Neurotrophins as Potential Biomarkers for Active Disease and Poor Outcome in Pediatric Acute Lymphoblastic Leukemia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Biological Samples
2.2. Enzyme-Linked Immunosorbent Assay (ELISA) Assay
2.3. Exploratory Overall Survival Analysis
2.4. Primary Cell Culture
2.5. Microarray Data and Gene Expression Analysis
3. Results
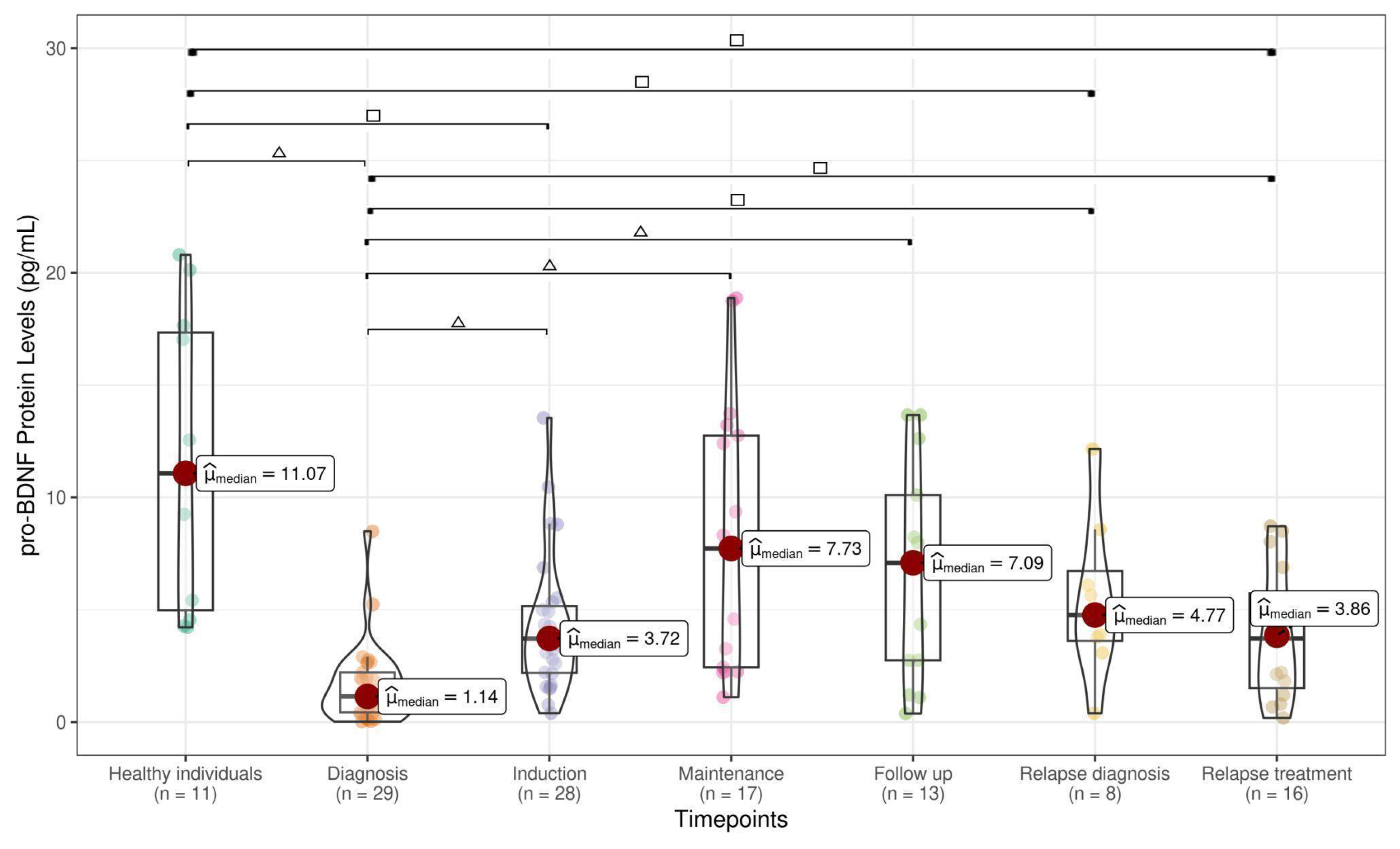
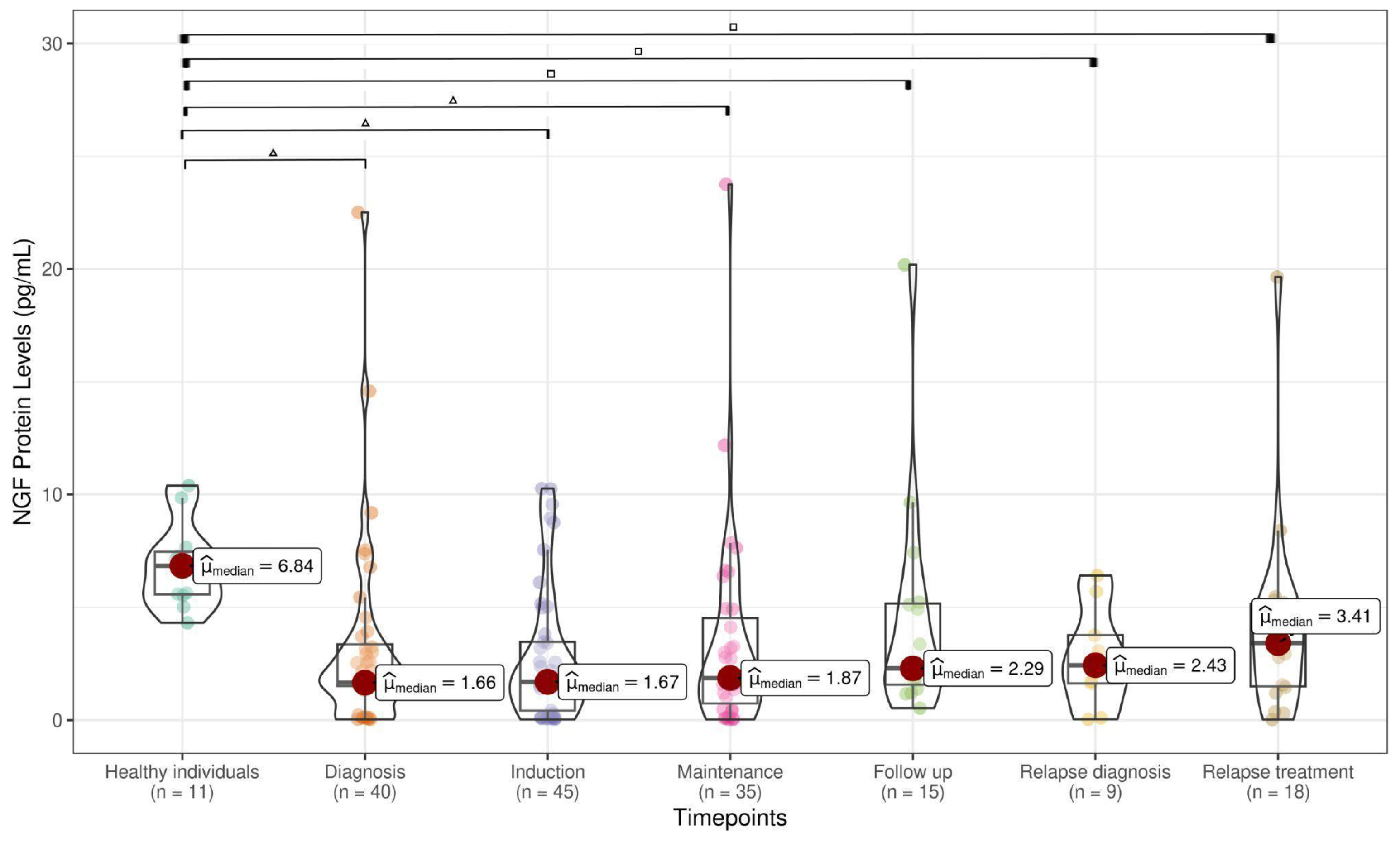
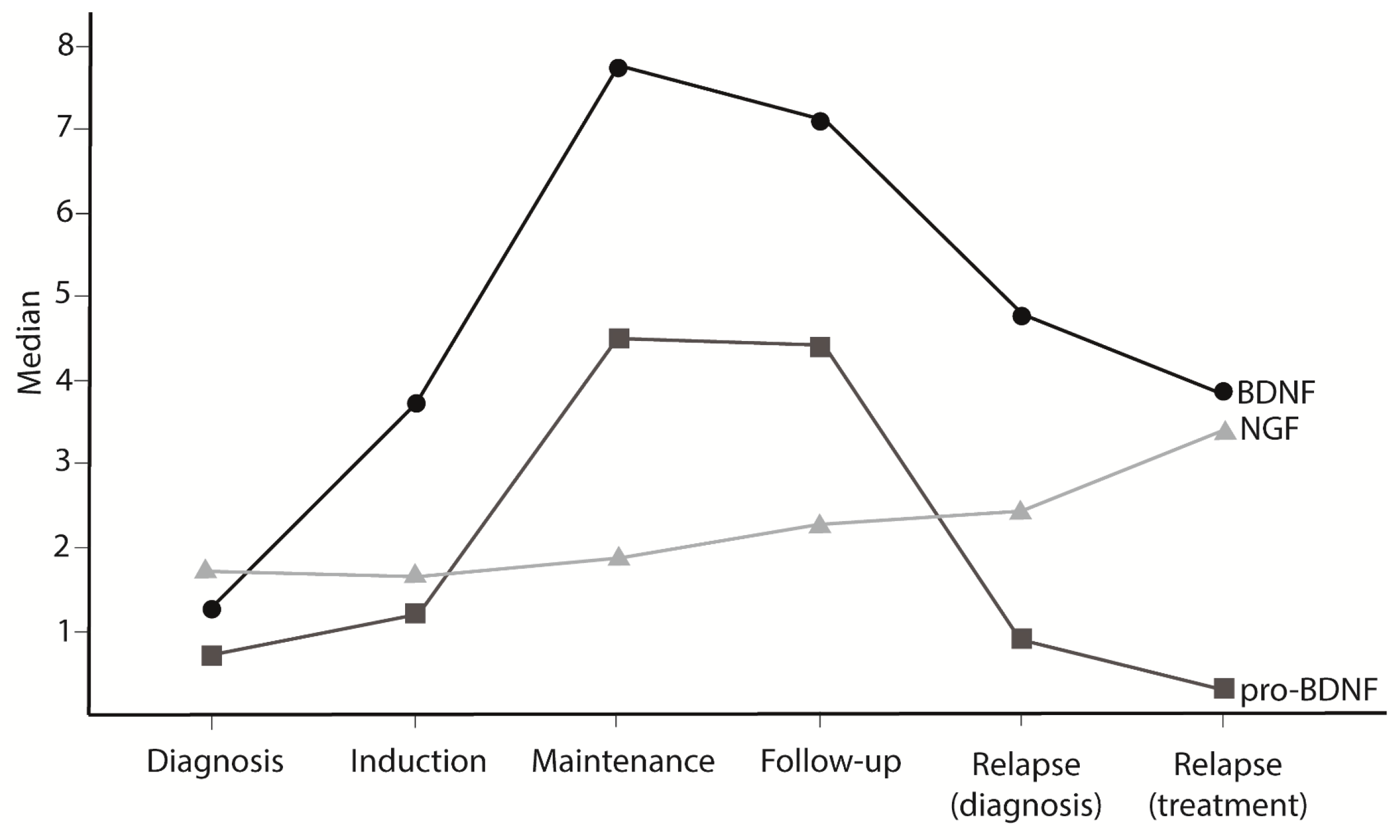
3.1. NT Levels Are Reduced in ALL Patients at Active Phases of the Disease
3.2. BDNF May Reduce Viability of Leukemia Cells
3.3. BDNF and NGF Expression in ALL Gene Datasets
3.4. NT Receptors Expression in ALL Gene Datasets
3.5. Metalloproteinases Gene Expression in ALL Gene Datasets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALL | Acute Lymphoblastic Leukemia |
| BDNF | Brain-Derived Neurotrophic Factor |
| BFM | Berlin–Frankfurt–Münster (treatment protocol) |
| BM | Bone Marrow |
| CNS | Central Nervous System |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EDTA | Ethylenediaminetetraacetic Acid |
| FDR | Benjamini–Hochberg false discovery rate (FDR) |
| GEO | Gene Expression Omnibus |
| HI | Healthy Individuals |
| IQR | Interquartile Range |
| MMP | Matrix Metalloproteinase |
| MSC | Mesenchymal Stromal Cells |
| NGF | Nerve Growth Factor |
| NT | Neurotrophin |
| PB | Peripheral Blood |
| PBS | Phosphate Buffered Saline |
| PLG | Plasmine |
| p75NTR | p75 Neurotrophin Receptor |
| RMA | Robust Microarray Analysis |
| SORT1 | Sortilin-1 |
| T-ALL | T-cell Acute Lymphoblastic Leukemia |
| TrkA | Tropomyosin Receptor Kinase A |
| TrkB | Tropomyosin Receptor Kinase B |
| TrkC | Tropomyosin Receptor Kinase C |
References
- Hillis, J.; O’Dwyer, M.; Gorman, A.M. Neurotrophins and B-cell malignancies. Cell. Mol. Life Sci. 2015, 73, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Vega, J.A.; García-Suárez, O.; Hannestad, J.; Pérez-Pérez, M.; Germanà, A. Neurotrophins and the immune system. J. Anat. 2003, 203, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.O.; Perestrelo, T.; Almeida, R.D. PROneurotrophins and CONSequences. Mol. Neurobiol. 2017, 55, 2934–2951. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.; Ellis, V. Activation of pro-BDNF by the pericellular serine protease plasmin. FEBS Lett. 2008, 582, 907–910. [Google Scholar] [CrossRef]
- Lee, R.; Kermani, P.; Teng, K.K.; Hempstead, B.L. Regulation of cell survival by secreted proneurotrophins. Science 2001, 294, 1945–1948. [Google Scholar] [CrossRef]
