1. Introduction
Skin diseases encompass a large spectrum of inflammatory and neoplastic diseases, including commonly diagnosed conditions such as basal cell carcinoma (BCC) or squamous cell carcinoma (SCC), as well as a multitude of less common and even rare dermatological disorders. The rising incidence of skin diseases in general, with nearly one in two Europeans experiencing a dermatological condition in the past twelve months, underscores the urgent need for precise diagnostic tools that can enhance the efficiency of finding the correct diagnosis and timely treatment planning [
1]. Besides clinical examination, especially in rare skin disorders, dermatohistopathological examination of excised tissue proves to be the gold standard. However, the histological process is often time-consuming and may delay the initiation of appropriate treatments [
2,
3].
Ex vivo confocal laser scanning microscopy (EVCM) is a promising imaging modality, offering a real-time, high-resolution visualization of skin structures at cellular and subcellular levels within a few minutes. Thanks to the novel digital staining software, the acquired microscopic images show a remarkable resemblance to conventional dermatohistopathologic haematoxylin-eosin (H&E) staining. This improvement makes it even more accessible for trained dermatohistopathologists to use EVCM effectively.
While EVCM has already demonstrated its operational readiness in the examination of healthy skin [
4], most common skin tumors [
5,
6], inflammatory diseases [
7], infectious diseases [
8], and in combination with fluorescent-labeled antibodies [
8,
9], its use in the assessment of uncommon and even rare skin diseases remains with exception of a few case reports [
10,
11] relatively unexplored. Hence, we have chosen the following skin conditions that have not yet undergone comprehensive investigation with EVCM and generally exhibit low incidences: dermatofibrosarcoma protuberans (DFSP) (N = 3), atypical fibroxanthoma (AFX) (N = 3), myxofibrosarcoma, leiomyoma (N = 2), sarcoma, fibrosarcoma, syphilis, lymphoma, cutaneous T-cell lymphoma, pseudolymphoma, prurigo nodularis, cylindroma, undifferentiated SCC, pigmented purpuric dermatosis (Morbus Schamberg) and granulation tissue (
Table 1).
Our main task in the study was to determine whether the pattern recognition known from dermatohistopathology was also possible in EVCM in less common or rare diseases that had never been closely studied or described in EVCM yet compared to more common diseases already well studied in EVCM. The second task was to compare the performances of three examiners with different backgrounds, experiences, and areas of expertise. This information is crucial for further use of EVCM for bedside histology, since common diagnoses such as SCC or BCC must be securely distinguished from other rare diseases.
2. Materials and Methods
The study was conducted per the Declaration of Helsinki and was approved by the Ludwig Maximilian University Ethics Committee, Munich, Germany (Protocol Nr. 19-150). Each patient gave written informed consent before inclusion in the study.
From November 2020 to April 2023, N = 50 tissue samples were collected from 50 patients enrolled at the Department of Dermatology and Allergy, University Hospital, LMU Munich, Germany. The patient cohort included 10 healthy controls, 10 BCC, 10 SCC, and 20 rare skin diseases that EVCM has not studied in detail until now.
Table 1 shows an overview of the investigated diseases and their reported incidence [
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22].
Table 1.
Overview of investigated diseases and their incidence, n.a. = non applicable.
Table 1.
Overview of investigated diseases and their incidence, n.a. = non applicable.
