Real-World Management of High-Risk Prostate Cancer Post-Radical Prostatectomy: Insights from a Regional Quality Collaborative
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Study Outcomes
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Borregales, L.D.; DeMeo, G.; Gu, X.; Cheng, E.; Dudley, V.; Schaeffer, E.M.; Nagar, H.; Carlsson, S.; Vickers, A.; Hu, J.C. Grade Migration of Prostate Cancer in the United States During the Last Decade. JNCI J. Natl. Cancer Inst. 2022, 114, 1012–1019. [Google Scholar] [CrossRef]
- Stephenson, A.J.; Scardino, P.T.; Eastham, J.A.; Bianco, F.J.; Dotan, Z.A.; Fearn, P.A.; Kattan, M.W. Preoperative Nomogram Predicting the 10-Year Probability of Prostate Cancer Recurrence After Radical Prostatectomy. JNCI J. Natl. Cancer Inst. 2006, 98, 715–717. [Google Scholar] [CrossRef]
- Moschini, M.; Sharma, V.; Zattoni, F.; Boorjian, S.A.; Frank, I.; Gettman, M.T.; Thompson, R.H.; Tollefson, M.K.; Kwon, E.D.; Karnes, R.J. Risk Stratification of pN+ Prostate Cancer after Radical Prostatectomy from a Large Single Institutional Series with Long-Term Followup. J. Urol. 2016, 195, 1773–1778. [Google Scholar] [CrossRef]
- Kneebone, A.; Fraser-Browne, C.; Duchesne, G.M.; Fisher, R.; Frydenberg, M.; Herschtal, A.; Williams, S.G.; Brown, C.; Delprado, W.; Haworth, A.; et al. Adjuvant radiotherapy versus early salvage radiotherapy following radical prostatectomy (TROG 08.03/ANZUP RAVES): A randomised, controlled, phase 3, non-inferiority trial. Lancet Oncol. 2020, 21, 1331–1340. [Google Scholar] [CrossRef]
- Sargos, P.; Chabaud, S.; Latorzeff, I.; Magné, N.; Benyoucef, A.; Supiot, S.; Pasquier, D.; Abdiche, M.S.; Gilliot, O.; Graff-Cailleaud, P.; et al. Adjuvant radiotherapy versus early salvage radiotherapy plus short-term androgen deprivation therapy in men with localised prostate cancer after radical prostatectomy (GETUG-AFU 17): A randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1341–1352. [Google Scholar] [CrossRef]
- Parker, C.C.; Clarke, N.W.; Cook, A.D.; Kynaston, H.G.; Petersen, P.M.; Catton, C.; Cross, W.; Logue, J.; Parulekar, W.; Payne, H.; et al. Timing of radiotherapy after radical prostatectomy (RADICALS-RT): A randomised, controlled phase 3 trial. Lancet 2020, 396, 1413–1421. [Google Scholar] [CrossRef]
- Vale, C.L.; Fisher, D.; Kneebone, A.; Parker, C.; Pearse, M.; Richaud, P.; Sargos, P.; Sydes, M.R.; Brawley, C.; Brihoum, M.; et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: A prospectively planned systematic review and meta-analysis of aggregate data. Lancet 2020, 396, 1422–1431. [Google Scholar] [CrossRef]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part II: Principles of Active Surveillance, Principles of Surgery, and Follow-Up. J. Urol. 2022, 208, 19–25. [Google Scholar] [CrossRef]
- Cornford, P.; Bergh, R.C.v.D.; Briers, E.; Broeck, T.V.D.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer—2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef]
- Morgan, T.M.; Boorjian, S.A.; Buyyounouski, M.K.; Chapin, B.F.; Chen, D.Y.T.; Cheng, H.H.; Chou, R.; Jacene, H.A.; Kamran, S.C.; Kim, S.K.; et al. Salvage Therapy for Prostate Cancer: AUA/ASTRO/SUO Guideline Part I: Introduction and Treatment Decision-Making at the Time of Suspected Biochemical Recurrence after Radical Prostatectomy. J. Urol. 2024, 211, 509–517. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer. EAU Guidelines. 2024. Available online: https://uroweb.org/guidelines/prostate-cancer (accessed on 30 December 2024).