- Shi, J. Regulatory networks between neurotrophins and miRNAs in brain diseases and cancers. Acta Pharmacol. Sin. 2015, 36, 149–157. [Google Scholar] [CrossRef]
- Junior, V.S.; Fernandes, G.M.D.M.; Oliveira-Cucolo, J.G.D.; Pavarino, E.C.; Goloni-Bertollo, E.M. Role of Tropomyosin-related kinase B receptor and brain-derived neurotrophic factor in cancer. Cytokine 2020, 136, 155270. [Google Scholar] [CrossRef]
- Griffin, N.; Faulkner, S.; Jobling, P.; Hondermarck, H. Targeting neurotrophin signaling in cancer: The renaissance. Pharmacol. Res. 2018, 135, 12–17. [Google Scholar] [CrossRef]
- Kaplan, J.A. Leukemia in Children. Pediatr. Rev. 2019, 40, 319–331. [Google Scholar] [CrossRef]
- de Sousa, D.W.L.; de Almeida Ferreira, F.V.; Cavalcante Félix, F.H.; de Oliveira Lopes, M.V. Acute lymphoblastic leukemia in children and adolescents: Prognostic factors and analysis of survival. Rev. Bras. Hematol. E Hemoter. 2015, 37, 223–229. [Google Scholar] [CrossRef]
- Pui, C.H.; Robison, L.L.; Look, A.T. Acute lymphoblastic leukemia. Lancet 2008, 371, 1030–1043. [Google Scholar] [CrossRef] [PubMed]
- Curado, M.P.; Pontes, T.; Guerra-Yi, M.E.; de Camargo Cancela, M. Leukemia mortality trends among children, adolescents, and young adults in Latin America. Rev. Panam. Salud Publica 2011, 29, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Portich, J.P.; Gil, M.S.; Dos Santos, R.P.; Goulart, B.K.; Ferreira, M.B.; Loss, J.F.; Gregianin, L.J.; Brunetto, A.L.; Brunetto, A.T.; Roesler, R.; et al. Low brain-derived neurotrophic factor levels are associated with active disease and poor prognosis in childhood acute leukemia. Cancer Biomark. 2016, 17, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Jaboin, J.; Kim, C.J.; Kaplan, D.R.; Thiele, C.J. Brain-derived neurotrophic factor activation of TrkB protects neuroblastoma cells from chemotherapy-induced apoptosis via phosphatidylinositol 3’-kinase pathway. Cancer Res. 2002, 62, 6756–6763. [Google Scholar]
- Eggert, A.; Grotzer, M.A.; Ikegaki, N.; Zhao, H.; Cnaan, A.; Brodeur, G.M.; Evans, A.E. Expression of the neurotrophin receptor TrkB is associated with unfavorable outcome in Wilms’ tumor. J. Clin. Oncol. 2001, 19, 689–696. [Google Scholar] [CrossRef]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and Synaptic Plasticity, Cognitive Function, and Dysfunction. In Neurotrophic Factors Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 223–250. [Google Scholar] [CrossRef]
- Edgar, R. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef]
