96, 443–448. [Google Scholar] [CrossRef]
- Kerner, H.; Lichtig, C. Lobular cancerization: Incidence and differential diagnosis with lobular carcinoma in situ of breast. Histopathology 1986, 10, 621–629. [Google Scholar] [CrossRef]
- Fechner, R.E. Ductal carcinoma involving the lobule of the breast. A source of confusion with lobular carcinoma in situ. Cancer 1971, 28, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Azzopardi, J.G.; Ahmed, A.; Millis, R.R. Problems in breast pathology. Major Probl. Pathol. 1979, 11, 1–466. [Google Scholar]
- Fechner, R.E. Epithelial alterations in the extralobular ducts of breasts with lobular carcinoma. Arch. Pathol. 1972, 93, 164–171. [Google Scholar]
- Foote, F.W., Jr.; Stewart, F.W. A histologic classification of carcinoma of the breast. Surgery 1946, 19, 74–99. [Google Scholar] [PubMed]
- Beguinot, M.; Dauplat, M.M.; Kwiatkowski, F.; Lebouedec, G.; Tixier, L.; Pomel, C.; Penault-Llorca, F.; Radosevic-Robin, N. Analysis of tumour-infiltrating lymphocytes reveals two new biologically different subgroups of breast ductal carcinoma in situ. BMC Cancer 2018, 18, 129. [Google Scholar] [CrossRef]
- Huo, L.; Sneige, N.; Hunt, K.K.; Albarracin, C.T.; Lopez, A.; Resetkova, E. Predictors of invasion in patients with core-needle biopsy-diagnosed ductal carcinoma in situ and recommendations for a selective approach to sentinel lymph node biopsy in ductal carcinoma in situ. Cancer 2006, 107, 1760–1768. [Google Scholar] [CrossRef]
- Renshaw, A.A. Predicting invasion in the excision specimen from breast core needle biopsy specimens with only ductal carcinoma in situ. Arch. Pathol. Lab. Med. 2002, 126, 39–41. [Google Scholar] [CrossRef]
- Go, E.M.L.; Chan, S.-K.; Vong, J.S.L.; Lui, P.C.W.; Chan, A.W.H.; Ma, T.K.F.; Ang, M.A.; Law, B.K.B.; Tan, P.-H.; Tse, G.M. Predictors of invasion in needle core biopsies of the breast with ductal carcinoma in situ. Mod. Pathol. 2010, 23, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Schuyler, P.A.; Dupont, W.D.; Page, D.L. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer 2005, 103, 2481–2484. [Google Scholar] [CrossRef]
- Collins, L.C.; Tamimi, R.M.; Baer, H.J.; Connolly, J.L.; Colditz, G.A.; Schnitt, S.J. Outcome of patients with ductal carcinoma in situ untreated after diagnostic biopsy: Results from the Nurses’ Health Study. Cancer 2005, 103, 1778–1784. [Google Scholar] [CrossRef]
- Page, D.L.; Dupont, W.D.; Rogers, L.W.; Landenberger, M. Intraductal carcinoma of the breast: Follow-up after biopsy only. Cancer 1982, 49, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Betsill, W.L.; Rosen, P.P.; Lieberman, P.H.; Robbins, G.F. Intraductal carcinoma: Long-term follow-up after treatment by biopsy alone. JAMA 1978, 239, 1863–1867. [Google Scholar] [CrossRef]
- Hernandez, L.; Wilkerson, P.M.; Lambros, M.B.; Campion-Flora, A.; Rodrigues, D.N.; Gauthier, A.; Cabral, C.; Pawar, V.; Mackay, A.; A’Hern, R. Genomic and mutational profiling of ductal carcinomas in situ and matched adjacent invasive breast cancers reveals intra-tumour genetic heterogeneity and clonal selection. J. Pathol. 2012, 227, 42–52. [Google Scholar] [CrossRef]
