1. Introduction
Squamous cell carcinoma (SCC) is a common form of epithelial cancer that shows features of squamous differentiation. It can arise in multiple locations throughout the body, including the skin, mucosal surfaces of the head and neck, esophagus, and cervix, reflecting its broad anatomical distribution. The clinical presentation of SCC varies significantly, with some lesions remaining localized and indolent, particularly on the skin, while others, especially in mucosal sites, exhibit aggressive behavior and a higher propensity for metastasis [
1,
2,
3,
4,
5,
6,
7,
8].
Historically, SCC diagnosis has been based on histopathological examination using hematoxylin and eosin (H&E) staining. However, this method alone often proves insufficient in specific clinical contexts, such as small biopsies, poorly differentiated tumors, or uncommon histologic variants like basaloid, sarcomatoid, or clear cell SCC. Moreover, conventional morphology lacks the molecular resolution required for accurate tumor stratification or treatment prediction [
5,
8,
9,
10,
11].
In this context, immunohistochemistry (IHC) has emerged as a key ancillary technique, enabling the identification of lineage-specific proteins, proliferation markers, and stemness indicators in formalin-fixed, paraffin-embedded tissue specimens. Markers such as p40, p63, and CK5/6 have become indispensable in confirming squamous differentiation. Others, including Ki-67, CD44, and p16, provide valuable insights for prognostic stratification and tumor staging. At the same time, exclusion markers like Ber-EP4, CK7, TTF-1, and S100 play an essential role in the differential diagnosis of SCC against entities such as adenocarcinomas, basal cell carcinomas, and melanomas [
7,
12,
13,
14,
15,
16,
17,
18,
19,
20].
Despite the widespread use of IHC in SCC diagnostics, there is a lack of structured syntheses that summarize marker performance and specificity across different SCC variants and anatomical contexts. Most publications to date have focused on isolated markers or site-specific subtypes, without offering an integrative diagnostic and prognostic framework.
The aim of this review is to synthesize and systematically organize available evidence regarding the most relevant immunohistochemical markers used in squamous cell carcinoma. We focus on their role in positive diagnosis, differential diagnosis, and prognostic stratification across various SCC subtypes and anatomical sites. Through qualitative synthesis of original studies and case reports, this review intends to provide a practical reference for pathologists and clinicians and to support a more standardized, evidence-based approach in SCC evaluation.
To achieve this, we conducted a structured narrative review based on predefined inclusion and exclusion criteria, focusing on original studies involving immunohistochemical markers applied in SCC diagnosis, differential diagnosis, and staging.
2. Materials and Methods
2.1. Protocol and Registration
This systematic review was conducted in accordance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The review protocol was not prospectively registered in PROSPERO or any other systematic review registry.
2.2. Eligibility Criteria
Inclusion criteria were defined prior to data collection and were as follows:
Original peer-reviewed articles, clinical case series, or case reports involving immunohistochemical (IHC) analysis in squamous cell carcinoma (SCC);
Articles published in English between January 2000 and March 2025;
Studies reporting the diagnostic, differential, or prognostic role of IHC markers in SCC;
Inclusion of conventional SCC or histologic variants such as basaloid, sarcomatoid, or clear cell subtypes;
Availability of full text and reporting of marker expression patterns, sensitivity, specificity, or clinical utility.
Exclusion criteria included the following:
Articles lacking any immunohistochemical component;
Narrative reviews, editorials, and expert opinions without original data or methodology;
Studies focused exclusively on non-squamous malignancies;
Publications not available in full text or published in languages other than English.
2.3. Information Sources and Search Strategy
A systematic literature search was performed across three major databases: PubMed, ScienceDirect, and Google Scholar. The search included articles published up to March 2025. Search terms were structured using Boolean logic and MeSH keywords related to squamous cell carcinoma and immunohistochemistry. Representative search terms included the following:
“squamous cell carcinoma AND immunohistochemistry”;
“p40 OR p63 OR CK5/6 AND SCC”;
“IHC AND squamous cell carcinoma AND prognostic markers”;
“basaloid SCC AND differential diagnosis”;
“desmoglein-3 AND SCC”.
Additional articles were retrieved through backward citation tracking of the included studies.
2.4. Study Selection
All retrieved records were imported into a shared screening database. Duplicate entries were removed manually. Two independent reviewers (V.M.V. and R.M.M.) screened titles and abstracts for relevance. Full texts of potentially eligible studies were reviewed to determine final inclusion. Disagreements were resolved through discussion with a third reviewer (F.S.).
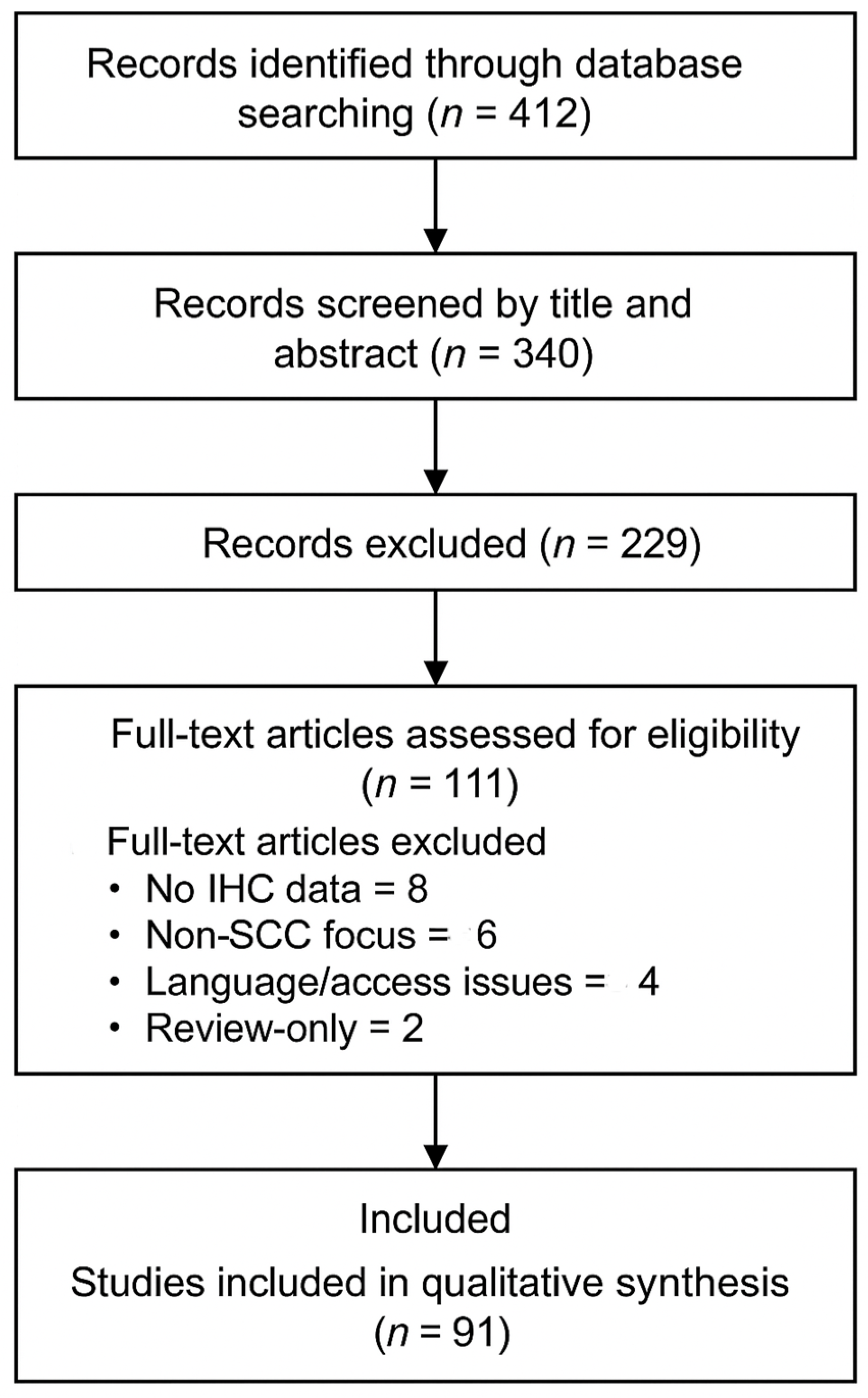
In total, 412 records were identified, of which 72 were duplicates. After title and abstract screening, 229 records were excluded. A total of 111 full-text articles were assessed for eligibility. Of these, 20 were excluded for the following reasons: absence of IHC data (n = 8), focus on non-SCC tumors (n = 6), language or access limitations (n = 4), and lack of original data (n = 2). Finally, 91 studies were included in the qualitative synthesis. The complete selection process is illustrated in the PRISMA 2020 flow diagram (
Figure 1).
2.5. Data Extraction and Management
A standardized data extraction form was used to capture relevant variables from each included article. The following data were extracted:
Anatomical site of the tumor;
SCC subtype (conventional or variant);
IHC markers assessed;
Reported sensitivity, specificity, and staining patterns;
Diagnostic, differential, or prognostic applications.
Data were extracted independently by two reviewers and compiled into comparative tables to facilitate thematic synthesis. No automation tools were used.
2.6. Risk of Bias and Quality Assessment
Given the heterogeneity of included studies in terms of design and reporting, no formal risk of bias tool (e.g., QUADAS-2) was applied. Studies were appraised qualitatively based on methodological clarity, cohort size, and completeness of IHC reporting.
2.7. Data Synthesis
A qualitative synthesis was performed. Studies were grouped thematically according to the function of the IHC markers: diagnostic confirmation, differential diagnosis from morphologic mimics, and prognostic stratification. Marker-specific sensitivity, specificity, and staining features were summarized narratively and presented in structured summary tables. No meta-analysis or pooled statistical analysis was conducted due to variability in marker targets, tissue sites, and reporting standards.