- Moschini, M.; Sharma, V.; Zattoni, F.; Quevedo, J.F.; Davis, B.J.; Kwon, E.; Karnes, R.J. Natural History of Clinical Recurrence Patterns of Lymph Node–Positive Prostate Cancer After Radical Prostatectomy. Eur. Urol. 2016, 69, 135–142. [Google Scholar] [CrossRef]
- Boorjian, S.A.; Thompson, R.H.; Siddiqui, S.; Bagniewski, S.; Bergstralh, E.J.; Karnes, R.J.; Frank, I.; Blute, M.L. Long-Term Outcome After Radical Prostatectomy for Patients with Lymph Node Positive Prostate Cancer in the Prostate Specific Antigen Era. J. Urol. 2007, 178, 864–871. [Google Scholar] [CrossRef]
- Touijer, K.A.; Mazzola, C.R.; Sjoberg, D.D.; Scardino, P.T.; Eastham, J.A. Long-term Outcomes of Patients with Lymph Node Metastasis Treated with Radical Prostatectomy Without Adjuvant Androgen-deprivation Therapy. Eur. Urol. 2014, 65, 20–25. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Su, M.Z.; Kneebone, A.B.; Woo, H.H. Adjuvant versus salvage radiotherapy following radical prostatectomy: Do the AUA/ASTRO guidelines have all the answers? Expert Rev. Anticancer Ther. 2014, 14, 1265–1270. [Google Scholar] [CrossRef]
- Sineshaw, H.M.; Gray, P.J.; Efstathiou, J.A.; Jemal, A. Declining Use of Radiotherapy for Adverse Features After Radical Prostatectomy: Results From the National Cancer Data Base. Eur. Urol. 2015, 68, 768–774. [Google Scholar] [CrossRef]
- Triner, D.; Daignault-Newton, S.; Singhal, U.; Sessine, M.; Dess, R.T.; Caram, M.E.V.; Borza, T.; Ginsburg, K.B.; Lane, B.R.; Morgan, T.M. Variation in management of lymph node positive prostate cancer after radical prostatectomy within a statewide quality improvement consortium. Urol. Oncol. 2024, 42, e1–e220. [Google Scholar] [CrossRef]
- Pooli, A.; Salmasi, A.; Faiena, I.; Lenis, A.T.; Johnson, D.C.; Lebacle, C.; Drakaki, A.; Gollapudi, K.; Blumberg, J.; Pantuck, A.J.; et al. Variation in surgical treatment patterns for patients with prostate cancer in the United States: Do patients in academic hospitals fare better? Urol. Oncol. 2019, 37, 63–70. [Google Scholar] [CrossRef]
- Jackson, W.C.; Johnson, S.B.; Li, D.; Foster, C.; Foster, B.; Song, Y.; Schipper, M.; Shilkrut, M.; Sandler, H.M.; Morgan, T.M.; et al. A prostate-specific antigen doubling time of <6 months is prognostic for metastasis and prostate cancer-specific death for patients receiving salvage radiation therapy post radical prostatectomy. Radiat. Oncol. 2013, 8, 170. [Google Scholar] [CrossRef]