- Iakovlev, V.V.; Arneson, N.C.; Wong, V.; Wang, C.; Leung, S.; Iakovleva, G.; Warren, K.; Pintilie, M.; Done, S.J. Genomic differences between pure ductal carcinoma in situ of the breast and that associated with invasive disease: A calibrated aCGH study. Clin. Cancer Res. 2008, 14, 4446–4454. [Google Scholar] [CrossRef] [PubMed]
- Pareja, F.; Brown, D.N.; Lee, J.Y.; Da Cruz Paula, A.; Selenica, P.; Bi, R.; Geyer, F.C.; Gazzo, A.; da Silva, E.M.; Vahdatinia, M.; et al. Whole-Exome Sequencing Analysis of the Progression from Non-Low-Grade Ductal Carcinoma In Situ to Invasive Ductal Carcinoma. Clin. Cancer Res. 2020, 26, 3682–3693. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, A.; Mallo, D.; Rupp, S.M.; King, L.M.; Hardman, T.; Lo, J.Y.; Hall, A.; Marks, J.R.; Hwang, E.S.; Maley, C.C. A new method to accurately identify single nucleotide variants using small FFPE breast samples. Brief. Bioinform. 2021, 22, bbab221. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Luo, M.; Huang, J.; Zhang, K.; Zheng, S.; Zhang, S.; Zhou, J. Progression from ductal carcinoma in situ to invasive breast cancer: Molecular features and clinical significance. Signal Transduct. Target. Ther. 2024, 9, 83. [Google Scholar] [CrossRef]
- Dano, H.; Altinay, S.; Arnould, L.; Bletard, N.; Colpaert, C.; Dedeurwaerdere, F.; Dessauvagie, B.; Duwel, V.; Floris, G.; Fox, S.; et al. Interobserver variability in upfront dichotomous histopathological assessment of ductal carcinoma in situ of the breast: The DCISion study. Mod. Pathol. 2020, 33, 354–366. [Google Scholar] [CrossRef]
- Takada, K.; Kashiwagi, S.; Asano, Y.; Goto, W.; Morisaki, T.; Takahashi, K.; Fujita, H.; Takashima, T.; Tomita, S.; Hirakawa, K.; et al. Factors predictive of invasive ductal carcinoma in cases preoperatively diagnosed as ductal carcinoma in situ. BMC Cancer 2020, 20, 513. [Google Scholar] [CrossRef]
- Bagnall, M.J.; Evans, A.J.; Wilson, A.R.M.; Pinder, S.E.; Denley, H.; Geraghty, J.G.; Ellis, I.O. Predicting invasion in mammographically detected microcalcification. Clin. Radiol. 2001, 56, 828–832. [Google Scholar] [CrossRef]
- Hoorntje, L.E.; Schipper, M.E.; Peeters, P.H.; Bellot, F.; Storm, R.K.; Borel Rinkes, I.H. The finding of invasive cancer after a preoperative diagnosis of ductal carcinoma-in-situ: Causes of ductal carcinoma-in-situ underestimates with stereotactic 14-gauge needle biopsy. Ann. Surg. Oncol. 2003, 10, 748–753. [Google Scholar] [CrossRef] [PubMed]
- King, T.A.; Farr, G.H., Jr.; Cederbom, G.J.; Smetherman, D.H.; Bolton, J.S.; Stolier, A.J.; Fuhrman, G.M. A mass on breast imaging predicts coexisting invasive carcinoma in patients with a core biopsy diagnosis of ductal carcinoma in situ. Am. Surg. 2001, 67, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.E.; Nguyen, K.; Gray, R.J.; Salud, C.; Ku, N.; Dupont, E.; Hutson, L.; Peltz, E.; Whitehead, G.; Reintgen, D. Importance of lymphatic mapping in ductal carcinoma in situ (DCIS): Why map DCIS? Am. Surg. 2001, 67, 513–521. [Google Scholar] [CrossRef]