3. Results
3.1. Diagnostic Markers Supporting Squamous Differentiation
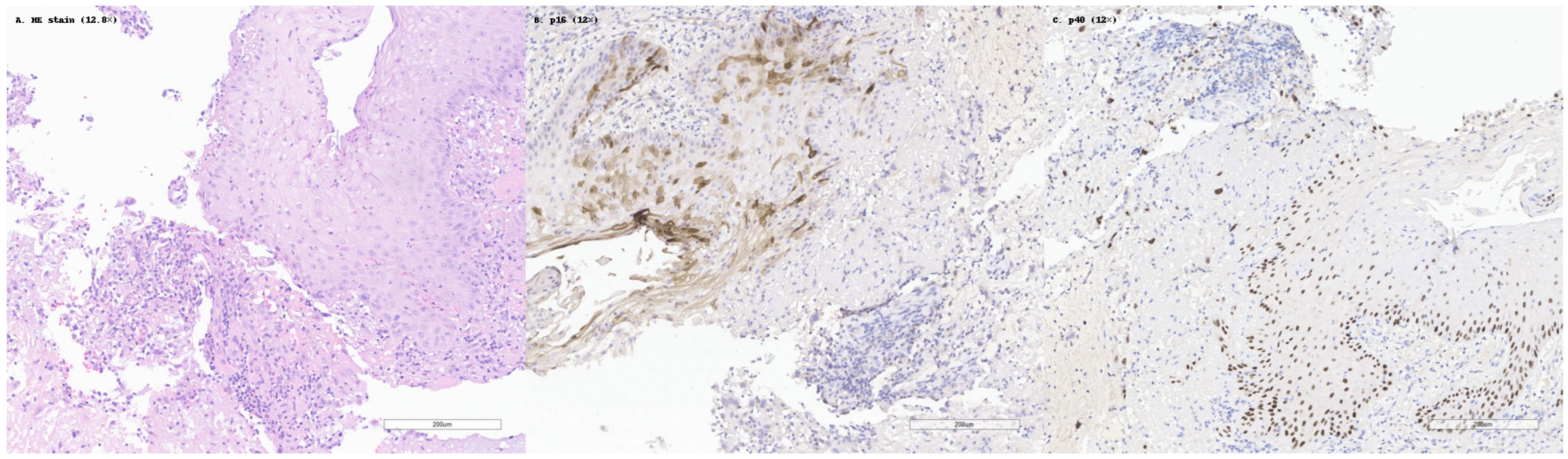
Among the studies analyzed, several immunohistochemical markers emerged as consistently useful in confirming squamous differentiation in SCC. The most frequently reported and validated markers were p40, p63, CK5/6, and desmoglein-3 (DSG3). These have become standard components in diagnostic panels, especially in cases with ambiguous morphology or limited squamous features. This is illustrated in
Figure 2, which shows a case of moderately differentiated SCC with classic histologic features, diffuse p40 nuclear staining, and negative p16 expression.
The p40 antibody, which binds specifically to the ΔNp63 isoform of the p63 protein, is considered the most specific marker for squamous differentiation. Although both p40 and p63 are highly sensitive in detecting squamous carcinomas, p40 offers superior specificity, minimizing false positives in non-squamous tumors. For example, in pulmonary SCC, p40 stained 100% of cases, without cross-reactivity, in large B-cell lymphomas and with only occasional staining in adenocarcinomas [
12]. In spindle cell SCC of the head and neck, p40 retained ~82% sensitivity in the sarcomatoid component, with patchy nuclear staining [
4,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25].
p63, while equally sensitive, displayed broader expression across both squamous and non-squamous neoplasms, including myoepithelial tumors and certain lymphomas. Thus, while not as specific, p63 remains valuable when interpreted within a panel, especially due to its diffuse and strong nuclear staining pattern in most SCC cases, including basaloid and clear cell variants [
13,
23,
25,
26,
27,
28].
Cytokeratin 5/6 (CK5/6), which identifies basal-type keratin expression, typically displays strong cytoplasmic staining in both conventional and variant forms of SCC. This marker remains detectable even in sarcomatoid and basaloid subtypes, and its expression is generally absent or limited in adenocarcinomas, enhancing its diagnostic utility [
13,
29,
30].
Desmoglein-3 (DSG3), a desmosomal adhesion protein, was noted in several studies as a sensitive and complementary marker, with membranous staining in SCC nests. In esophageal SCC, DSG3 was positive in 100% of cases and proved especially useful in well-differentiated tumors with weak or absent p40 expression [
13,
31,
32,
33,
34,
35,
36,
37].
A comparative summary of marker performance is provided below (
Table 1):
These markers retained diagnostic value across SCC variants:
Basaloid SCC showed strong p63 and CK5/6 expression, aiding distinction from adenoid cystic carcinoma [
39,
40,
41].
Sarcomatoid SCC demonstrated focal positivity for p63/p40 and AE1/AE3 in the spindle component, confirming epithelial origin [
9,
24,
25,
28].
Clear cell SCC expressed p63, p40, and CK5/6, though some studies noted decreased p40 intensity in clear areas [
13,
23,
28,
30].
In summary, a panel-based approach—typically combining p40 and CK5/6, optionally with p63 or DSG3—ensures the highest diagnostic accuracy in both conventional and variant SCC.
3.2. Markers Used in Differential Diagnosis
The immunohistochemical distinction between squamous cell carcinoma (SCC) and its histologic mimics is a critical step in diagnostic accuracy, especially in small biopsies or poorly differentiated tumors. Based on the analyzed studies, a consistent set of “exclusion markers” was employed to differentiate SCC from morphologically overlapping entities such as basal cell carcinoma (BCC), adenocarcinoma, and melanoma [
42].
One of the most reliable markers for this purpose is Ber-EP4, an epithelial cell adhesion molecule. SCC is consistently negative for Ber-EP4, whereas BCC shows strong membranous positivity, making this marker particularly valuable in the differential diagnosis of cutaneous tumors. In documented cases of collision tumors, Ber-EP4 selectively marked the BCC component, while the adjacent SCC remained negative [
14].
Epithelial membrane antigen (EMA), a general epithelial marker, is typically positive in SCC and negative in BCC, further supporting their distinction when p63 is positive in both. EMA is especially helpful in basaloid variants of SCC, where it can differentiate SCC from p63-positive myoepithelial tumors, which are usually EMA-negative [
26].
In differentiating SCC from adenocarcinoma, especially in the lung and salivary glands, CK7, TTF-1, and napsin A serve as key markers. SCC usually lacks expression of CK7, while adenocarcinomas are CK7-positive, often with co-expression of TTF-1 and napsin A. A CK7−/p40+ profile strongly supports SCC, whereas CK7+/TTF-1+ suggests glandular origin [
19,
30,
38].
TTF-1 (thyroid transcription factor-1) and napsin A are highly specific for lung adenocarcinomas and are negative in SCC. Their expression, especially when paired with CK7, is used to exclude non-squamous pulmonary neoplasms in thoracic pathology [
12,
18,
19,
20,
30].
To exclude melanoma, especially amelanotic or spindle cell variants, S100 and SOX10 are indispensable. Both are negative in SCC, including sarcomatoid variants, but strongly positive in melanocytic tumors. Their use is essential in the evaluation of undifferentiated or spindle cell tumors to avoid misclassification [
9,
15,
43,
44,
45].
A comparative table based on the reviewed literature is presented below (
Table 2):
In several reports, these markers were used effectively in challenging contexts:
In a poorly differentiated spindle cell tumor, negativity for S100 and SOX10 combined with patchy p63 and pan-cytokeratin positivity confirmed a diagnosis of sarcomatoid squamous cell carcinoma, excluding melanoma or sarcoma [
9,
15,
43,
47].
In a maxillary gingival lesion suspected of BCC, Ber-EP4 positivity defined the BCC component, while EMA positivity and Ber-EP4 negativity in adjacent nests confirmed SCC [
14,
48].
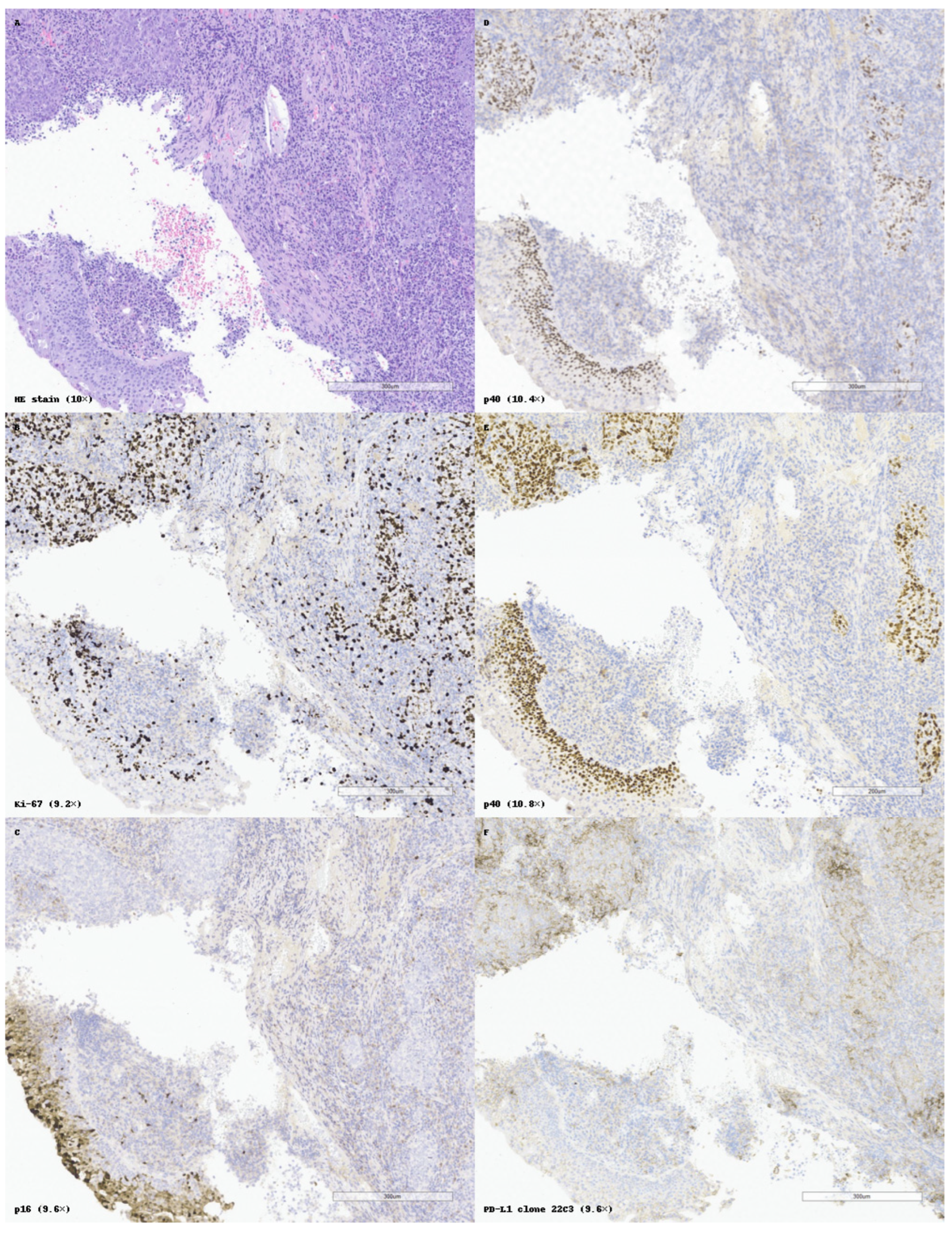
Figure 3 exemplifies a well-differentiated keratinizing SCC with classical histology, diffuse p40 positivity, p16 negativity, and low membranous PD-L1 expression, consistent with limited immunotherapeutic potential.