| | Sample Size (N) | Incidence |
|---|
| Healthy skin | 10 | n.a. |
| Basal cell carcinoma | 10 | 100–800/100,000 [12] |
| Squamous cell carcinoma | 10 | 5–500/100,000 [13] |
| Rare/unstudied skin diseases | 20 | n.a. |
| Dermatofibrosacoma protuberans | 3 | ≈1–5/100,000 [14] |
| Atypical fibrosarcoma | 3 | ≈2.5/100,000 [15] |
| Myxofibrosarcoma | 1 | <1/100,000 [16] |
| Cutaneous leiomyoma | 2 | <1/100,000 [17] |
| Pleomorphic dermal sarcoma | 1 | <1/100,000 [23] |
| Fibrosarcoma | 1 | <1/100,000 [24] |
| Syphilis | 1 | 8.9/100,000 [25] |
| Primary cutaneous B-cell lymphoma | 1 | 10–15/100,000 [26] |
| Cutaneous T-cell lymphoma | 1 | ≈0.5–2/100,000 [18] |
| Cutaneous pseudolymphoma | 1 | <1/100,000 [19] |
| Prurigo nodularis | 1 | ≈72/100,000 [20] |
| Cylindroma | 1 | <1/100,000 [21] |
| Undifferentiated SCC | 1 | n.a. |
| Morbus Schamberg | 1 | ≈50/100,000 [22] |
| Granulation tissue | 1 | n.a. |
EVCM examination: Freshly excised tissue was immediately stained and scanned following a standardized protocol: The staining process consists of immersing the probes in Acridine Orange (0.1 mmol/L, Sigma-Aldrich, St. Louis, MO, USA) for 30 s, followed by a 30-s rinse with phosphate-buffered saline (0.1 mmol/L, Dulbecco’s Phosphate Buffered Saline; PBS; pH 7.4, Sigma-Aldrich, St. Louis, MO, USA) in order to remove excess stain. Subsequently, the tissue probes were coated 30 s with citric acid (0.1 mmol/L) for aceto-whitening. Afterward, the tissue probes were positioned on object slides, mounted with sponges and magnets [
27], and examined in vertical mode. The parameters for imaging were standardized across all samples to ensure consistency and comparability.
The commercially available EVCM Vivascope 2500 G-4 device (Vivascope, Munich, Germany) is equipped with two different lasers with wavelengths of 488 nm (blue) and 638 nm (red). It examines the samples simultaneously in reflectance mode (RM), fluorescence mode (FM), overlay mode (OM), and digital staining mode (DHE). This study was performed solely in DHE mode, as digital H&E-like images are easier to interpret for EVCM-unexperienced dermatohistopathologists. Further technical details regarding the EVCM, tissue preparation, and examination are provided elsewhere [
3,
27,
28]. Following the EVCM analysis, all samples were fixed in a formaldehyde solution for a gold standard dermatohistopathologcial examination and were independently analyzed in the Dermatohistopathological Department of the Department of Dermatology and Allergy, University Hospital, LMU Munich.
Image evaluation: Overview and detailed DHE EVCM images were presented in a randomized order. Three blinded investigators, an EVCM-trained dermatohistopathologist (D.H.), an EVCM-unexperienced dermatohistopathologist (M.M.), and an EVCM-trained dermatologist with no experience in dermatohistopathology (M.D.), were asked to categorize the images into “rare skin diseases”, BCC, SCC, or healthy control group. Subsequently, the investigators were instructed to analyze the cellular morphology and tissue architecture to describe characteristic morphologic features and patterns of the shown skin diseases.
Comparative Analysis and Dermatohistopathology: In parallel, all skin samples underwent conventional dermatohistopathological examination to establish the gold standard diagnosis. The dermatohistopathological assessments were conducted by independent, experienced dermatohistopathologists following standardized protocols, including the incorporation of immunostaining techniques. Dermatohistopathological results were then compared to the results obtained from EVCM analysis in order to determine the concordance and discrepancy between the two diagnostic modalities. Descriptive statistical calculations were performed using Microsoft Excel 2016 (Microsoft, Redmond, WA, USA).
3. Results
EVCM demonstrated high diagnostic efficacy in identifying characteristic features of uncommon and even rare skin diseases. The mean concordance with dermatohistopathology of all three investigators combined was 80%, while the EVCM-trained dermatohistopathologist (D.H.), performed best (46/50 = 92%) compared to the EVCM-trained dermatologist with no experience in dermatohistopathology (M.D.) (44/50 = 88%) and the EVCM-unexperienced dermatohistopathologist (M.M.) (30/50 = 60%).
Table 2 illustrates the performance of each examiner subdivided into four classification groups (BCC, SCC, rare skin diseases, and healthy control). Regarding the evaluation of BCC, the EVCM-trained dermatohistopathologist demonstrated a sensitivity of 0.90, specificity of 0.98, positive predictive value (PPV) of 0.90, and negative predictive value (NPV) of 0.98. Regarding SCC image interpretation, a sensitivity of 0.82, specificity of 0.95, PPV of 0.82, and NPV of 0.95 could be reached. In the classification of rare skin diseases, this investigator achieved a sensitivity of 0.95, specificity of 0.97, PPV of 0.95, and NPV of 0.97, and correctly identified all healthy controls with sensitivity, specificity, PPV, and NPV of 1.00.