- Rohan, T.E.; Zhang, C.; Wang, Y.; Couch, F.J.; Greenlee, R.T.; Honda, S.; Stark, A.; White, L.L.; Chitale, D.A.; Xue, X.; et al. p16, COX-2, and Ki67 protein expression in DCIS and risk of ipsilateral invasive breast cancer. Cancer Epidemiol. Biomark. Prev. 2025, OF1–OF4. [Google Scholar] [CrossRef]
- Shan, M.; Zhang, X.; Liu, X.; Qin, Y.; Liu, T.; Liu, Y.; Wang, J.; Zhong, Z.; Zhang, Y.; Geng, J.; et al. P16 and p53 play distinct roles in different subtypes of breast cancer. PLoS ONE 2013, 8, e76408. [Google Scholar] [CrossRef]
- Polchai, N.; Thongvitokomarn, S. Extensive intraductal component as a factor determining local recurrence of breast cancer: A systematic review and meta-analysis. Gland. Surg. 2023, 12, 1336–1347. [Google Scholar] [CrossRef]
- Holland, R.; Connolly, J.L.; Gelman, R.; Mravunac, M.; Hendriks, J.; Verbeek, A.; Schnitt, S.J.; Silver, B.; Boyages, J.; Harris, J.R. The presence of an extensive intraductal component following a limited excision correlates with prominent residual disease in the remainder of the breast. J. Clin. Oncol. 1990, 8, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Van Goethem, M.; Schelfout, K.; Kersschot, E.; Colpaert, C.; Verslegers, I.; Biltjes, I.; Tjalma, W.; De Schepper, A.; Weyler, J.; Parizel, P. MR mammography is useful in the preoperative locoregional staging of breast carcinomas with extensive intraductal component. Eur. J. Radiol. 2007, 62, 273–282. [Google Scholar] [CrossRef]
- Stomper, P.; Connolly, J. Mammographic features predicting an extensive intraductal component in early-stage infiltrating ductal carcinoma. AJR. Am. J. Roentgenol. 1992, 158, 269–272. [Google Scholar] [CrossRef]
- Goldstein, N.S.; Lacerna, M.; Vicini, F. Cancerization of lobules and atypical ductal hyperplasia adjacent to ductal carcinoma in situ of the breast. Am. J. Clin. Pathol. 1998, 110, 357–367. [Google Scholar] [CrossRef][Green Version]
- Marinovich, M.L.; Azizi, L.; Macaskill, P.; Irwig, L.; Morrow, M.; Solin, L.J.; Houssami, N. The Association of Surgical Margins and Local Recurrence in Women with Ductal Carcinoma In Situ Treated with Breast-Conserving Therapy: A Meta-Analysis. Ann. Surg. Oncol. 2016, 23, 3811–3821. [Google Scholar] [CrossRef] [PubMed]
- Wärnberg, F.; Garmo, H.; Emdin, S.; Hedberg, V.; Adwall, L.; Sandelin, K.; Ringberg, A.; Karlsson, P.; Arnesson, L.-G.; Anderson, H. Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized SweDCIS Trial. J. Clin. Oncol. 2014, 32, 3613–3618. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Sestak, I.; Pinder, S.E.; Ellis, I.O.; Forsyth, S.; Bundred, N.J.; Forbes, J.F.; Bishop, H.; Fentiman, I.S.; George, W.D. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: Long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011, 12, 21–29. [Google Scholar] [CrossRef]
- Donker, M.; Litiere, S.; Werutsky, G.; Julien, J.-P.; Fentiman, I.S.; Agresti, R.; Rouanet, P.; de Lara, C.T.; Bartelink, H.; Duez, N. Breast-conserving treatment with or without radiotherapy in ductal carcinoma in situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J. Clin. Oncol. 2013, 31, 4054–4059. [Google Scholar] [CrossRef]