Taken together, this exclusion-based panel approach enhances diagnostic specificity and prevents major diagnostic pitfalls. While markers like p40 and CK5/6 confirm squamous lineage, it is the negative staining for lineage-specific mimics (melanocytic, adnexal, or glandular) that ensures diagnostic precision.
3.3. Prognostic and Staging-Related Markers
Beyond their utility in confirming squamous differentiation, several immunohistochemical (IHC) markers have demonstrated value in assessing proliferative activity, invasiveness, and tumor aggressiveness in SCC. These biomarkers complement traditional TNM staging by providing functional and molecular data, thereby supporting more nuanced patient stratification.
The most widely studied marker in this context is Ki-67, a nuclear protein expressed during active phases of the cell cycle. High Ki-67 labeling index correlates with advanced tumor stage, poor histologic differentiation, and reduced overall survival, especially in oral and cervical SCC [
1]. In dysplastic mucosa, elevated Ki-67 levels have also been associated with a greater risk of progression to invasive carcinoma, underscoring its relevance in early-stage assessment [
17,
49,
50,
51,
52,
53,
54,
55].
In mucosal SCCs, such as those in the cervix and oropharynx, p16 functions as an indirect indicator of high-risk HPV involvement. Its widespread, intense nuclear and cytoplasmic expression is often associated with more advanced disease, including regional lymph node spread and an aggressive clinical course in HPV-associated cancers. However, in non-HPV-related SCC, p16 expression is variable and less clearly prognostic [
1,
17,
50,
51,
53].
CD44, a cell-surface glycoprotein, plays a role in both cell adhesion and the epithelial–mesenchymal transition. Its expression progressively increases from normal tissue to dysplastic changes and finally to invasive SCC, where it has been linked to enhanced invasiveness, metastatic behavior, and potential resistance to treatment. Co-expression of CD44 with TGF-β has been proposed as an indicator of malignant transformation risk in oral premalignant lesions [
1,
2,
56,
57,
58].
Another prognostically relevant marker is xCT (SLC7A11), a cystine/glutamate antiporter that regulates oxidative stress responses. In oral SCC, high xCT expression—particularly when co-expressed with CD44—was associated with chemoresistance and poor clinical outcomes. This phenotype defines a subpopulation of tumor cells with enhanced survival capacity under oxidative stress [
56,
59].
Additional emerging markers include ARL4C and YAP, which have been investigated in rare histological subtypes such as clear cell SCC. Loss of expression of these regulators has been associated with dedifferentiation, more aggressive histology, and poor prognosis, although current evidence remains limited to small studies or case series [
10,
60,
61,
62].
These findings highlight the growing relevance of IHC not only in confirming diagnosis but also in informing prognosis and guiding management. Though not yet standardized across all pathology protocols, these markers show promise for integration into future clinical staging systems and risk-based treatment models.
3.4. Role of IHC in Early Detection and Lesion Stratification
Several studies have demonstrated that IHC is useful not only in diagnosing overt SCC but also in characterizing premalignant lesions and identifying those with higher malignant transformation risk. For instance, CD44 expression has been reported to increase progressively from normal epithelium to dysplasia and invasive SCC, with the most intense and diffuse staining seen in high-grade lesions [
2,
56,
57]. This supports its role as a biomarker for early carcinogenic progression, particularly in oral leukoplakia or erythroplakia.
Similarly, Ki-67 is frequently used to assess epithelial proliferation. A high Ki-67 index in dysplastic mucosa correlates with increased likelihood of progression to SCC. The combination of Ki-67 and p16 is especially informative in HPV-related cervical or oropharyngeal premalignant lesions, where strong dual positivity signals viral integration and higher-grade intraepithelial neoplasia [
1,
16,
17,
54,
63,
64].
These applications demonstrate that IHC, when applied early, can support screening, risk stratification, and biopsy triage in lesions that may not yet meet the morphological criteria for carcinoma.
4. Discussion
This review provides a comprehensive synthesis of the most frequently used immunohistochemical (IHC) markers in the diagnosis and staging of squamous cell carcinoma (SCC), offering a practical, structured overview of their diagnostic and prognostic roles. The data extracted from 91 peer-reviewed articles reveal a clear trend: IHC has become indispensable in the accurate identification and subclassification of SCC, particularly in cases with ambiguous histology or poorly differentiated features.
4.1. Diagnostic Value of IHC in SCC
The findings of this review reinforce the importance of specific immunohistochemical markers in identifying squamous cell carcinoma and its variants. p40, in particular, stands out for its exceptional nuclear specificity and sensitivity, not only in conventional SCC but also in less-differentiated forms such as sarcomatoid and basaloid subtypes. Notably, p40 maintains immunoreactivity in sarcomatoid areas where other epithelial markers may be reduced, making it a key tool for confirming epithelial lineage in tumors with spindle morphology. Although p63 remains highly sensitive, its broader expression in non-squamous neoplasms—such as myoepithelial tumors and lymphomas—limits its diagnostic specificity. This supports the current consensus favoring p40 over p63 for SCC confirmation. Nevertheless, p63 retains value within a panel due to its robust nuclear staining and widespread expression across SCC subtypes, including clear cell and basaloid variants [
4,
9,
12,
19,
22,
28,
39].
As a marker of basal keratin expression, CK5/6 typically exhibits strong cytoplasmic staining in both well-differentiated and poorly differentiated squamous tumors. Its utility is especially pronounced in cases where nuclear markers are inconsistently expressed, such as minimally keratinized tumors. Moreover, its specificity is relatively high, with limited staining observed in adenocarcinomas [
9,
13,
65,
66,
67].
DSG3 enhances the diagnostic panel by providing distinct membranous staining, particularly along cell junctions in tumor nests. It is especially valuable in well-differentiated SCCs where other markers, such as p40 or p63, may be less intensely expressed. This makes DSG3 a valuable adjunct in cases with preserved architecture but weak nuclear reactivity [
13,
31,
36,
68].
The combination of these markers in a standardized panel allows for more confident diagnostic classification, especially in small biopsies, incisional samples, or cases with overlapping morphology. Importantly, histologic variants of SCC—including sarcomatoid, basaloid, and clear cell forms—retain sufficient expression of these markers to permit accurate immunophenotypic confirmation, although intensity and distribution may vary.
4.2. IHC in Differential Diagnosis: Avoiding Pitfalls
An important aspect highlighted by this review is the utility of immunohistochemical analysis in differentiating SCC from tumors with similar histological features. This is especially crucial when the tumor morphology is unclear or the degree of differentiation is limited, making histopathological evaluation alone insufficient [
14]. This was particularly evident in reported “collision tumors”, where IHC permitted clear separation of dual tumor populations.
In pulmonary or salivary gland pathology, the challenge often lies in differentiating SCC from adenocarcinoma. Here, a panel including CK7, TTF-1, and napsin A is critical: these markers are generally absent in SCC but positive in adenocarcinomas, especially of lung origin. A CK7−/p40+ profile strongly supports squamous differentiation, while CK7+/TTF-1+ favors a glandular phenotype [
20,
38,
46,
65,
69,
70].
Another group of neoplasms that may resemble SCC includes amelanotic melanomas and spindle cell tumors. In such cases, the application of melanocytic markers like S100 and SOX10 is critical. These markers are typically not expressed in SCC, including its sarcomatoid forms, but are characteristically present in melanomas, thereby facilitating accurate exclusion [
9,
45,
47,
71].
4.3. Prognostic and Staging Relevance
Beyond diagnosis, IHC has demonstrated increasing utility in assessing tumor biology and staging parameters. The Ki-67 index serves as an important measure of tumor proliferation and has been associated with more advanced disease and unfavorable clinical outcomes. In HPV-driven mucosal SCCs, p16 expression, originally used as a proxy for viral presence, has also been linked to regional lymph node metastasis and more aggressive tumor behavior [
1]. HPV-independent cervical SCCs are frequently diagnosed at an advanced stage, and they have a higher rate of lymph node metastasis, which confers a reduced disease-free and overall survival [
72,
73,
74,
75].
CD44, as a marker of cancer stemness and EMT, and xCT, related to redox metabolism and chemoresistance, identify high-risk SCC subpopulations that may require more aggressive treatment strategies. These findings suggest that IHC is not only diagnostic but stratificational, with real potential to impact patient management when incorporated into staging systems [
2,
56,
76,
77].
Immune checkpoint molecules, particularly PD-L1 (programmed death-ligand 1), have also emerged as clinically significant in non-keratinizing nasopharyngeal SCC. Immunohistochemical detection using the 22C3 clone, which highlights membranous PD-L1 expression, is now routinely applied to evaluate eligibility for checkpoint blockade therapy. A 2024 meta-analysis by Xu et al. demonstrated that PD-L1 positivity (≥1%) in nasopharyngeal carcinoma correlates with improved response rates and progression-free survival under anti-PD-1/PD-L1 therapy. This supports the role of PD-L1 as a predictive and biologically stratifying biomarker, especially in advanced-stage or recurrent disease [
78,
79,
80,
81]. This pattern is exemplified in
Figure 4, which illustrates a poorly differentiated non-keratinizing SCC of the nasopharynx, with high Ki-67 labeling index, absence of p16 expression, and diffuse but heterogeneous PD-L1 positivity.