- Carvalho, B.S.; Irizarry, R.A. A framework for oligonucleotide microarray preprocessing. Bioinformatics 2010, 26, 2363–2367. [Google Scholar] [CrossRef]
- Kauffmann, A.; Gentleman, R.; Huber, W. arrayQualityMetrics—A bioconductor package for quality assessment of microarray data. Bioinformatics 2008, 25, 415–416. [Google Scholar] [CrossRef]
- Bolstad, B.; Irizarry, R.; Astrand, M.; Speed, T. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003, 19, 185–193. [Google Scholar] [CrossRef]
- Irizarry, R.A.; Irizarry, R.A.; Bolstad, B.M.; Collin, F.; Cope, L.M.; Hobbs, B.; Speed, T.P. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003, 31, e15. [Google Scholar] [CrossRef]
- Irizarry, R.A.; Hobbs, B.; Collin, F.; Beazer-Barclay, Y.D.; Antonellis, K.J.; Scherf, U.; Speed, T.P. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003, 4, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Dormady, S.P.; Bashayan, O.; Dougherty, R.; Zhang, X.-M.; Basch, R.S. Immortalized Multipotential Mesenchymal Cells and the Hematopoietic Microenvironment. J. Hematotherapy Stem Cell Res. 2001, 10, 125–140. [Google Scholar] [CrossRef]
- Tessarollo, L. Pleiotropic Functions of Neurotrophins in Development. Cytokine Growth Factor Rev. 1998, 9, 125–137. [Google Scholar] [CrossRef]
- Simone, M.D.; De Santis, S.; Vigneti, E.; Papa, G.; Amadori, S.; Aloe, L. Nerve growth factor: A survey of activity on immune and hematopoietic cells. Hematol. Oncol. 1999, 17, 1–10. [Google Scholar] [CrossRef]
- Aloe, L.; Bracci-Laudiero, L.; Bonini, S.; Manni, L. The expanding role of nerve growth factor: From neurotrophic activity to immunologic diseases. Allergy 1997, 52, 883–894. [Google Scholar] [CrossRef]
- Schenone, A.; Gill, J.S.; Zacharias, D.A.; Windebank, A.J. Expression of high- and low-affinity neurotrophin receptors on human transformed B lymphocytes. J. Neuroimmunol. 1996, 64, 141–149. [Google Scholar] [CrossRef]
- Schuhmann, B.; Dietrich, A.; Sel, S.; Hahn, C.; Klingenspor, M.; Lommatzsch, M.; Gudermann, T.; Braun, A.; Renz, H.; Nockher, W.A. A role for brain-derived neurotrophic factor in B cell development. J. Neuroimmunol. 2005, 163, 15–23. [Google Scholar] [CrossRef]
- Fauchais, A.-L.; Lalloué, F.; Lise, M.-C.; Boumediene, A.; Preud’homme, J.-L.; Vidal, E.; Jauberteau, M.-O. Role of Endogenous Brain-Derived Neurotrophic Factor and Sortilin in B Cell Survival. J. Immunol. 2008, 181, 3027–3038. [Google Scholar] [CrossRef]