- Wapnir, I.L.; Dignam, J.J.; Fisher, B.; Mamounas, E.P.; Anderson, S.J.; Julian, T.B.; Land, S.R.; Margolese, R.G.; Swain, S.M.; Costantino, J.P. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J. Natl. Cancer Inst. 2011, 103, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative, G.; Correa, C.; McGale, P.; Taylor, C.; Wang, Y.; Clarke, M.; Davies, C.; Peto, R.; Bijker, N.; Solin, L.; et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J. Natl. Cancer Inst. Monogr. 2010, 2010, 162–177. [Google Scholar] [CrossRef]
- Mamtani, A.; Van Zee, K.J. Treatment of ductal carcinoma in situ: Considerations for tailoring therapy in the contemporary era. Curr. Breast Cancer Rep. 2020, 12, 98–106. [Google Scholar] [CrossRef]


| Clinicopathological Features | Total Patients | COL-NO | COL-YES | p-Value |
|---|---|---|---|---|
| EIC (n = 106) | ||||
| No | 69 | 54 (85.7%) | 15 (34.9%) | <0.001 * |
| Yes | 37 | 9(14.3%) | 28 (65.1%) | |
| % of blocks/slides with DCIS (n = 171) | ||||
| ≤30% | 111 | 83 (84.7%) | 28 (38.4%) | <0.001 * |
| >30% | 60 | 15 (15.3%) | 45 (61.6%) | |
| Necrosis (n = 171) | ||||
| Absent | 61 | 43 (43.9%) | 18 (24.6%) | 0.008 * |
| Present/Focal | 49 | 29 (29.6%) | 20 (27.4%) | |
| Present/Comedo | 61 | 26 (26.5%) | 35 (48.0%) | |
| Margin status for DCIS (n = 171) | ||||
| Present with 2 mm | 38 | 14 (14.3%) | 24 (32.9%) | 0.004 * |
| More than 2 mm away | 133 | 84 (85.7%) | 49 (67.1%) | |
| DCIS grade (n = 166) | ||||
| 1 | 22 | 18 (19.3%) | 4 (5.5%) | 0.006 * |
| 2 | 102 | 58 (62.4%) | 44 (60.3%) | |
| 3 | 42 | 17 (18.3%) | 25 (34.2%) | |
| Invasion (n = 171) | ||||
| Absent | 65 | 35 (35.7%) | 30 (41.1%) | 0.566 |
| Present | 106 | 63 (64.3%) | 43 (58.9%) | |
| Invasive component type (n = 106) | ||||
| IC, NST | 98 | 61 (96.8%) | 37 (86%) | 0.150 |
| ILC | 1 | 1 (1.6%) | 0 (0%) | |
| TC | 3 | 0 (0%) | 3 (6.8%) | |
| IMC | 3 | 1 (1.6%) | 2 (4.6%) | |
| ILC AND TC | 1 | 0 (0%) | 1 (2.3%) | |
| Invasive component grade (n = 103) | ||||
| 1 | 26 | 16 (26.2%) | 10 (23.8%) | 0.615 |
| 2 | 66 | 40 (64.6%) | 26 (61.9%) | |
| 3 | 11 | 5 (8.2%) | 6 (14.3%) | |
| Margin status for invasive component (n = 106) | ||||
| Negative | 103 | 63 (100%) | 40 (93.0%) | 0.083 |
| Positive | 3 | 0 (0.0%) | 3 (7.0%) | |
| pT (n = 171) | ||||
| pTis | 65 | 35 (35.7%) | 30 (41.1%) | 0.522 |
| pT1mi | 4 | 2 (2.0%) | 2 (2.7%) | |
| pT1a | 12 | 6 (6.1%) | 6 (8.2%) | |
| pT1b | 35 | 21 (21.4%) | 14 (19.2%) | |
| pT1c | 33 | 22 (22.4%) | 11 (15.1%) | |
| pT2 | 18 | 10 (10.2%) | 8 (10.9%) | |
| pT3 | 2 | 0 (0.0%) | 2 (2.7%) | |
| pT4a | 0 | 0 (0.0%) | 0 (0.0%) | |
| pT4b | 2 | 2 (2.0%) | 0 (0.0%) | |
| pN (n = 171) | ||||
| pNx | 55 | 34 (34.7%) | 21 (28.7%) | 0.801 |
| pN0 | 88 | 49 (50.0%) | 39 (53.4%) | |
| pN0 (i+) | 1 | 1 (1.0%) | 0 (0.0%) | |
| pN1a | 14 | 7 (7.1%) | 7 (9.6%) | |
| pN1mi | 9 | 4 (4.0%) | 5 (6.8%) | |
| pN2a | 3 | 2 (2.0%) | 1 (1.4%) | |
| pN3a | 1 | 1 (1.0%) | 0 (0.0%) |
| Clinicopathological Features | p-Value | OR |
|---|---|---|
| EIC | <0.001 * | 11.20 |
| % of blocks/slides with DCIS | <0.001 * | 8.89 |
| Necrosis, focal | 0.22 | 1.65 |
| Necrosis, comedo | 0.002 * | 3.26 |