Notably, even in rare variants such as clear cell SCC, expression of ARL4C and YAP provided clues to dedifferentiation and clinical behavior, expanding the utility of IHC beyond traditional morphology-based grading [
10].
4.4. Toward Standardized Interpretation and Panels
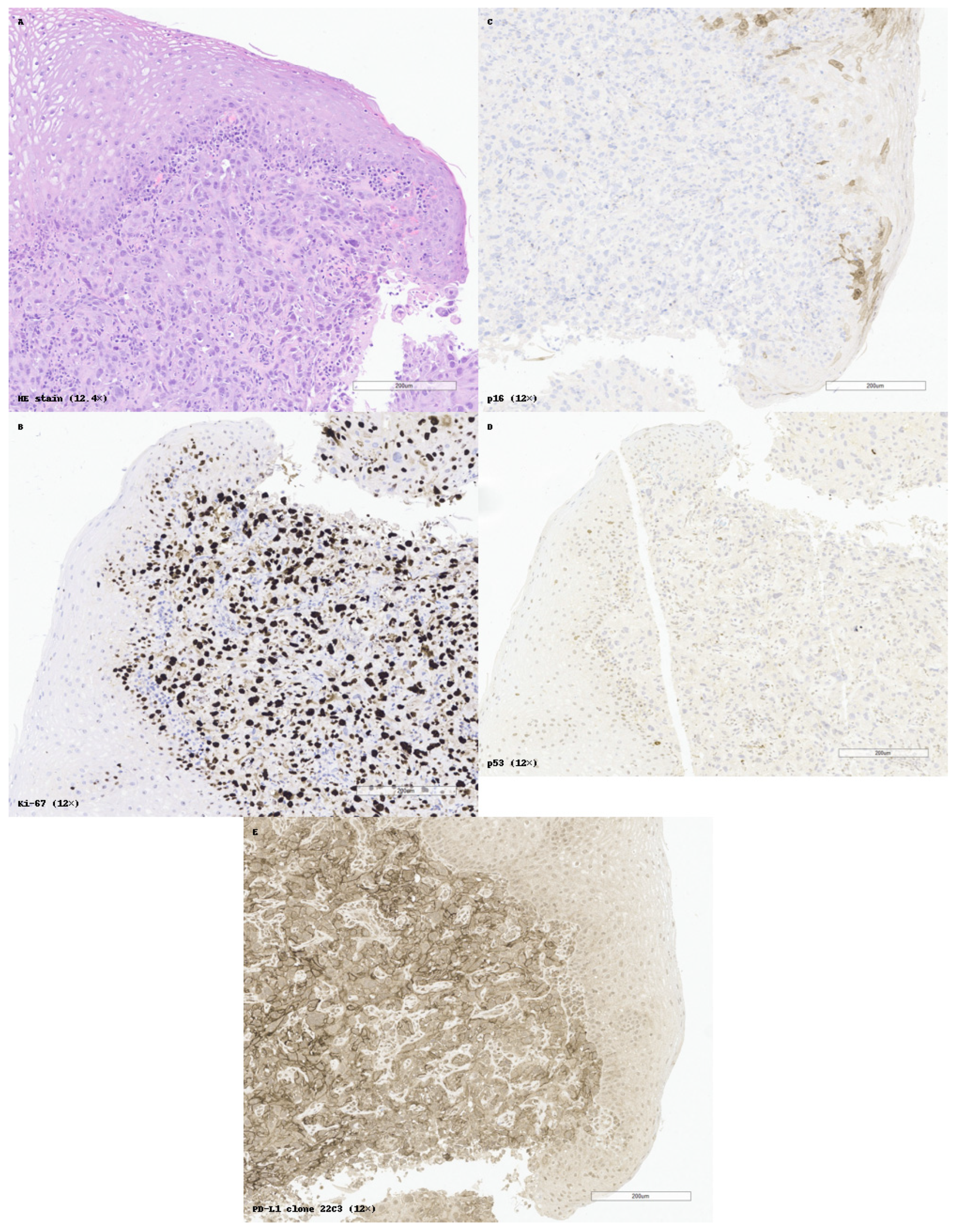
Figure 5 illustrates a practical example of standardized IHC application, with p16/Ki-67 dual interpretation, PD-L1 assessment, and internal controls supporting diagnostic accuracy in SCC of the epiglottis. Despite the availability of numerous immunohistochemical (IHC) markers for squamous cell carcinoma (SCC), lack of standardization in marker selection and interpretation remains a challenge in daily diagnostic practice. One of the key findings of this review is the variability with which markers are applied across institutions and anatomical sites, often depending on individual pathologist preference, tissue availability, or clinical context.
A standardized panel-based approach, tailored to site and morphology, could significantly improve both diagnostic consistency and clinical relevance. For example:
In pulmonary SCC, the minimal diagnostic panel often includes p40, CK5/6, and TTF-1, allowing reliable differentiation from adenocarcinoma [
12,
30].
In head and neck lesions, especially where basaloid features are present, a combination of p63 or p40, EMA, and Ber-EP4 can reliably separate SCC from adnexal tumors or BCC [
14,
46].
In spindle cell or undifferentiated tumors, the addition of S100 and SOX10 is crucial to exclude melanoma, while Ki-67 and pan-cytokeratin confirm epithelial lineage and assess proliferation [
9,
45,
71].
These examples support the idea that marker interpretation should be algorithmic and context-driven, rather than fragmentary. This also includes an awareness of marker limitations—for instance, p63 cross-reactivity in myoepithelial tumors or CK5/6 expression in some basal-type adenocarcinomas—which underscores the need for multi-marker strategies and internal controls [
30,
66,
67,
82].
An additional approach that may enhance diagnostic precision and tissue economy is the use of dual immunohistochemical stains. By combining two markers within the same tissue section—such as p40/napsin A or CK5/6/TTF-1—dual staining enables simultaneous interpretation based on subcellular localization (e.g., nuclear vs. cytoplasmic) and reduces tissue consumption. In a comparative study involving 58 NSCLC cases, dual-marker staining achieved 100% concordance with single-marker protocols in identifying SCC and adenocarcinoma, while offering a time-saving and cost-efficient workflow—particularly in limited biopsy material [
18,
30,
83,
84,
85,
86].
Another well-established application of dual staining is in HPV-related squamous neoplasia, where p16/Ki-67 co-expression has demonstrated strong correlation with high-grade intraepithelial lesions. In cervical cytology, this dual stain significantly improves specificity and positive predictive value compared to cytology or HPV DNA testing alone. In a study of 93 women, dual p16/Ki-67 positivity was strongly associated with CIN2+ histology, supporting its use in triage protocols to reduce unnecessary colposcopies and overtreatment in young patients with low-grade findings [
16,
17,
63,
87,
88,
89,
90].
Moreover, the lack of uniform scoring systems for many prognostic markers (e.g., Ki-67, p16, CD44) limits reproducibility across studies. Adoption of semi-quantitative tools such as the H-score, Allred score, or defined positivity thresholds could enhance both diagnostic and research utility.
This review contributes by synthesizing a framework in which diagnostic, differential, and prognostic markers are organized by function and clinical use. Future work should focus on prospective validation of standardized panels, ideally stratified by anatomical site, histologic variant, and molecular subtype. This would facilitate the broader incorporation of IHC into multidimensional staging and personalized care models.
4.5. Quality Control and Technical Solutions in Conventional IHC
While immunohistochemistry has become central to SCC diagnostics, its effectiveness depends on high technical quality and consistent interpretation. Several challenges persist in conventional IHC practice, particularly in relation to sample integrity, incomplete staining, and interpretive variability. Suboptimal fixation (e.g., under- or over-fixation) may result in epitope degradation or masking, leading to weak or false-negative staining, especially for labile antigens like Ki-67 or PD-L1 [
91,
92]. Similarly, inadequate antigen retrieval or improper antibody titration can lead to in-consistent results, affecting reproducibility across laboratories.
To address these issues, standardized protocols for pre-analytical handling—including immediate fixation in 10% neutral-buffered formalin for 6–72 h—are strongly recommended [
93,
94]. The use of internal positive controls within the same histological section (e.g., non-neoplastic basal cells or inflammatory infiltrates) is critical to confirm staining reliability and detect technical failure [
95]. All IHC stains included in this review were performed on formalin-fixed, paraffin-embedded tissues using validated protocols and included appropriate internal controls. Automated IHC platforms have also demonstrated improved consistency by minimizing user variability, offering precise control over incubation, reagent distribution, and slide handling [
96,
97].
Another key development is the integration of quality assurance programs (QAPs), such as external quality assessments (EQA) and digital slide archiving for remote peer validation. Studies have shown that participation in such programs significantly reduces diagnostic discrepancies and helps identify recurrent technical flaws [
98,
99].
Lastly, digital pathology tools have started to play an increasing role in monitoring staining completeness and uniformity. Image analysis software can detect under-stained areas, quantify marker intensity, and identify artifacts or edge effects—thereby enhancing slide-level quality assurance and reducing interpretive error [
100,
101].
Addressing these practical factors is essential to ensure that IHC provides not only diagnostic information but also reproducible, actionable results across laboratories and patient cohorts.
4.6. The Potential Role of Digital Immunohistochemistry in SCC
The interpretation of immunohistochemical markers in squamous cell carcinoma (SCC) is often subjective and prone to interobserver variability, especially for markers with complex or borderline expression patterns, such as PD-L1. In recent years, digital immunohistochemistry (digital IHC) has gained increasing attention as a strategy to improve standardization, reproducibility, and quantification in IHC evaluation [
102].
Digital IHC employs whole slide imaging combined with artificial intelligence or algorithm-based analysis to objectively assess marker expression. Several studies have shown that digital platforms can improve the consistency of scoring for PD-L1, Ki-67, and p53, particularly in tumors with heterogeneous staining or challenging morphology. Moreover, it offers the possibility of remote consultation and consensus scoring, which is relevant for rare tumor subtypes or borderline IHC results [
103].