- Roberts, S.G.; Blute, M.L.; Bergstralh, E.J.; Slezak, J.M.; Zincke, H. PSA doubling time as a predictor of clinical progression after biochemical failure following radical prostatectomy for prostate cancer. Mayo Clin. Proc. 2001, 76, 576–581. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Moul, J.W.; Carroll, P.R.; Sun, L.; Lubeck, D.; Chen, M.H. Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J. Natl. Cancer Inst. 2003, 95, 1376–1383. [Google Scholar] [CrossRef]
- Stephenson, A.J.; Scardino, P.T.; Kattan, M.W.; Pisansky, T.M.; Slawin, K.M.; Klein, E.A.; Anscher, M.S.; Michalski, J.M.; Sandler, H.M.; Lin, D.W.; et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J. Clin. Oncol. 2007, 25, 2035–2041. [Google Scholar] [CrossRef]
- Messing, E.M.; Manola, J.; Yao, J.; Kiernan, M.; Crawford, D.; Wilding, G.; di’SantAgnese, P.A.; Trump, D. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006, 7, 472–479. [Google Scholar] [CrossRef]
- Ballas, L.K.; Reddy, C.A.; Han, H.R.; Makar, J.B.; Mian, O.; Broughman, J.; de Bustamante, C.; Eggener, S.; Liauw, S.L.; Abramowitz, M.; et al. Patterns of recurrence following radiation and ADT for pathologic lymph node positive prostate cancer: A multi-institutional study. Pract. Radiat. Oncol. 2024, in press. [Google Scholar] [CrossRef]
- Marra, G.; Lesma, F.; Montefusco, G.; Filippini, C.; Olivier, J.; Affentranger, A.; Grogg, J.B.; Hermanns, T.; Afferi, L.; Fankhauser, C.D.; et al. Observation with or Without Subsequent Salvage Therapy for Pathologically Node-positive Prostate Cancer with Negative Conventional Imaging: Results From a Large Multicenter Cohort. Eur. Urol. Open Sci. 2024, 68, 32–39. [Google Scholar] [CrossRef]
- Aguiar, J.A.; Li, E.V.; Ho, A.; Bennett, R.; Li, Y.; Neill, C.; Schaeffer, E.M.; Patel, H.D.; Ross, A.E. Ultrasensitive PSA: Rethinking post-surgical management for node positive prostate cancer. Front. Oncol. 2024, 14, 1363009. [Google Scholar] [CrossRef]
- Carlsson, S.V.; Tafe, L.J.; Chade, D.C.; Sjoberg, D.D.; Passoni, N.; Shariat, S.F.; Eastham, J.; Scardino, P.T.; Fine, S.W.; Touijer, K.A. Pathological features of lymph node metastasis for predicting biochemical recurrence after radical prostatectomy for prostate cancer. J. Urol. 2013, 189, 1314–1318. [Google Scholar] [CrossRef]
- Briganti, A.; Karnes, J.R.; Da Pozzo, L.F.; Cozzarini, C.; Gallina, A.; Suardi, N.; Bianchi, M.; Freschi, M.; Doglioni, C.; Fazio, F.; et al. Two positive nodes represent a significant cut-off value for cancer specific survival in patients with node positive prostate cancer. A new proposal based on a two-institution experience on 703 consecutive N+ patients treated with radical prostatectomy, extended pelvic lymph node dissection and adjuvant therapy. Eur. Urol. 2009, 55, 261–270. [Google Scholar] [CrossRef]