- Linker, R.A.; Lee, D.-H.; Flach, A.-C.; Litke, T.; Brandt, J.V.D.; Reichardt, H.M.; Lingner, T.; Bommhardt, U.; Sendtner, M.; Gold, R.; et al. Thymocyte-derived BDNF influences T-cell maturation at the DN3/DN4 transition stage. Eur. J. Immunol. 2015, 45, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, D.; Horowitz, N.A. Brain-derived neurotrophic factor in hematological malignancies: From detrimental to potentially beneficial. Blood Rev. 2021, 51, 100871. [Google Scholar] [CrossRef] [PubMed]
- Talbot, H.; Saada, S.; Barthout, E.; Gallet, P.F.; Gachard, N.; Abraham, J.; Jaccard, A.; Troutaud, D.; Lalloué, F.; Naves, T.; et al. BDNF belongs to the nurse-like cell secretome and supports survival of B chronic lymphocytic leukemia cells. Sci. Rep. 2020, 10, 12572. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Maz. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Pius-Sadowska, E.; Machaliński, B. BDNF—A key player in cardiovascular system. J. Mol. Cell. Cardiol. 2017, 110, 54–60. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, W.; Deng, Q.; Zhang, X.; Zhang, J. Protective effects of BDNF overexpression bone marrow stromal cell transplantation in rat models of traumatic brain injury. J. Mol. Neurosci. 2013, 49, 409–416. [Google Scholar] [CrossRef]
- Sharma, G.P.; Frei, A.C.; Narayanan, J.; Gasperetti, T.; Veley, D.; Amjad, A.; Albano, K.; Fish, B.L.; Himburg, H.A. Brain-derived neurotrophic factor promotes immune reconstitution following radiation injury via activation of bone marrow mesenchymal stem cells. PLoS ONE 2021, 16, e0259042. [Google Scholar] [CrossRef]
- Weishaupt, N.; Blesch, A.; Fouad, K. BDNF: The career of a multifaceted neurotrophin in spinal cord injury. Exp. Neurol. 2012, 238, 254–264. [Google Scholar] [CrossRef]
- Haba, M.Ș.C.; Tudorancea, I.; Mihai, C.T.; Onofrei, V.; Costache, I.I.; Petriș, A.O.; Șorodoc, L. Brain-Derived Neurotrophic Factor Expression in Patients with Acute Pulmonary Embolism Compared to the General Population: Diagnostic and Prognostic Implications. J. Clin. Med. 2022, 11, 4948. [Google Scholar] [CrossRef]
- Karantali, E.; Kazis, D.; Papavasileiou, V.; Prevezianou, A.; Chatzikonstantinou, S.; Petridis, F.; McKenna, J.; Luca, A.C.; Trus, C.; Ciobica, A.; et al. Serum BDNF Levels in Acute Stroke: A Systematic Review and Meta-Analysis. Medicina 2021, 57, 297. [Google Scholar] [CrossRef]