Despite these advantages, digital IHC still faces important limitations, including variable validation of algorithms, high implementation costs, and limited integration into routine pathology workflows. Nevertheless, its future role in SCC diagnosis and biomarker-based therapeutic decisions appears promising, especially in the context of increasing use of PD-L1 immunotherapy and standardized reporting [
104].
5. Limitations
While this review provides a structured synthesis of immunohistochemical (IHC) markers in squamous cell carcinoma (SCC), several limitations must be acknowledged with respect to methodology, data heterogeneity, and generalizability of the findings.
5.1. Narrative Design and Absence of Quantitative Synthesis
This work was designed as a narrative, literature-based review, rather than a fully systematic review with meta-analysis. As a result, the sensitivity and specificity values discussed were derived from individual studies and interpreted qualitatively, without pooled statistical analysis.
Additionally, several of the included studies were descriptive in nature—including case reports and small case series—which may not accurately reflect population-level trends. The lack of standardized IHC scoring systems (e.g., Allred score, H-score, or defined cut-offs) in many reports limited cross-study comparisons of staining intensity and diagnostic thresholds.
5.2. Heterogeneity of Tumor Sites and Antibody Clones
SCC arises in a variety of anatomical locations, each with distinct molecular and etiological features. This review included data from cutaneous, oral, esophageal, laryngeal, cervical, and pulmonary SCCs. While this broad inclusion improves generalizability, it also introduces biological heterogeneity, particularly in relation to HPV-driven vs. HPV-independent tumorigenesis.
In addition, differences in antibody clones and staining protocols across studies (e.g., p63 clones 4A4 vs. 7JUL; CK5/6 clones D5/16B4 vs. MA5-12596) may affect reproducibility. Many reports did not specify the antibody clones used, antigen retrieval methods, or dilution parameters, further complicating the interpretation of inter-study variability.
5.3. Limited Representation of Rare Variants
While the review included several articles discussing variant histologies of SCC (e.g., basaloid, sarcomatoid, clear cell), these were often limited to single case reports or small series, making it difficult to draw firm conclusions regarding IHC marker performance in these subtypes. The rarity of these variants inherently limits the evidence base, and future larger studies or registries would be needed to validate the diagnostic strategies proposed here.
5.4. Lack of Outcome Correlation in Many Studies
Although markers such as Ki-67, CD44, and p16 have recognized prognostic value, many studies reviewed here did not include clinical follow-up or survival analysis. As such, conclusions about the predictive or staging value of these markers were drawn primarily from associative data. This limits the ability to integrate IHC findings into evidence-based treatment algorithms or prognostic models, which would require prospective validation in larger cohorts.
5.5. Potential Publication Bias
Finally, as with any literature-based review, there is a risk of publication bias, whereby studies reporting positive or confirmatory results are more likely to be published and indexed. Negative findings or studies with ambiguous IHC expression patterns may be underrepresented, skewing the perceived utility of some markers. Moreover, the review did not include unpublished studies, abstracts, or gray literature, which may omit emerging data from recent conference presentations or ongoing trials.
Despite these limitations, the structured synthesis of available data provides practical, pathology-focused insights into the use of IHC in SCC and highlights the need for standardization and prospective validation of marker panels in diverse clinical contexts.
6. Conclusions
Squamous cell carcinoma (SCC) represents a morphologically and biologically diverse group of epithelial malignancies with wide anatomical distribution and variable clinical behavior. In this context, immunohistochemistry (IHC) has emerged as an indispensable tool, enabling not only accurate tumor identification but also histological subtyping and risk stratification, even in small or poorly differentiated specimens.
This review consolidates current evidence regarding the most frequently used IHC markers in SCC, highlighting their role in positive diagnosis, differential diagnosis, and prognostic assessment. By organizing the data across functional and clinical categories, this synthesis offers a practical and structured reference for pathologists, oncologists, and trainees involved in SCC diagnostics.
Although this review is not comprehensive of all available markers, it emphasizes the increasing importance of IHC in current cancer diagnostics. The adoption of marker panels tailored to tumor subtype and site can enhance accuracy, minimize diagnostic ambiguity, and help guide therapeutic decisions, even in uncommon forms of SCC.
Future Directions
Based on the current synthesis, several key areas for future research and clinical integration can be identified:
Prospective validation of IHC panels across different SCC variants and anatomical sites, with standardized cut-offs and scoring systems;
Development of semi-quantitative frameworks (e.g., Allred or H-score) for prognostic markers such as Ki-67, CD44, and p16;
Integration of IHC with molecular tools, including next-generation sequencing, transcriptomic profiling, or digital pathology platforms, to enhance personalized diagnostics;
Establishment of treatment algorithms that incorporate IHC-based stratification into routine clinical decision-making, especially in HPV-related and chemoresistant SCCs;
Exploration of real-time or non-invasive diagnostic methods, such as digital immunostaining, ex vivo confocal microscopy, or liquid biopsy correlates of IHC markers.
As IHC continues to evolve, its full potential lies in its integration—not only with histological assessment, but also with molecular tools, in the service of precision medicine.
In conclusion, immunohistochemistry remains the cornerstone of modern SCC diagnosis. When used in a targeted and systematic manner, IHC not only confirms tumor identity but also opens the door to biologically informed patient care. Its full potential lies in its integration not only with morphology but with molecular tools and clinical data, in the service of precision oncology.
Author Contributions
Conceptualization, V.-M.V., F.S. and R.-M.M.; literature research, V.-M.V. and S.D.; image acquisition, S.D.; writing—original draft preparation, R.-M.M.; writing—review and editing, V.-M.V. and F.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the support of the Foundation for Cellular and Molecular Medicine, Bucharest, in facilitating the publication of this review.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mehdi, H.K.; Raju, K.; Sheela, S.R. Association of P16, Ki-67, and CD44 expression in high-grade squamous intraepithelial neoplasia and squamous cell carcinoma of the cervix. J. Cancer Res. Ther. 2023, 19, S260–S267. [Google Scholar] [CrossRef]
- Ghazi, N.; Saghravanian, N.; Shakeri, M.T.; Jamali, M. Evaluation of CD44 and TGF-B Expression in Oral Carcinogenesis. J. Dent. 2021, 22, 33–40. [Google Scholar]
- Scarini, J.F.; Mariz, B.A.; de Lima-Souza, R.A.; Lavareze, L.; Emerick, C.; Tincani, A.J.; Egal, É.S.; Altemani, A.; Mariano, F.V. Spindle cell carcinoma of the lip: An immunohistochemical study of a challenging case. Oral Oncol. 2022, 125, 105680. [Google Scholar] [CrossRef]
- Kim, N.I.; Lee, J.S. Greater specificity of p40 compared with p63 in distinguishing squamous cell carcinoma from adenocarcinoma in effusion cellblocks. CytoJournal 2020, 17, 13. [Google Scholar] [CrossRef]
- Zhurauliou, A.; Vasyukova, O. Perianal Cutaneous Spindle Cell Squamous Cell Carcinoma with Myxoid Stroma: Case Report and Literature Review. Am. J. Dermatopathol. 2023, 45, 650–653. [Google Scholar] [CrossRef]
- Romanach, M.J.; Azevedo, R.S.; Carlos, R.; De Almeida, O.P.; Pires, F.R. Clinicopathological and immunohistochemical features of oral spindle cell carcinoma. J. Oral Pathol. Med. 2010, 39, 335–341. [Google Scholar] [CrossRef]
- Bălășescu, E.; Gheorghe, A.C.; Moroianu, A.; Turcu, G.; Brînzea, A.; Antohe, M.; Hodorogea, A.; Manea, L.; Balaban, M.; Andrei, R.; et al. Role of immunohistochemistry in the diagnosis and staging of cutaneous squamous-cell carcinomas (Review). Exp. Ther. Med. 2022, 23, 383. [Google Scholar] [CrossRef]
- Jaiswal, A.; Singh, R. Loss of Epidermal Homeostasis Underlies the Development of Squamous Cell Carcinoma. Stem Cell Rev. Rep. 2023, 19, 667–679. [Google Scholar] [CrossRef]
- Murali Mohan, R.; Sahu, K.K.; Suresh, P.K. Immunohistochemical comparison of p63 and p40 in head and neck Spindle Cell Carcinoma. Pathol. Res. Pract. 2022, 229, 153733. [Google Scholar] [CrossRef]
- Hasegawa, K.; Fujii, S.; Kurppa, K.J.; Maehara, T.; Oobu, K.; Nakamura, S.; Kiyoshima, T. Clear cell squamous cell carcinoma of the tongue exhibits characteristics as an undifferentiated squamous cell carcinoma. Pathol. Res. Pract. 2022, 235, 153909. [Google Scholar] [CrossRef]
- Polo, V.; Pasello, G.; Frega, S.; Favaretto, A.; Koussis, H.; Conte, P.; Bonanno, L. Squamous cell carcinomas of the lung and of the head and neck: New insights on molecular characterization. Oncotarget 2016, 7, 25050–25063. [Google Scholar] [CrossRef][Green Version]
- Bishop, J.A.; Teruya-Feldstein, J.; Westra, W.H.; Pelosi, G.; Travis, W.D.; Rekhtman, N. P40 (ΔNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod.Pathol. 2012, 25, 405–415. [Google Scholar] [CrossRef]
- Chi, Z.; Balani, J.; Gopal, P.; Hammer, S.; Lewis, C.M.; Peng, L. Characterization of Desmoglein 3 (DSG3) as a Sensitive and Specific Marker for Esophageal Squamous Cell Carcinoma. Gastroenterol. Res.Pract. 2022, 2022, 2220940. [Google Scholar] [CrossRef]
- Trudrung, M.A.; Bacig, C.; Vander Zee, B.; Potter, H. Basal cell carcinoma and squamous cell carcinoma of the conjunctiva in a single lesion. Am. J. Ophthalmol. Case Rep. 2025, 38, 102300. [Google Scholar] [CrossRef]
- Hu, Y.; Han, Y.; He, M.; Zhang, Y.; Zou, X. S100 proteins in head and neck squamous cell carcinoma (Review). Oncol. Lett. 2023, 26, 362. [Google Scholar] [CrossRef]
- P53, p16 and Ki67 Immunoexpression in Cutaneous Squamous Cell Carcinoma and Its Precursor Lesions—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27833960/ (accessed on 20 April 2025).