- Pozdnyakov, A.; Kulanthaivelu, R.; Bauman, G.; Ortega, C.; Veit-Haibach, P.; Metser, U. The impact of PSMA PET on the treatment and outcomes of men with biochemical recurrence of prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic. Dis. 2023, 2, 240–248. [Google Scholar] [CrossRef]
- Leapman, M.S.; Ho, J.; Liu, Y.; Filson, C.; Zhao, X.; Hakansson, A.; Proudfoot, J.A.; Davicioni, E.; Martin, D.T.; An, Y.; et al. Association Between the Decipher Genomic Classifier and Prostate Cancer Outcome in the Real-world Setting. Eur. Urol. Oncol. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total (n = 605) | Adjuvant (n = 230) | Salvage (n = 375) | p Value |
|---|---|---|---|---|
| Age Range (years) | 0.96 | |||
| 49 or under | 6 (1.0%) | 3 (1.3%) | 3 (0.8%) | |
| 50–59 | 45 (7.4%) | 17 (7.4%) | 28 (7.5%) | |
| 60–69 | 265 (43.8%) | 101 (43.9%) | 164 (43.7%) | |
| 70–79 | 259 (42.8%) | 99 (43.0%) | 160 (42.7%) | |
| 80–89 | 30 (5.0%) | 10 (4.3%) | 20 (5.3%) | |
| Race | 0.14 | |||
| African American | 117 (19.3%) | 51 (22.2%) | 66 (17.6%) | |
| Asian | 12 (2.0%) | 7 (3.0%) | 5 (1.3%) | |
| Caucasian | 458 (75.7%) | 163 (70.9%) | 295 (78.7%) | |
| Hawaiian/Pacific Islander | 1 (0.2%) | 1 (0.4%) | 0 (0%) | |
| Other/Unknown/Refused | 17 (2.8%) | 8 (3.5%) | 9 (2.4%) | |
| Ethnicity | 0.12 | |||
| Non-Hispanic | 586 (96.9%) | 219 (95.2%) | 367 (97.9%) | |
| Hispanic | 14 (2.3%) | 9 (3.9%) | 5 (1.3%) | |
| Unknown/Refused | 5 (0.8%) | 2 (0.9%) | 3 (0.8%) | |
| Family History of CaP | 0.34 | |||
| 1st degree | 121 (20.0%) | 46 (20.0%) | 75 (20.0%) | |
| 2nd degree | 37 (6.1%) | 16 (7.0%) | 21 (5.6%) | |
| Both | 15 (2.5%) | 4 (1.7%) | 11 (2.9%) | |
| Positive, relation unknown | 2 (0.3%) | 0 (0%) | 2 (0.5%) | |
| Unknown | 43 (7.1%) | 11 (4.8%) | 32 (8.5%) | |
| None | 387 (64.0%) | 153 (66.5%) | 234 (62.4%) | |
| Insurance | <0.001 | |||
| Private | 159 (26.3%) | 81 (35.2%) | 78 (20.8%) | |
| Medicare/Medicaid | 149 (24.6%) | 66 (28.7%) | 83 (22.1%) | |
| Tricare/VA | 8 (1.3%) | 1 (0.4%) | 7 (1.9%) | |
| Self-pay | 5 (0.8%) | 1 (0.4%) | 4 (1.1%) | |
| Other | 96 (15.9%) | 12 (5.2%) | 84 (22.4%) | |
| Unknown | 188 (31.1%) | 69 (30.0%) | 119 (31.7%) | |
| Marital Status | 0.31 | |||
| Single | 37 (6.1%) | 19 (8.3%) | 18 (4.8%) | |
| Married/Partnered | 285 (47.1%) | 110 (47.8%) | 175 (46.7%) | |
| Separated/Divorced | 24 (4.0%) | 7 (3.0%) | 17 (4.5%) | |
| Widowed | 10 (1.7%) | 5 (2.2%) | 5 (1.3%) | |
| Unknown | 249 (41.2%) | 89 (38.7%) | 160 (42.7%) | |
| Comorbidities | ||||
| Cerebrovascular Disease | 31 (5.1%) | 15 (6.5%) | 16 (4.3%) | 0.22 |
| Chronic Pulmonary Disease | 30 (5.0%) | 10 (4.3%) | 20 (5.3%) | 0.59 |