- Peng, S.; Wuu, J.; Mufson, E.J.; Fahnestock, M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J. Neurochem. 2005, 93, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Szudy-Szczyrek, A.; Mlak, R.; Bury-Kamińska, M.; Mielnik, M.; Podgajna, M.; Kuśmierczuk, K.; Mazurek, M.; Homa-Mlak, I.; Szczyrek, M.; Krawczyk, J.; et al. Serum brain-derived neurotrophic factor (BDNF) concentration predicts polyneuropathy and overall survival in multiple myeloma patients. Br. J. Haematol. 2020, 191, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, D.; Herishanu, Y.; Shapiro, M.; Brandshaft, Y.; Suriu, C.; Akria, L.; Braester, A. Elevated serum BDNF levels are associated with favorable outcome in CLL patients: Possible link to CXCR4 downregulation. Exp. Hematol. 2018, 63, 17–21.e1. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, S.; Praloran, V.; Smith, C.; Macgrogan, D.; Ip, N.; Yancopoulos, G.; Brachet, P.; Pouplard, A.; Gascan, H. Expression and functionality of the trkA proto-oncogene product/NGF receptor in undifferentiated hematopoietic cells. Blood 1994, 83, 1479–1485. [Google Scholar] [CrossRef]
- Labouyrie, E.; Dubus, P.; Groppi, A.; Mahon, F.X.; Ferrer, J.; Parrens, M.; Reiffers, J.; de Mascarel, A.; Merlio, J.P. Expression of Neurotrophins and their Receptors in Human Bone Marrow. Am. J. Pathol. 1999, 154, 405–415. [Google Scholar] [CrossRef]
- Nykjaer, A.; Lee, R.; Teng, K.K.; Jansen, P.; Madsen, P.; Nielsen, M.S.; Jacobsen, C.; Kliemannel, M.; Schwarz, E.; Willnow, T.E.; et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature 2004, 427, 843–848. [Google Scholar] [CrossRef]
- Miknyoczki, S.J.; Lang, D.; Huang, L.; Klein-Szanto, A.J.; Dionne, C.A.; Ruggeri, B.A. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: Expression patterns and effects on in vitro invasive behavior. Int. J. Cancer 1999, 81, 417–427. [Google Scholar] [CrossRef]
- Ricci, A.; Greco, S.; Mariotta, S.; Felici, L.; Bronzetti, E.; Cavazzana, A.; Cardillo, G.; Amenta, F.; Bisetti, A.; Barbolini, G. Neurotrophins and neurotrophin receptors in human lung cancer. Am. J. Respir. Cell Mol. Biol. 2001, 25, 439–446. [Google Scholar] [CrossRef]
- Krygier, S.; Djakiew, D. Neurotrophin receptor p75(NTR) suppresses growth and nerve growth factor-mediated metastasis of human prostate cancer cells. Int. J. Cancer 2002, 98, 1–7. [Google Scholar] [CrossRef]
- Troeger, A.; Gudowius, S.; Escherich, G.; den Boer, M.L.; Glouchkova, L.; Ackermann, B.; Meisel, R.; Laws, H.J.; Groeger, M.; Wessalowski, R.; et al. High nerve growth factor receptor (p75NTR) expression is a favorable prognostic factor in pediatric B cell precursor-acute lymphoblastic leukemia. Br. J. Haematol. 2007, 139, 450–457. [Google Scholar] [CrossRef]
- Farahi, L.; Ghaemimanesh, F.; Milani, S.; Razavi, S.M.; Akhondi, M.M.; Rabbani, H. Sortilin as a Novel Diagnostic and Therapeutic Biomarker in Chronic Lymphocytic Leukemia. Avicenna J. Med. Biotechnol. 2019, 11, 270–276. [Google Scholar] [PubMed] [PubMed Central]