- Vîlcea, A.M.; Stoica, L.E.; Andreiana, B.C.; Mirea, C.S.; Cojan, T.Ş.; Vîlcea, I.C.; Mărgăritescu, C. Immunoexpression of Ki67, P16 and Beta-catenin in precursor lesions of cutaneous squamous cell carcinoma. Rom. J. Morphol. Embryol. 2024, 65, 19. [Google Scholar] [CrossRef]
- Sethi, S.; Geng, L.; Shidham, V.B.; Archuletta, P.; Bandyophadhyay, S.; Feng, J.; Madan, S.; Shi, D.; Tranchida, P.; Giorgadze, T. Dual color multiplex TTF-1 + Napsin A and p63 + CK5 immunostaining for subcategorizing of poorly differentiated pulmonary non-small carcinomas into adenocarcinoma and squamous cell carcinoma in fine needle aspiration specimens. Cytojournal 2012, 9, 10. [Google Scholar] [CrossRef]
- Van Zyl, A.; Schubert, P.T.; Koegelenberg, C.F.N. The utility of TTF-1, napsin A, CK5 and p63 staining in the sub-classification of non-small cell carcinoma of the lung. Cytopathology 2019, 30, 586–591. [Google Scholar] [CrossRef]
- Ao, M.H.; Zhang, H.; Sakowski, L.; Sharma, R.; Illei, P.B.; Gabrielson, E.; Askin, F.; Li, Q.K. The utility of a novel triple marker (combination of TTF1, napsin A, and p40) in the subclassification of non-small cell lung cancer. Hum. Pathol. 2014, 45, 926–934. [Google Scholar] [CrossRef]
- Tacha, D.; Bremer, R.; Haas, T.; Qi, W. An immunohistochemical analysis of a newly developed, mouse monoclonal p40 (BC28) antibody in lung, bladder, skin, breast, prostate, and head and neck cancers. Arch. Pathol. Lab. Med. 2014, 138, 1358–1364. [Google Scholar] [CrossRef]
- Chi, Z.; Balani, J.; Gopal, P.; Peng, L.; Hammer, S. Variations of P40 and P63 Immunostains in Different Grades of Esophageal Squamous Cell Carcinoma: A Potential Diagnostic Pitfall. Appl. Immunohistochem. Mol. Morphol. AIMM 2021, 29, 674–679. [Google Scholar] [CrossRef]
- Alomari, A.K.; Glusac, E.J.; McNiff, J.M. p40 is a more specific marker than p63 for cutaneous poorly differentiated squamous cell carcinoma. J. Cutan. Pathol. 2014, 41, 839–845. [Google Scholar] [CrossRef]
- Bishop, J.A.; Montgomery, E.A.; Westra, W.H. Use of p40 and p63 immunohistochemistry and human papillomavirus testing as ancillary tools for the recognition of head and neck sarcomatoid carcinoma and its distinction from benign and malignant mesenchymal processes. Am. J. Surg. Pathol. 2014, 38, 257–264. [Google Scholar] [CrossRef]
- Ha Lan, T.T.; Chen, S.J.; Arps, D.P.; Fullen, D.R.; Patel, R.M.; Siddiqui, J.; Carskadon, S.; Palanisamy, N.; Harms, P.W. Expression of the p40 isoform of p63 has high specificity for cutaneous sarcomatoid squamous cell carcinoma. J. Cutan. Pathol. 2014, 41, 831–838. [Google Scholar] [CrossRef]
- Neha, B.; Shashi, D.; Seema, R. Spindle Cell Squamous Cell Carcinoma of Head and Neck Region: A Clinicopathological and Immunohistochemical Study. Indian J. Surg. Oncol. 2021, 12, 699. [Google Scholar] [CrossRef]
- Lilo, M.T.; Allison, D.; Wang, Y.; Ao, M.; Gabrielson, E.; Geddes, S.; Zhang, H.; Askin, F.; Li, Q.K. Expression of P40 and P63 in lung cancers using fine needle aspiration cases. Understanding clinical pitfalls and limitations. J. Am. Soc. Cytopathol. 2016, 5, 123–132. [Google Scholar] [CrossRef]
- Smirnov, A.; Anemona, L.; Novelli, F.; Piro, C.M.; Annicchiarico-Petruzzelli, M.; Melino, G.; Candi, E. p63 Is a Promising Marker in the Diagnosis of Unusual Skin Cancer. Int. J. Mol. Sci. 2019, 20, 5781. [Google Scholar] [CrossRef]
- Hirai, E.; Yamamoto, K.; Yamamoto, N.; Yamashita, Y.; Kounoe, T.; Kondo, Y.; Yonemasu, H.; Takahashi, T.; Kurokawa, H. Basaloid squamous cell carcinoma of the mandible: Report of two cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, e54–e58. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Katzenstein, A.L.A. Subclassification of non-small cell lung carcinomas lacking morphologic differentiation on biopsy specimens: Utility of an immunohistochemical panel containing TTF-1, napsin A, p63, and CK5/6. Am. J. Surg. Pathol. 2011, 35, 15–25. [Google Scholar] [CrossRef]
- Troeltzsch, M.; Künzel, V.; Haidari, S.; Troeltzsch, M.; Otto, S.; Ehrenfeld, M.; Probst, F.; Knösel, T. Desmoglein-3 overexpression in oral squamous cell carcinoma is associated with metastasis formation and early recurrence: An immunohistochemical study. J. Craniomaxillofac. Surg. 2022, 50, 281–288. [Google Scholar] [CrossRef]
- Wang, L.; Liu, T.; Wang, Y.; Cao, L.; Nishioka, M.; Aguirre, R.L.; Ishikawa, A.; Geng, L.; Okada, N. Altered expression of desmocollin 3, desmoglein 3, and beta-catenin in oral squamous cell carcinoma: Correlation with lymph node metastasis and cell proliferation. Virchows Arch. 2007, 451, 959–966. [Google Scholar] [CrossRef]
- Apu, E.H.; Akram, S.U.; Rissanen, J.; Wan, H.; Salo, T. Desmoglein 3—Influence on oral carcinoma cell migration and invasion. Exp. Cell Res. 2018, 370, 353–364. [Google Scholar]
- Savci-Heijink, C.D.; Kosari, F.; Aubry, M.-C.; Caron, B.L.; Sun, Z.; Yang, P.; Vasmatzis, G. The role of desmoglein-3 in the diagnosis of squamous cell carcinoma of the lung. Am. J. Pathol. 2009, 174, 1629–1637. [Google Scholar] [CrossRef]
- Agackiran, Y.; Ozcan, A.; Akyurek, N.; Memis, L.; Findik, G.; Kaya, S. Desmoglein-3 and Napsin A double stain, a useful immunohistochemical marker for differentiation of lung squamous cell carcinoma and adenocarcinoma from other subtypes. Appl. Immunohistochem. Mol. Morphol. AIMM 2012, 20, 350–355. [Google Scholar] [CrossRef]
- Viehweger, F.; Azem, A.; Gorbokon, N.; Uhlig, R.; Lennartz, M.; Rico, S.D.; Kind, S.; Reiswich, V.; Kluth, M.; Hube-Magg, C.; et al. Desmoglein 3 (Dsg3) expression in cancer: A tissue microarray study on 15,869 tumors. Pathol. Res. Pract. 2022, 240, 154200. [Google Scholar] [CrossRef]
- Fang, W.-K.; Chen, B.; Xu, X.-E.; Liao, L.-D.; Wu, Z.-Y.; Wu, J.-Y.; Shen, J.; Xu, L.-Y.; Li, E.-M. Altered expression and localization of desmoglein 3 in esophageal squamous cell carcinoma. Acta Histochem. 2014, 116, 803–809. [Google Scholar] [CrossRef]
- Kim, B.R.; Coyaud, E.; Laurent, E.M.; St-Germain, J.; Van de Laar, E.; Tsao, M.-S.; Raught, B.; Moghal, N. Identification of the SOX2 Interactome by BioID Reveals EP300 as a Mediator of SOX2-dependent Squamous Differentiation and Lung Squamous Cell Carcinoma Growth. Mol. Cell. Proteom. 2017, 16, 1864–1888. [Google Scholar] [CrossRef]
- Emanuel, P.; Wang, B.; Wu, M.; E Burstein, D. p63 Immunohistochemistry in the distinction of adenoid cystic carcinoma from basaloid squamous cell carcinoma. Mod. Pathol. 2005, 18, 645–650. [Google Scholar] [CrossRef]
- Ishida, H.; Kasajima, A.; Yamauchi, T.; Akaishi, R.; Ueki, S.; Taniyama, Y.; Fujishima, F.; Koike, T.; Kamei, T.; Lam, A.K.-Y.; et al. A diagnostic pitfall; Small cell carcinoma-like features in basaloid squamous cell carcinoma of the esophagus. Histol. Histopathol. 2023, 38, 155–163. [Google Scholar] [CrossRef]
- Xie, S.; Bredell, M.; Yang, H.; Shen, S.; Yang, H. Basaloid squamous cell carcinoma of the maxillary gingiva: A case report and review of the literature. Oncol. Lett. 2014, 8, 1287–1290. [Google Scholar] [CrossRef]
- Alaeddini, M.; Etemad-Moghadam, S. Cell kinetic markers in cutaneous squamous and basal cell carcinoma of the head and neck. Braz. J. Otorhinolaryngol. 2022, 88, 529–532. [Google Scholar] [CrossRef]
- Raffat, M.A.; Hadi, N.I.; Hosein, M.; Mirza, S.; Ikram, S.; Akram, Z. S100 proteins in oral squamous cell carcinoma. Clin. Chim. Acta 2018, 480, 143–149. [Google Scholar] [CrossRef]
- Rye, P.; Nustad, K.; Stigbrand, T. Tumor marker workshops. Tumour Biol. 2003, 24, 165–171. [Google Scholar] [CrossRef]
- Behrens, E.L.; Boothe, W.; D’Silva, N.; Walterscheid, B.; Watkins, P.; Tarbox, M. SOX-10 staining in dermal scars. J. Cutan. Pathol. 2019, 46, 579–585. [Google Scholar] [CrossRef]
- Clarke, L.E.; Conway, A.B.; Warner, N.M.; Barnwell, P.N.; Sceppa, J.; Helm, K.F. Expression of CK7, Cam 5.2 and Ber-Ep4 in cutaneous squamous cell carcinoma. J. Cutan. Pathol. 2013, 40, 646–650. [Google Scholar] [CrossRef]
- Qi, J.; Hu, Z.; Xiao, H.; Liu, R.; Guo, W.; Yang, Z.; Ma, K.; Su, S.; Tang, P.; Zhou, X.; et al. SOX10—A Novel Marker for the Differential Diagnosis of Breast Metaplastic Squamous Cell Carcinoma. Cancer Manag. Res. 2020, 12, 4039–4044. [Google Scholar] [CrossRef]
- Tope, W.D.; Nowfar-Rad, M.; Kist, D.A. Ber-EP4-positive phenotype differentiates actinic keratosis from superficial basal cell carcinoma. Dermatol. Surg. 2000, 26, 415–418. [Google Scholar] [CrossRef]
- Ouh, Y.-T.; Kim, H.Y.; Yi, K.W.; Lee, N.-W.; Kim, H.-J.; Min, K.-J. Enhancing Cervical Cancer Screening: Review of p16/Ki-67 Dual Staining as a Promising Triage Strategy. Diagnostics 2024, 14, 451. [Google Scholar] [CrossRef]
- Aromseree, S.; Wongjumpa, W.; Ekalaksananan, T.; Temtanakitpaisan, A.; Kleebkaow, P.; Srisathaporn, S.; Tongchai, P.; Pientong, C. P16/Ki-67 Dual Staining in Positive Human Papillomavirus DNA Testing for Predictive Diagnosis of Abnormal Cervical Lesions in Northeastern Thai Women. Asian Pac. J. Cancer Prev. 2022, 23, 3405–3411. [Google Scholar] [CrossRef]
- Wentzensen, N.; Fetterman, B.; Castle, P.E.; Schiffman, M.; Wood, S.N.; Stiemerling, E.; Tokugawa, D.; Bodelon, C.; Poitras, N.; Lorey, T.; et al. p16/Ki-67 Dual Stain Cytology for Detection of Cervical Precancer in HPV-Positive Women. J. Natl. Cancer Inst. 2015, 107, djv257. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Wu, Z.; Li, T.; Yu, L.; Zhang, S.K.; Zhang, X.; Qu, P.; Sun, P.; Xi, M.R.; Liu, X.; et al. Performance of HPV genotyping combined with p16/Ki-67 in detection of cervical precancer and cancer among HPV-positive Chinese women. Cancer Prev. Res. 2020, 13, 163–171. [Google Scholar] [CrossRef]
- Expression of the p16 and Ki67 in Cervical Squamous Intraepithelial Lesions and Cancer—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27509952/ (accessed on 20 April 2025).