| Diabetes without Organ Damage | 76 (12.6%) | 30 (13.0%) | 46 (12.3%) | 0.78 |
| Diabetes with Organ Damage | 7 (1.2%) | 2 (0.9%) | 5 (1.3%) | 0.60 |
| Myocardial Infarction | 18 (3.0%) | 3 (1.3%) | 15 (4.0%) | 0.06 |
| Peripheral Vascular Disease | 10 (1.7%) | 5 (2.2%) | 5 (1.3%) | 0.43 |
| Facility | <0.001 | |||
| A | 183 (30.2%) | 108 (47.0%) | 75 (20.0%) | |
| B | 12 (2.0%) | 9 (3.9%) | 3 (0.8%) | |
| C | 225 (37.2%) | 26 (11.3%) | 199 (53.1%) | |
| D | 29 (4.8%) | 21 (9.1%) | 8 (2.1%) | |
| E | 4 (0.7%) | 3 (1.3%) | 1 (0.3%) | |
| F | 11 (1.8%) | 8 (3.5%) | 3 (0.8%) | |
| G | 34 (5.6%) | 21 (9.1%) | 13 (3.5%) | |
| H | 40 (6.6%) | 14 (6.1%) | 26 (6.9%) | |
| I | 66 (10.9%) | 19 (8.3%) | 47 (12.5%) | |
| J | 1 (0.2%) | 1 (0.4%) | 0 (0.0%) |
| Variable | Total (n = 605) | Adjuvant (n = 230) | Salvage (n = 375) | p Value |
|---|---|---|---|---|
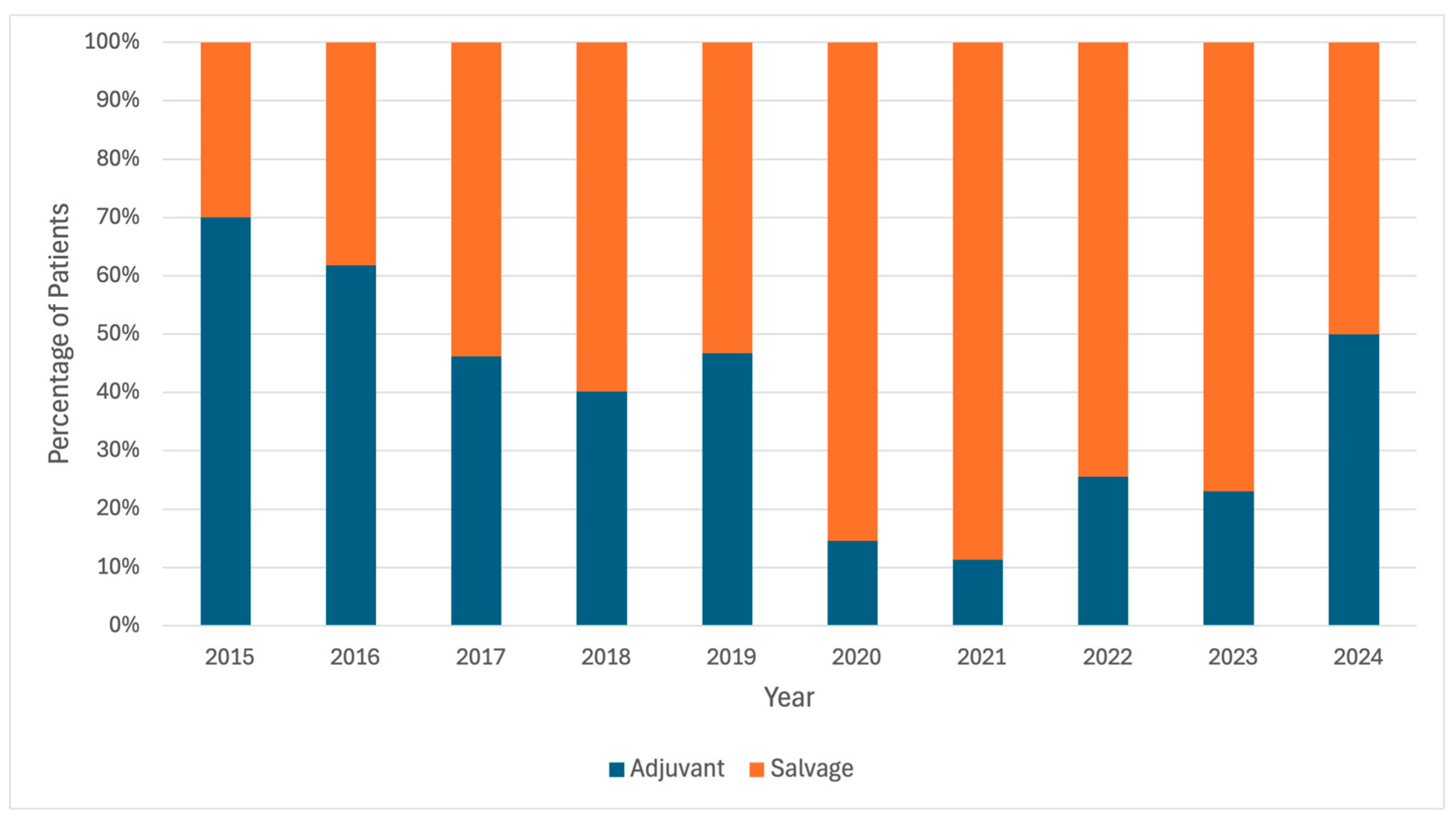
| Year of RP | 0.03 | |||
| 2015 | 57 (9.4%) | 25 (10.9%) | 32 (8.5%) | |
| 2016 | 113 (18.7%) | 44 (19.1%) | 69 (18.4%) | |
| 2017 | 165 (27.3%) | 57 (24.8%) | 108 (28.8%) | |
| 2018 | 106 (17.5%) | 51 (22.2%) | 55 (14.7%) | |
| 2019 | 78 (12.9%) | 27 (11.7%) | 51 (13.6%) | |
| 2020 | 41 (6.8%) | 6 (2.6%) | 35 (9.3%) | |
| 2021 | 28 (4.6%) | 12 (5.2%) | 16 (4.3%) | |
| 2022 | 15 (2.5%) | 7 (3.0%) | 8 (2.1%) | |
| 2023 | 2 (0.3%) | 1 (0.4%) | 1 (0.3%) | |
| Year of RP (pre/post-2020) | 0.11 | |||
| <2020 | 519 (85.8%) | 204 (88.7%) | 315 (84.0%) | |
| ≥2020 | 86 (14.2%) | 26 (11.3%) | 60 (16.0%) | |
| pT | <0.001 | |||
| T2 | 156 (25.8%) | 36 (15.7%) | 120 (32.0%) | |
| T3a | 196 (32.4%) | 83 (36.1%) | 113 (30.1%) | |
| T3b | 251 (41.5%) | 111 (48.3%) | 140 (37.3%) | |
| T4 | 2 (0.3%) | 0 (0%) | 2 (0.5%) | |
| pN | 0.003 | |||
| N0 | 499 (82.5%) | 176 (76.5%) | 323 (86.1%) | |
| N1 | 106 (17.5%) | 54 (23.5%) | 52 (13.9%) | |
| Gleason Score | 0.01 | |||
| 6 | 3 (0.5%) | 2 (0.9%) | 1 (0.3%) | |
| 7 | 291 (49.3%) | 95 (42.2%) | 196 (53.7%) | |
| 8 | 103 (17.5%) | 37 (16.4%) | 66 (18.1%) | |
| 9 | 187 (31.7%) | 87 (38.7%) | 100 (27.4%) | |
| 10 | 6 (1.0%) | 4 (1.8%) | 2 (0.5%) | |
| Surgical Margin | 0.003 | |||
| Negative | 272 (45.0%) | 86 (37.4%) | 186 (49.7%) | |
| Positive | 332 (55.0%) | 144 (62.6%) | 188 (50.3%) | |
| Pre-op PSA (ng/mL) | 8.20 (5.40–14.18) | 7.90 (5.19–14.54) | 8.31 (5.50–14.01) | 0.46 |
| Pre-op PSA Range (ng/mL) | 0.97 | |||
| PSA < 10 | 340 (59.3%) | 126 (59.4%) | 214 (59.3%) | |
| PSA ≥ 10 | 233 (40.7%) | 86 (40.6%) | 147 (40.7%) | |
| Post-op PSA (ng/mL) | 0.09 (0.09–0.40) | 0.10 (0.06–0.55) | 0.09 (0.09–0.30) | 0.45 |
| Post-op PSA Range (ng/mL) | <0.001 | |||