- Saada, S.; Marget, P.; Fauchais, A.-L.; Lise, M.-C.; Chemin, G.; Sindou, P.; Martel, C.; Delpy, L.; Vidal, E.; Jaccard, A.; et al. Differential Expression of Neurotensin and Specific Receptors, NTSR1 and NTSR2, in Normal and Malignant Human B Lymphocytes. J. Immunol. 2012, 189, 5293–5303. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-H.; Su, S.-C.; Lin, C.-W.; Chao, Y.-H.; Yang, W.-E.; Yang, S.-F. Pathological and therapeutic aspects of matrix metalloproteinases: Implications in childhood leukemia. Cancer Metastasis Rev. 2019, 38, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Khalil, M.; Abdellateif, M.S.; Ebeid, E.; Madney, Y.; Kandeel, E.Z. Role of matrix metalloproteinase MMP-2, MMP-9 and tissue inhibitor of metalloproteinase (TIMP-1) in the clinical progression of pediatric acute lymphoblastic leukemia. Hematology 2021, 26, 758–768. [Google Scholar] [CrossRef]
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Mol. Cancer Ther. 2018, 17, 1147–1155. [Google Scholar] [CrossRef]
- Schneider, P.; Costa, O.; Legrand, E.; Bigot, D.; Lecleire, S.; Grassi, V.; Vannier, J.P.; Vasse, M. In vitro secretion of matrix metalloprotease 9 is a prognostic marker in childhood acute lymphoblastic leukemia. Leuk. Res. 2010, 34, 24–31. [Google Scholar] [CrossRef]
- Pan, Y.X.; Yang, L.; Wen, S.P.; Liu, X.J.; Luo, J.M. Expression and clinical significance of MMP-2 and MMP-9 in B acute lymphoblastic leukemia. J. Exp. Hematol. 2014, 22, 640–643. [Google Scholar] [CrossRef]
- Kuittinen, O.; Savolainen, E.R.; Koistinen, P.; Möttönen, M.; Turpeenniemi-Hujanen, T. MMP-2 and MMP-9 expression in adult and childhood acute lymphatic leukemia (ALL). Leuk. Res. 2001, 25, 125–131. [Google Scholar] [CrossRef]
- Li, H.; Yang, F.; Chai, L.; Zhang, L.; Li, S.; Xu, Z.; Kong, L. CCAAT/Enhancer Binding Protein β-Mediated MMP3 Upregulation Promotes Esophageal Squamous Cell Cancer Invasion In Vitro and Is Associated with Metastasis in Human Patients. Genet. Test. Mol. Biomark. 2019, 23, 304–309. [Google Scholar] [CrossRef]
- Frieling, J.S.; Li, T.; Tauro, M.; Lynch, C.C. Prostate cancer-derived MMP-3 controls intrinsic cell growth and extrinsic angiogenesis. Neoplasia 2020, 22, 511–521. [Google Scholar] [CrossRef]