- Gothwal, M.; Nalwa, A.; Singh, P.; Yadav, G.; Bhati, M.; Samriya, N. Role of Cervical Cancer Biomarkers p16 and Ki67 in Abnormal Cervical Cytological Smear. J. Obstet. Gynaecol. India 2021, 71, 72–77. [Google Scholar] [CrossRef]
- Woźniak, M.; Nahajowski, M.; Hnitecka, S.; Rutkowska, M.; Marek, G.; Agrawal, A.; Makuch, S.; Agrawal, S.; Ziółkowski, P. A comparative study of osteopontin expression, Ki67 index and prognosis in squamous cell carcinoma and cysts of the oral cavity. Transl. Cancer Res. 2020, 9, 795–808. [Google Scholar] [CrossRef]
- Lee, J.R.; Roh, J.; Lee, S.M.; Park, Y.; Cho, K.; Choi, S.; Nam, S.Y.; Kim, S.Y. Overexpression of cysteine-glutamate transporter and CD44 for prediction of recurrence and survival in patients with oral cavity squamous cell carcinoma. Head Neck 2018, 40, 2340–2346. [Google Scholar] [CrossRef]
- Evaluation of CD24 and CD44 as Cancer Stem Cell Markers in Squamous Cell Carcinoma and Epithelial Dysplasia of the Oral Cavity by q- RT-PCR—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/32774798/ (accessed on 28 March 2025).
- De Barros, F.D.; Torres, L.C.; Araujo, C.; Marinho, F.d.S.; Dubourcq, B.C.; Dubourcq, L.C.; Guimarães, G.C. Prognostic utility of SOX2, STAT3, and CD44high/CD24low expression in penile cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 2081–2094. [Google Scholar] [CrossRef]
- Okazaki, S.; Umene, K.; Yamasaki, J.; Suina, K.; Otsuki, Y.; Yoshikawa, M.; Minami, Y.; Masuko, T.; Kawaguchi, S.; Nakayama, H.; et al. Glutaminolysis-related genes determine sensitivity to xCT-targeted therapy in head and neck squamous cell carcinoma. Cancer Sci. 2019, 110, 3453–3463. [Google Scholar] [CrossRef]
- Fujii, S.; Shinjo, K.; Matsumoto, S.; Harada, T.; Nojima, S.; Sato, S.; Usami, Y.; Toyosawa, S.; Morii, E.; Kondo, Y.; et al. Epigenetic upregulation of ARL4C, due to DNA hypomethylation in the 3′-untranslated region, promotes tumorigenesis of lung squamous cell carcinoma. Oncotarget 2016, 7, 81571–81587. [Google Scholar] [CrossRef]
- Vincent-Mistiaen, Z.; Elbediwy, A.; Vanyai, H.; Cotton, J.; Stamp, G.; Nye, E.; Spencer-Dene, B.; Thomas, G.J.; Mao, J.; Thompson, B.; et al. YAP drives cutaneous squamous cell carcinoma formation and progression. eLife 2018, 7, e33304. [Google Scholar] [CrossRef]
- Liu, S.; Knochelmann, H.M.; Lomeli, S.H.; Hong, A.; Richardson, M.; Yang, Z.; Lim, R.J.; Wang, Y.; Dumitras, C.; Krysan, K.; et al. Response and recurrence correlates in individuals treated with neoadjuvant anti-PD-1 therapy for resectable oral cavity squamous cell carcinoma. Cell Rep. Med. 2021, 2, 100411. [Google Scholar] [CrossRef]
- Celewicz, A.; Celewicz, M.; Wężowska, M.; Chudecka-Głaz, A.; Menkiszak, J.; Urasińska, E. Clinical efficacy of p16/Ki-67 dual-stained cervical cytology in secondary prevention of cervical cancer. Pol. J. Pathol. 2018, 69, 42–47. [Google Scholar] [CrossRef]
- Singh, P.; Kaushik, S.; Thakur, B.; Acharya, S.; Bhardwaj, A.; Bahal, N. Evaluation of variable p16 immunostaining patterns, Ki-67 indices and HPV status in cervical SILs and squamous cell carcinomas: An institutional experience. Indian J. Pathol. Microbiol. 2023, 66, 63–69. [Google Scholar] [CrossRef]
- Hoff, P.M.; Coudry, R.; Moniz, C.M.V. Pathology of Anal Cancer. Surg. Oncol. Clin. N. Am. 2017, 26, 57–71. [Google Scholar] [CrossRef]
- Downey, P.; Cummins, R.; Moran, M.; Gulmann, C. If it’s not CK5/6 positive, TTF-1 negative it’s not a squamous cell carcinoma of lung. APMIS 2008, 116, 526–529. [Google Scholar] [CrossRef]
- Xu, X.Y.; Yang, G.Y.; Yang, J.H.; Li, J. Analysis of clinical characteristics and differential diagnosis of the lung biopsy specimens in 99 adenocarcinoma cases and 111 squamous cell carcinoma cases: Utility of an immunohistochemical panel containing CK5/6, CK34βE12, p63, CK7 and TTF-1. Pathol. Res. Pract. 2014, 210, 680–685. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chang, J.T.; Lee, L.; Wang, H.-M.; Liao, C.-T.; Chiu, C.-C.; Chen, P.-J.; Cheng, A.-J. DSG3 is overexpressed in head neck cancer and is a potential molecular target for inhibition of oncogenesis. Oncogene 2007, 26, 467–476. [Google Scholar] [CrossRef]
- Pulitzer, M.; Desman, G.; Busam, K.J. CK7 expression in primary cutaneous squamous cell carcinoma. J. Cutan. Pathol. 2010, 37, 966–972. [Google Scholar] [CrossRef]
- Woods, R.S.R.; Callanan, D.; Jawad, H.; Molony, P.; Werner, R.; Heffron, C.; Feeley, L.; Sheahan, P. Cytokeratin 7 and 19 expression in oropharyngeal and oral squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2022, 279, 1435–1443. [Google Scholar] [CrossRef]
- Rooper, L.M.; McCuiston, A.M.; Westra, W.H.; Bishop, J.A. SOX10 Immunoexpression in Basaloid Squamous Cell Carcinomas: A Diagnostic Pitfall for Ruling out Salivary Differentiation. Head Neck Pathol. 2019, 13, 543–547. [Google Scholar] [CrossRef]
- Rodríguez-Carunchio, L.; Soveral, I.; Steenbergen, R.; Torné, A.; Martinez, S.; Fusté, P.; Pahisa, J.; Marimon, L.; Ordi, J.; del Pino, M. HPV-negative carcinoma of the uterine cervix: A distinct type of cervical cancer with poor prognosis. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 119–127. [Google Scholar] [CrossRef]
- Nicolás, I.; Marimon, L.; Barnadas, E.; Saco, A.; Rodríguez-Carunchio, L.; Fusté, P.; Martí, C.; Rodriguez-Trujillo, A.; Torne, A.; del Pino, M.; et al. HPV-negative tumors of the uterine cervix. Mod. Pathol. 2019, 32, 1189–1196. [Google Scholar] [CrossRef]
- Menon, S.; Moch, H.; Berney, D.; Cree, I.; Srigley, J.; Tsuzuki, T.; Compérat, E.; Hartmann, A.; Netto, G.; Rubin, M.; et al. WHO 2022 classification of penile and scrotal cancers: Updates and evolution. Histopathology 2023, 82, 508–520. [Google Scholar] [CrossRef]
- Rimini, M.; Franco, P.; Bertolini, F.; Berardino, D.B.; Giulia, Z.M.; Stefano, V.; Andrikou, K.; Arcadipane, F.; Napolitano, M.; Buno, L.V.; et al. The Prognostic Role of Baseline Eosinophils in HPV-Related Cancers: A Multi-institutional Analysis of Anal SCC and OPC Patients Treated with Radical CT-RT. J. Gastrointest. Cancer 2023, 54, 662–671. [Google Scholar] [CrossRef]
- Rodrigues, M.F.S.D.; Xavier, F.C.d.A.; Andrade, N.P.; Lopes, C.; Luiz, L.M.; Sedassari, B.T.; Ibarra, A.M.C.; López, R.V.M.; Schmerling, C.K.; Moyses, R.A.; et al. Prognostic implications of CD44, NANOG, OCT4, and BMI1 expression in tongue squamous cell carcinoma. Head Neck 2018, 40, 1759–1773. [Google Scholar] [CrossRef]
- Zhao, S.; He, J.-L.; Qiu, Z.-X.; Chen, N.-Y.; Luo, Z.; Chen, B.-J.; Li, W.-M. Prognostic value of CD44 variant exon 6 expression in non-small cell lung cancer: A meta-analysis. Asian Pac. J. Cancer Prev. 2014, 15, 6761–6766. [Google Scholar] [CrossRef]
- Xu, R.; Wong, C.H.; Chan, K.S.; Chiang, C.L. PD-L1 expression as a potential predictor of immune checkpoint inhibitor efficacy and survival in patients with recurrent or metastatic nasopharyngeal cancer: A systematic review and meta-analysis of prospective trials. Front. Oncol. 2024, 14, 1386381. [Google Scholar] [CrossRef]