| PSA < 0.1 | 310 (51.2%) | 98 (42.6%) | 212 (56.5%) | |
| PSA ≥ 0.1 | 295 (48.8%) | 132 (57.4%) | 163 (43.5%) | |
| Pre-Secondary Tx PSA (ng/mL) | 0.26 (0.12–0.70) | 0.24 (0.09–0.80) | 0.27 (0.16–0.68) | 0.02 |
| Secondary Tx Type | <0.001 | |||
| ADT | 162 (26.8%) | 82 (35.7%) | 80 (21.3%) | |
| Chemotherapy | 5 (0.8%) | 0 (0%) | 5 (1.3%) | |
| EBRT | 438 (72.4%) | 148 (64.3%) | 290 (77.3%) | |
| Year Secondary Tx Initiated | <0.001 | |||
| 2015 | 10 (1.7%) | 7 (3.0%) | 3 (0.8%) | |
| 2016 | 55 (9.1%) | 34 (14.8%) | 21 (5.6%) | |
| 2017 | 104 (17.2%) | 48 (20.9%) | 56 (14.9%) | |
| 2018 | 167 (27.6%) | 67 (29.1%) | 100 (26.7%) | |
| 2019 | 92 (15.2%) | 43 (18.7%) | 49 (13.1%) | |
| 2020 | 62 (10.2%) | 9 (3.9%) | 53 (14.1%) | |
| 2021 | 53 (8.8%) | 6 (2.6%) | 47 (12.5%) | |
| 2022 | 47 (7.8%) | 12 (5.2%) | 35 (9.3%) | |
| 2023 | 13 (2.1%) | 3 (1.3%) | 10 (2.7%) | |
| 2024 | 2 (0.3%) | 1 (0.4%) | 1 (0.3%) | |
| Secondary Tx Initiated Before/After 2020 | <0.001 | |||
| <2020 | 428 (70.7%) | 199 (86.5%) | 229 (61.1%) | |
| ≥2020 | 177 (29.3%) | 31 (13.5%) | 146 (38.9%) | |
| Days From RP to Secondary Tx | 243 (140–470) | 166 (113–253) | 350 (185–658) | <0.001 |
| Variable | OR | p Value | CI |
|---|---|---|---|
| Insurance | |||
| Private (ref.) | 1 | – | – |
| Medicare/Medicaid | 0.87 | 0.66 | 0.46–1.62 |
| Tricare/VA | 5.39 | 0.16 | 0.51–56.41 |
| Self-pay | 0.13 | 0.11 | 0.01–1.54 |
| Other | 0.92 | 0.87 | 0.33–2.54 |
| Unknown | 1.12 | 0.73 | 0.58–2.17 |
| Facility | |||
| A | 0.74 | 0.53 | 0.28–1.92 |
| B | 0.41 | 0.31 | 0.07–2.29 |
| C | 5.26 | 0.003 | 1.73–15.93 |
| D | 0.47 | 0.27 | 0.13–1.78 |
| E | 1.81 | 0.73 | 0.06–51.97 |
| F | 0.98 | 0.98 | 0.17–5.53 |
| G | 0.75 | 0.64 | 0.22–2.57 |
| H (ref.) | 1 | – | – |
| I | 2.63 | 0.09 | 0.86–7.97 |
| J | 1 | – | – |
| pT | |||
| T2 (ref.) | 1 | – | – |
| T3 or T4 | 1.06 | 0.81 | 0.65–1.73 |
| pN | |||
| N0 (ref.) | 1 | – | – |
| N+ | 0.78 | 0.45 | 0.41–1.48 |
| Gleason Score | 0.95 | 0.69 | 0.73–1.23 |
| Margin | |||
| Negative (ref.) | 1 | – | – |
| Positive | 0.85 | 0.52 | 0.52–1.39 |
| Pre-op PSA Range | |||
| <10 ng/mL (ref.) | 1 | – | – |
| ≥10 ng/mL | 2.15 | 0.002 | 1.31–3.53 |
| Post-op PSA Range | |||
| <0.1 ng/mL (ref.) | 1 | – | – |
| ≥0.1 ng/mL | 0.39 | 0.004 | 0.20–0.74 |
| Pre-Secondary Tx PSA Range (ng/mL) | |||
| PSA < 0.1 | 0.2 | <0.001 | 0.09–0.44 |
| 0.1 ≤ PSA < 0.2 (ref.) | 1 | – | – |
| PSA ≥ 0.2 | 1.48 | 0.26 | 0.75–2.93 |
| Secondary Tx Initiated Before/After 2020 | |||
| <2020 (ref.) | 1 | – | – |
| ≥2020 | 3.41 | <0.001 | 1.75–6.66 |
| Secondary Tx Type | |||
| ADT (ref.) | 1 | – | – |
| Chemotherapy | 1 | – | – |