- Vosseler, S.; Lederle, W.; Airola, K.; Obermueller, E.; Fusenig, N.E.; Mueller, M.M. Distinct progression-associated expression of tumor and stromal MMPs in HaCaT skin SCCs correlates with onset of invasion. Int. J. Cancer 2009, 125, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Didiasova, M.; Wujak, L.; Wygrecka, M.; Zakrzewicz, D. From Plasminogen to Plasmin: Role of Plasminogen Receptors in Human Cancer. Int. J. Mol. Sci. 2014, 15, 21229–21252. [Google Scholar] [CrossRef] [PubMed]
- Jaaks, P.; Bernasconi, M. The proprotein convertase furin in tumor progression. Int. J. Cancer 2017, 141, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-Y.; Hu, Y.; Wang, H.-F.; He, W.-J.; Wang, Y.-D.; Wu, T. Brain-derived neurotrophic factor inducing angiogenesis through modulation of matrix-degrading proteases. Chin. Med. J. 2006, 119, 589–595. [Google Scholar] [CrossRef]
- Gutiérrez-Fernández, A.; Parmer, R.J.; Miles, L.A. Plasminogen gene expression is regulated by nerve growth factor. J. Thromb. Haemost. 2007, 5, 1715–1725. [Google Scholar] [CrossRef]


| Characteristics of Patients (n = 103) | Total (%) |
|---|---|
| Gender | |
| Male | 67 (65) |
| Female | 36 (35) |
| Age at diagnosis (years) | |
| Minimum | 0.5 |
| Maximum | 16 |
| Median | 6 |
| ALL Subtype | |
| B-ALL * | 87 (84) |
| T-ALL ** | 16 (16) |
| BFM Risk group | |
| High | 59 (57) |
| Standard | 24 (23) |
| Intermediary | 20 (20) |
| Outcome | |
| In treatment/remission | 78 (76) |
| Death | 25 (24) |
| Remission (Day 33 of initial treatment) | |
| Yes | 70 (68) |
| No | 21 (20) |
| Remission after relapse treatment | 5 (5) |
| Refractory | 7 (7) |
| Relapse (Early or late) | |
| No | 76 (74) |
| Yes | 27 (26) |
| CNS infiltration at diagnosis | |
| No | 91 (88) |
| Yes | 12 (12) |
| Local of relapse | |
| Bone marrow | 13 (42) |
| Testicle | 5 (16) |
| CNS | 5 (16) |
| CNS and bone marrow | 3 (10) |
| Bone marrow and testicle | 2 (7) |
| CNS, bone marrow and testicle | 1 (3) |
| Eye | 1 (3) |
| Missing | 1 (3) |
| GSE | Number of Samples | Leukemia Type | Sample Type | Compared Groups |
|---|---|---|---|---|
| GSE87070 | 633 | B-ALL, T-ALL | Bone marrow | B-ALL (n = 563)/T-ALL (n = 70) At diagnosis |
| GSE28460 | 92 | B-ALL | Bone marrow | Diagnosis (n = 44)/Recurrence (n = 48) |
| GSE26713 | 112 | T-ALL | Bone marrow | Diagnosis (n = 106)/HI (n = 6) |
| GSE47051 | 97 | B-ALL, T-ALL | Bone marrow and peripheral blood | Patient’s diagnosis bone marrow samples (n = 82)/Patient’s diagnosis peripheral blood (n = 15) |
| GSE7440 | 80 | B-ALL | Bone marrow | Early response to therapy (n = 41)/Slow response to therapy (n = 39) |
| GSE101425 | 51 | B-ALL | Mesenchymal Stromal Cells (MSCs) | Patients MSCs (n = 35)/ Healthy individuals MSCs (n = 16) |
| Genes | LogFC | p-Value | Status |
|---|---|---|---|