- Rajasekaran, N.; Wang, X.; Ravindranathan, S.; Chin, D.J.; Tseng, S.-Y.; Klakamp, S.L.; Widmann, K.; Kapoor, V.N.; Vexler, V.; Keegan, P.; et al. Toripalimab, a therapeutic monoclonal anti-PD-1 antibody with high binding affinity to PD-1 and enhanced potency to activate human T cells. Cancer Immunol. Immunother. 2024, 73, 60. [Google Scholar] [CrossRef]
- Huang, Z.; Zheng, S.; Ding, S.; Wei, Y.; Chen, C.; Liu, X.; Li, H.; Xia, Y. Prognostic role of programmed cell death ligand-1 expression in head and neck cancer treated with programmed cell death protein-1/programmed cell death ligand-1 inhibitors: A meta-analysis based on clinical trials. J. Cancer Res. Ther. 2021, 17, 676–687. [Google Scholar] [CrossRef]
- Sacco, A.G.; Chen, R.; Worden, F.P.; Wong, D.J.L.; Adkins, D.; Swiecicki, P.; Chai-Ho, W.; Oppelt, P.; Ghosh, D.; Bykowski, J.; et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: An open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021, 22, 883–892. [Google Scholar] [CrossRef]
- Hsieh, M.-H.; Choe, J.H.; Gadhvi, J.; Kim, Y.J.; Arguez, M.A.; Palmer, M.; Gerold, H.; Nowak, C.; Do, H.; Mazambani, S.; et al. p63 and SOX2 Dictate Glucose Reliance and Metabolic Vulnerabilities in Squamous Cell Carcinomas. Cell Rep. 2019, 28, 1860–1878.e9. [Google Scholar] [CrossRef]
- Guo, R.; Tian, Y.; Zhang, N.; Huang, H.; Huang, Y.; Yang, J. Use of dual-marker staining to differentiate between lung squamous cell carcinoma and adenocarcinoma. J. Int. Med. Res. 2020, 48, 0300060519893867. [Google Scholar] [CrossRef]
- Sharma, R.; Wang, Y.; Chen, L.; Gurda, G.T.; Geddes, S.; Gabrielson, E.; Askin, F.; Li, Q.K. Utility of a novel triple marker (combination of thyroid transcription factor 1, Napsin A, and P40) in the subclassification of non-small cell lung carcinomas using fine-needle aspiration cases. Hum. Pathol. 2016, 54, 8–16. [Google Scholar] [CrossRef]
- Nishino, M.; Hoang, M.P.; Della Pelle, P.; Morales-Oyarvide, V.; Huynh, T.G.; Mark, E.J.; Mino-Kenudson, M. Napsin A/p40 antibody cocktail for subtyping non-small cell lung carcinoma on cytology and small biopsy specimens. Cancer Cytopathol. 2016, 124, 472–484. [Google Scholar] [CrossRef]
- Nobre, A.R.; Albergaria, A.; Schmitt, F. p40: A p63 isoform useful for lung cancer diagnosis—a review of the physiological and pathological role of p63. Acta Cytol. 2013, 57, 1–8. [Google Scholar] [CrossRef]
- Balcere, A.; Sperga, M.; Čēma, I.; Lauskis, G.; Zolovs, M.; Kupfere, M.R.; Krūmiņa, A. Expression of p53, p63, p16, Ki67, Cyclin D, Bcl-2, and CD31 Markers in Actinic Keratosis, In Situ Squamous Cell Carcinoma and Normal Sun-Exposed Skin of Elderly Patients. J. Clin. Med. 2023, 12, 7291. [Google Scholar] [CrossRef]
- Ruhoy, S.M.; Guinee, D.G.; Nuovo, G. Squamous cell carcinoma arising in association with verruca vulgares and HPV-2: A clinicopathologic study with p16 and p53 immunohistochemical studies and human papillomavirus in situ hybridization studies. Appl. Immunohistochem. Mol. Morphol. AIMM 2014, 22, 253–261. [Google Scholar] [CrossRef]
- Kühn, J.P.; Speicher, S.; Linxweiler, B.; Körner, S.; Rimbach, H.; Wagner, M.; Solomayer, E.F.; Schick, B.; Linxweiler, M. Dual Sec62/Ki67 immunocytochemistry of liquid-based cytological preparations represents a highly valid biomarker for non-invasive detection of head and neck squamous cell carcinomas. Cytopathology 2024, 35, 113–121. [Google Scholar] [CrossRef]
- Accardi, R.; Gheit, T. Cutaneous HPV and skin cancer. Presse Med. 2014, 43, e435–e443. [Google Scholar] [CrossRef]
- Bussolati, G.; Leonardo, E. Technical pitfalls potentially affecting diagnoses in immunohistochemistry. J. Clin. Pathol. 2008, 61, 1184–1192. [Google Scholar] [CrossRef]
- Von Wasielewski, R.; Masood, S.; von Wasielewski, R.; Mengel, M.; Nolte, M.; Werner, M. Influence of fixation, antibody clones, and threshold value on Ki-67 labeling of breast cancer tissue. Histochem. Cell Biol. 2000, 113, 31–36. [Google Scholar]
- ASCO/CAP Guidelines. Recommendations for Optimal Fixation and Handling of Tissue Specimens for Pathology. Arch. Pathol. Lab. Med. 2020, 144, 426–437. [Google Scholar]
- Hewitt, S.M.; Lewis, F.A.; Cao, Y.; Conrad, R.C.; Cronin, M.; Danenberg, K.D.; Goralski, T.J.; Langmore, J.P.; Raja, R.G.; Williams, M.P.; et al. Tissue Handling and Specimen Preparation in Surgical Pathology: Issues Concerning the Recovery of Nucleic Acids from Formalin-Fixed, Paraffin-Embedded Tissue. Arch. Pathol. Lab. Med. 2008, 132, 579–586. [Google Scholar] [CrossRef]
- Taylor, C.R. Quality assurance and standardization in immunohistochemistry: A requirement for increased clinical use. J. Histochem. Cytochem. 2001, 49, 831–837. [Google Scholar]
- Leong, A.S. Quantitation in immunohistology: Fact or fiction? APMIS 2004, 112, 263–270. [Google Scholar]
- Prichard, J.W. Overview of automated immunohistochemistry. Arch. Pathol. Lab. Med. 2014, 138, 1578–1582. [Google Scholar] [CrossRef]
- UK NEQAS ICC & ISH Annual Reports. Available online: https://ukneqas.org.uk (accessed on 23 March 2025).
- Fitzgibbons, P.L.; Bradley, L.A.; Fatheree, L.A.; Alsabeh, R.; Fulton, R.S.; Goldsmith, J.D.; Haas, T.S.; Karabakhtsian, R.G.; Loykasek, P.A.; Marolt, M.J.; et al. Principles of Analytic Validation of Immunohistochemical Assay. Arch. Pathol. Lab. Med. 2014, 138, 1432–1443. [Google Scholar] [CrossRef]
- Veta, M.; van Diest, P.J.; Willems, S.M.; Wang, H.; Madabhushi, A.; Cruz-Roa, A.; Gonzalez, F.; Larsen, A.B.; Vestergaard, J.S.; Dahl, A.B.; et al. Assessment of algorithms for mitosis detection in breast cancer histopathology images. Med. Image Anal. 2015, 20, 237–248. [Google Scholar] [CrossRef]
- Parwani, A.V. Next-generation diagnostic pathology: Use of digital pathology and artificial intelligence tools to augment a pathologist’s diagnostic workflow. J. Pathol. Inform. 2019, 10, 32. [Google Scholar]
- Canini, V.; Eccher, A.; D’amati, G.; Fusco, N.; Maffini, F.; Lepanto, D.; Martini, M.; Cazzaniga, G.; Paliogiannis, P.; Lobrano, R.; et al. Digital Pathology Applications for PD-L1 Scoring in Head and Neck Squamous Cell Carcinoma: A Challenging Series. J. Clin. Med. 2024, 13, 1240. [Google Scholar] [CrossRef]
- Moratin, J.; Mock, A.; Obradovic, S.; Metzger, K.; Flechtenmacher, C.; Zaoui, K.; Fröhling, S.; Jäger, D.; Krauss, J.; Hoffmann, J.; et al. Digital Pathology Scoring of Immunohistochemical Staining Reliably Identifies Prognostic Markers and Anatomical Associations in a Large Cohort of Oral Cancers. Front. Oncol. 2021, 11, 712944. [Google Scholar] [CrossRef]
- Bai, B.; Yang, X.; Li, Y.; Zhang, Y.; Pillar, N.; Ozcan, A. Deep learning-enabled virtual histological staining of biological samples. Light Sci. Appl. 2023, 12, 57. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).