| EBRT | 2.75 | 0.001 | 1.52–5.00 |
| Variable | Total Salvage (n = 375) | <2020 (n = 229) | ≥2020 (n = 146) | p Value |
|---|---|---|---|---|
| Year of RP | <0.001 | |||
| 2015 | 32 (8.5%) | 30 (13.1%) | 2 (1.4%) | |
| 2016 | 69 (18.4%) | 65 (28.4%) | 4 (2.7%) | |
| 2017 | 108 (28.8%) | 89 (38.9%) | 19 (13.0%) | |
| 2018 | 55 (14.7%) | 38 (16.6%) | 17 (11.6%) | |
| 2019 | 51 (13.6%) | 7 (3.1%) | 44 (30.1%) | |
| 2020 | 35 (9.3%) | 0 (0%) | 35 (24.0%) | |
| 2021 | 16 (4.3%) | 0 (0%) | 16 (11.0%) | |
| 2022 | 8 (2.1%) | 0 (0%) | 8 (5.5%) | |
| 2023 | 1 (0.3%) | 0 (0%) | 1 (0.7%) | |
| Year of RP (pre/post-2020) | <0.001 | |||
| <2020 | 315 (84.0%) | 229 (100%) | 86 (58.9%) | |
| ≥2020 | 60 (16.0%) | 0 (0%) | 60 (41.1%) | |
| Days From RP to Secondary Tx | 350 (185–658) | 281 (170–489) | 488 (221–1064) | <0.001 |
| Pre-Secondary Tx PSA (ng/mL) | 0.27 (0.16–0.68) | 0.30 (0.16–0.77) | 0.22 (0.16–0.49) | 0.26 |
| Rising PSA | 0.04 | |||
| No | 32 (8.5%) | 25 (10.9%) | 7 (4.8%) | |
| Yes | 343 (91.5%) | 204 (89.1%) | 139 (95.2%) | |
| Pre-secondary Tx PSA Range (ng/mL) | 0.73 | |||
| PSA < 0.1 | 34 (9.3%) | 22 (9.9%) | 12 (8.3%) | |
| 0.1 ≤ PSA < 0.2 | 75 (20.4%) | 43 (19.3%) | 32 (22.2%) | |
| PSA ≥ 0.2 | 258 (70.3%) | 158 (70.9%) | 100 (69.4%) | |
| Pre-secondary Tx PSA Range (ng/mL) and Rising | 0.32 | |||
| PSA < 0.1, not rising | 8 (2.2%) | 6 (2.7%) | 2 (1.4%) | |
| 0.1 ≤ PSA < 0.2, not rising | 5 (1.4%) | 5 (2.2%) | 0 (0.0%) | |
| PSA ≥ 0.2, not rising | 17 (4.6%) | 12 (5.4%) | 5 (3.5%) | |
| PSA < 0.1 and rising | 26 (7.1%) | 16 (7.2%) | 10 (6.9%) | |
| 0.1 ≤ PSA < 0.2 and rising | 70 (19.1%) | 38 (17.0%) | 32 (22.2%) | |
| PSA ≥ 0.2 and rising | 241 (65.7%) | 146 (65.5%) | 95 (66.0%) |
| Variable | Total pN+ (n = 106) | Adjuvant (n = 54) | Salvage (n = 52) | p Value |
|---|---|---|---|---|
| Days From RP to Secondary Tx | 126 (77–202) | 102 (51–149) | 150 (108–248) | <0.001 |
| Secondary Tx Type | 0.06 | |||
| ADT | 60 (56.6%) | 36 (66.7%) | 24 (46.2%) | |
| Chemotherapy | 2 (1.9%) | 0 (0%) | 2 (3.8%) | |
| EBRT | 44 (41.5%) | 18 (33.3%) | 26 (50.0%) | |
| Gleason Score | 0.28 | |||
| 7 | 29 (28.2%) | 12 (23.1%) | 17 (33.3%) | |
| 8 | 19 (18.4%) | 10 (19.2%) | 9 (17.6%) | |
| 9 | 53 (51.5%) | 30 (57.7%) | 23 (45.1%) | |
| 10 | 2 (1.9%) | 0 (0%) | 2 (3.9%) | |
| Surgical Margin | 0.78 | |||
| Negative | 34 (32.1%) | 18 (33.3%) | 16 (30.8%) | |
| Positive | 72 (67.9%) | 36 (66.7%) | 36 (69.2%) | |
| pT | 0.30 | |||
| T2 | 10 (9.4%) | 3 (5.6%) | 7 (13.5%) | |
| T3a | 20 (18.9%) | 12 (22.2%) | 8 (15.4%) | |
| T3b | 76 (71.7%) | 39 (72.2%) | 37 (71.2%) | |