| B-ALL X T-ALL | |||
| BDNF | −0.171 | p < 0.001 | Overexpressed in T-ALL |
| FURIN | 0.148 | 0.009 | Overexpressed in B-ALL |
| MMP-2 | −0.114 | 0.008 | Overexpressed in T-ALL |
| p75NTR | 0.626 | p < 0.001 | Overexpressed in B-ALL |
| SORT1 | −0.487 | p < 0.001 | Overexpressed in T-ALL |
| PLG | 0.088 | 0.018 | Overexpressed in B-ALL |
| Tumor Tissue Diagnosis X Health Tissue | |||
| FURIN | −0.666 | 0.001 | Overexpressed in Healthy Tissue |
| SORT1 | −1.129 | p < 0.001 | Overexpressed in Healthy Tissue |
| NGF | −0.274 | 0.011 | Overexpressed in Healthy Tissue |
| MMP-9 | −4.902 | 0.086 | Overexpressed in Healthy Tissue |
| Diagnosis X Relapse | |||
| MMP-9 | −0.819 | 0.003 | Overexpressed in Relapse |
| Bone Marrow X Peripheral Blood | |||
| TrkB | 0.213 | 0.002 | Overexpressed in Bone Marrow |
| Health MSC X Tumor MSC | |||
| MMP3 | 1.782 | p < 0.001 | Overexpressed in Healthy Bone marrow mesenchymal stromal cells (MSC) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Andrade, K.P.; Michaelsen, G.L.; Dutra, L.F.; Marques, R.F.; Benincasa, D.E.R.; Portich, J.P.; Loss, J.F.; Gregianin, L.J.; Brunetto, A.T.; Sinigaglia, M.; et al. Neurotrophins as Potential Biomarkers for Active Disease and Poor Outcome in Pediatric Acute Lymphoblastic Leukemia. Cancers 2025, 17, 1623. https://doi.org/10.3390/cancers17101623
de Andrade KP, Michaelsen GL, Dutra LF, Marques RF, Benincasa DER, Portich JP, Loss JF, Gregianin LJ, Brunetto AT, Sinigaglia M, et al. Neurotrophins as Potential Biomarkers for Active Disease and Poor Outcome in Pediatric Acute Lymphoblastic Leukemia. Cancers. 2025; 17(10):1623. https://doi.org/10.3390/cancers17101623
Chicago/Turabian Stylede Andrade, Karine Pereira, Gustavo Lovatto Michaelsen, Lívia Fratini Dutra, Rebeca Ferreira Marques, Daniela Elaine Roth Benincasa, Júlia Plentz Portich, Jiseh Fagundes Loss, Lauro José Gregianin, André Tesainer Brunetto, Marialva Sinigaglia, and et al. 2025. "Neurotrophins as Potential Biomarkers for Active Disease and Poor Outcome in Pediatric Acute Lymphoblastic Leukemia" Cancers 17, no. 10: 1623. https://doi.org/10.3390/cancers17101623
APA Stylede Andrade, K. P., Michaelsen, G. L., Dutra, L. F., Marques, R. F., Benincasa, D. E. R., Portich, J. P., Loss, J. F., Gregianin, L. J., Brunetto, A. T., Sinigaglia, M., Roesler, R., da Cunha Jaeger, M., Land, M., & de Farias, C. B. (2025). Neurotrophins as Potential Biomarkers for Active Disease and Poor Outcome in Pediatric Acute Lymphoblastic Leukemia. Cancers, 17(10), 1623. https://doi.org/10.3390/cancers17101623