| Margin status for DCIS | 0.006 * | 2.94 |
| DCIS grade | 0.002 * | N/A |
| Invasion | 0.47 | 0.80 |
| Tumor stage | 0.57 | N/A |
| Lymph node status | 0.63 | 1.26 |
| Clinicopathological Features | EIC (p-Value, OR *) | Margin Status for DCIS (p-Value, OR *) | % of Blocks/Sides with DCIS (p-Value) | Necrosis, Comedo (p-Value, OR *) | DCIS Grade (p-Value) |
|---|---|---|---|---|---|
| COL | 0.002, 9.28 | 0.54 | <0.001 | 0.17 | 0.01 |
| DCIS Morphology | |||||
| Cribriform | 0.64 | 0.34 | 0.07 | 0.008, 6.96 | 0.004 |
| Solid | 0.07 | 0.26 | 0.03 | 0.019, 5.54 | 0.59 |
| Comedo | 0.31 | 0.23 | 0.01 | <0.001, 18.44 | 0.001 |
| Papillary | 0.84 | 0.23 | 0.06 | 0.15 | 0.28 |
| Micropapillary | 0.33 | 0.96 | 0.004 | 0.22 | 0.46 |
| Necrosis | 0.03, 4.73 | 0.11 | 0.34 | N/A | <0.001 |
| % Slides with DCIS | 0.06 | 0.08 | N/A | 0.95 | 0.57 |
| Patient Age | 0.39 | 0.94 | 0.96 | 0.30 | 0.31 |
| Invasion | N/A | 0.25 | 0.04 | 0.50 | 0.64 |
| Tumor Stage | 0.23 | 0.75 | 0.96 | 0.49 | 0.61 |
| Lymph Nodes Status | 0.014, 6.06 | 0.08 | 0.52 | 0.14 | 0.53 |
| Margin Status for DCIS | 0.042 | N/A | 0.09 | 0.65 | 0.58 |
| EIC | N/A | 0.02, 5.38 | 0.05 | 0.09 | 0.46 |
| DCIS Grade | 0.79 | 0.55 | 0.58 | 0.13 | N/A |
| Clinicopathological Features | p-Value | OR |
|---|---|---|
| DCIS Morphology | ||
| Cribriform | 0.87 | 0.93 |
| Solid | 0.38 | 0.65 |
| Comedo | 0.007 * | 0.15 |
| Papillary | 0.12 | 0.34 |
| Micropapillary | 0.21 | 0.46 |
| Necrosis | 0.83 | 1.07 |
| % Slides with DCIS | <0.001 * | >99 |
| Patient Age | 0.07 | 0.97 |
| Invasion | 0.63 | 0.65 |
| Tumor Stage | 0.45 | 0.84 |
| Lymph Nodes Status | 0.73 | 1.28 |
| Margin Status for DCIS | 0.35 | 1.64 |
| EIC | 0.001 * | 9.69 |
| DCIS Grade | 0.02 * | 2.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alloush, F.; Bahmad, H.F.; Deb, A.; Ocejo, S.; Valencia, A.-K.; Abulaban, A.; Krishnamurthy, K.; Alghamdi, S.; Poppiti, R. Influence of Cancerization of Lobules in Ductal Carcinoma In Situ of the Breast on the Pathological Outcomes in Mastectomy Specimens. Cancers 2025, 17, 1634. https://doi.org/10.3390/cancers17101634
Alloush F, Bahmad HF, Deb A, Ocejo S, Valencia A-K, Abulaban A, Krishnamurthy K, Alghamdi S, Poppiti R. Influence of Cancerization of Lobules in Ductal Carcinoma In Situ of the Breast on the Pathological Outcomes in Mastectomy Specimens. Cancers. 2025; 17(10):1634. https://doi.org/10.3390/cancers17101634
Chicago/Turabian StyleAlloush, Ferial, Hisham F. Bahmad, Arunima Deb, Stephanie Ocejo, Ann-Katrin Valencia, Amr Abulaban, Kritika Krishnamurthy, Sarah Alghamdi, and Robert Poppiti. 2025. "Influence of Cancerization of Lobules in Ductal Carcinoma In Situ of the Breast on the Pathological Outcomes in Mastectomy Specimens" Cancers 17, no. 10: 1634. https://doi.org/10.3390/cancers17101634
APA StyleAlloush, F., Bahmad, H. F., Deb, A., Ocejo, S., Valencia, A.-K., Abulaban, A., Krishnamurthy, K., Alghamdi, S., & Poppiti, R. (2025). Influence of Cancerization of Lobules in Ductal Carcinoma In Situ of the Breast on the Pathological Outcomes in Mastectomy Specimens. Cancers, 17(10), 1634. https://doi.org/10.3390/cancers17101634