| Pre-op PSA (ng/mL) | 11.47 (6.90–23.04) | 8.70 (5.30–18.20) | 13.70 (9.26–26.35) | 0.01 |
| Post-op PSA (ng/mL) | 0.50 (0.10–2.16) | 0.40 (0.09–1.34) | 0.59 (0.18–5.95) | 0.12 |
| Pre-Secondary Tx PSA (ng/mL) | 0.67 (0.20–3.12) | 0.69 (0.09–2.40) | 0.60 (0.20–6.60) | 0.26 |
| Rising PSA | <0.001 | |||
| No | 58 (54.7%) | 54 (100%) | 4 (7.7%) | |
| Yes | 48 (45.3%) | 0 (0%) | 48 (92.3%) | |
| Pre-secondary Tx PSA Range (ng/mL) | 0.06 | |||
| PSA < 0.1 | 15 (16.9%) | 11 (26.2%) | 4 (8.5%) | |
| 0.1 ≤ PSA < 0.2 | 7 (7.9%) | 2 (4.8%) | 5 (10.6%) | |
| PSA ≥ 0.2 | 67 (75.3%) | 29 (69.0%) | 38 (80.9%) | |
| Pre-secondary Tx PSA Range (ng/mL) and Rising | <0.001 | |||
| PSA < 0.1, not rising | 12 (13.5%) | 11 (26.2%) | 1 (2.1%) | |
| 0.1 ≤ PSA < 0.2, not rising | 3 (3.4%) | 2 (4.8%) | 1 (2.1%) | |
| PSA ≥ 0.2, not rising | 30 (33.7%) | 29 (69.0%) | 1 (2.1%) | |
| PSA < 0.1 and rising | 3 (3.4%) | 0 (0.0%) | 3 (6.4%) | |
| 0.1 ≤ PSA < 0.2 and rising | 4 (4.5%) | 0 (0.0%) | 4 (8.5%) | |
| PSA ≥ 0.2 and rising | 37 (41.6%) | 0 (0.0%) | 37 (78.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hochberg, A.R.; Ho, A.H.; Thompson, R.A.M.; Buck, M.B.; Lallas, C.D.; Ibilibor, C.; Tomaszewski, J.J.; Ginzburg, S.; Correa, A.; Uzzo, R.; et al. Real-World Management of High-Risk Prostate Cancer Post-Radical Prostatectomy: Insights from a Regional Quality Collaborative. Cancers 2025, 17, 1600. https://doi.org/10.3390/cancers17101600
Hochberg AR, Ho AH, Thompson RAM, Buck MB, Lallas CD, Ibilibor C, Tomaszewski JJ, Ginzburg S, Correa A, Uzzo R, et al. Real-World Management of High-Risk Prostate Cancer Post-Radical Prostatectomy: Insights from a Regional Quality Collaborative. Cancers. 2025; 17(10):1600. https://doi.org/10.3390/cancers17101600
Chicago/Turabian StyleHochberg, Aaron R., Annie H. Ho, Rasheed A. M. Thompson, Matthew B. Buck, Costas D. Lallas, Christine Ibilibor, Jeffrey J. Tomaszewski, Serge Ginzburg, Andres Correa, Robert Uzzo, and et al. 2025. "Real-World Management of High-Risk Prostate Cancer Post-Radical Prostatectomy: Insights from a Regional Quality Collaborative" Cancers 17, no. 10: 1600. https://doi.org/10.3390/cancers17101600
APA StyleHochberg, A. R., Ho, A. H., Thompson, R. A. M., Buck, M. B., Lallas, C. D., Ibilibor, C., Tomaszewski, J. J., Ginzburg, S., Correa, A., Uzzo, R., Smaldone, M. C., Danella, J. F., Guzzo, T. J., Lee, D. J., Belkoff, L., Walker, J., Raman, J. D., Clark, R. K., Reese, A., ... Shah, M. S. (2025). Real-World Management of High-Risk Prostate Cancer Post-Radical Prostatectomy: Insights from a Regional Quality Collaborative. Cancers, 17(10), 1600. https://doi.org/10.3390/cancers17101600