The EVCM-trained dermatologist achieved a sensitivity of 0.80 in the identification of SCC, a specificity of 0.95, a PPV of 0.80, and an NPV of 0.95. For the identification of BCC, this examiner saw a sensitivity of 0.73, specificity of 0.95, PPV of 0.80, and NPV of 0.93. The same examiner demonstrated a sensitivity of 0.95, specificity of 0.94, PPV of 0.90, and NPV of 0.97 in the group of rare skin diseases and also correctly identified all healthy controls with sensitivity, specificity, PPV, and NPV of 1.00.
The dermatohistopathologist achieved a sensitivity of 0.60, specificity of 0.93, PPV of 0.67, and NPV of 0.90 and sensitivity of 0.36, specificity of 0.85, PPV of 0.40, and NPV of 0.83 for BCC and SCC, respectively. In the assessment of rare skin diseases, the dermatohistopathologist presented a sensitivity of 0.68, specificity of 0.74, PPV of 0.62, and NPV of 0.79. The healthy controls were identified with a sensitivity of 0.70, specificity of 0.93, PPV of 0.70, and NPV of 0.93.
Notably, the EVCM imaging enabled the visualization of specific cellular and tissue patterns. Referring to conventional dermatohistopathologic descriptions, we observed unique patterns of each skin disease, facilitating their prompt and accurate identification for experienced users (
Table 3). In the following, we present the patterns observed in DHE EVCM images and their corresponding skin diseases, complemented by a schematic representation.
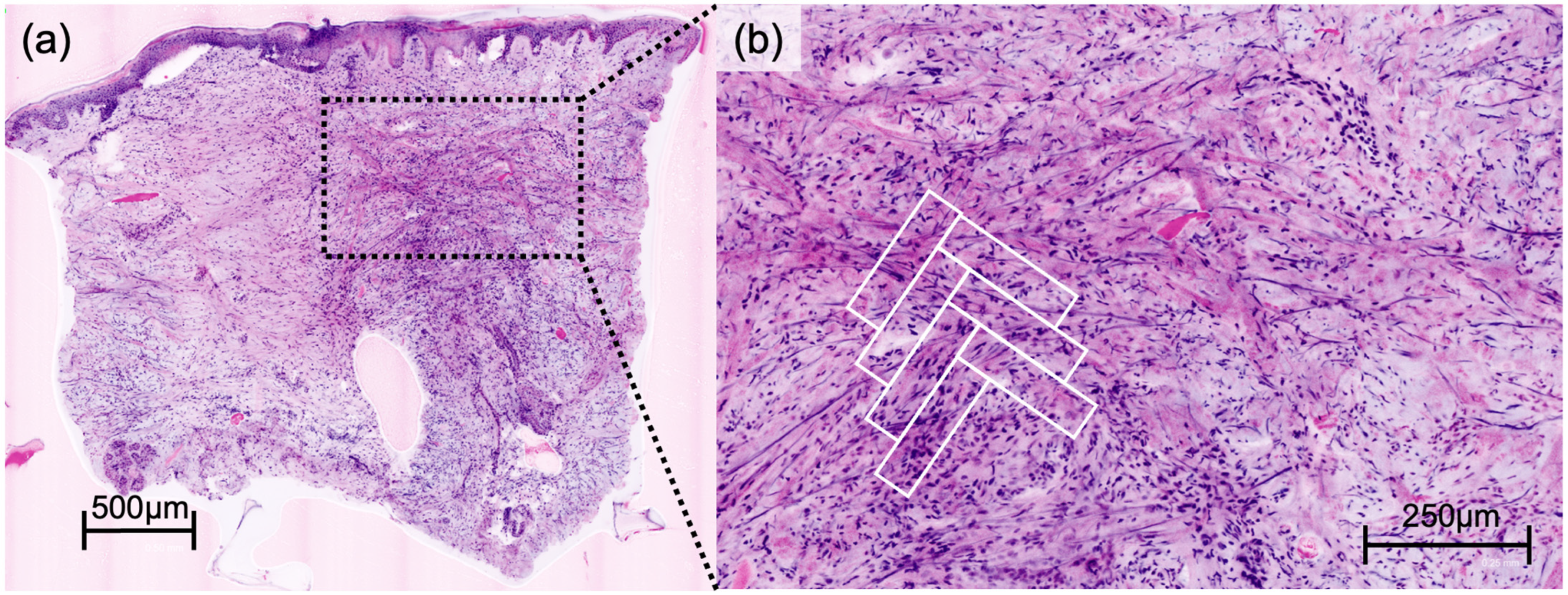
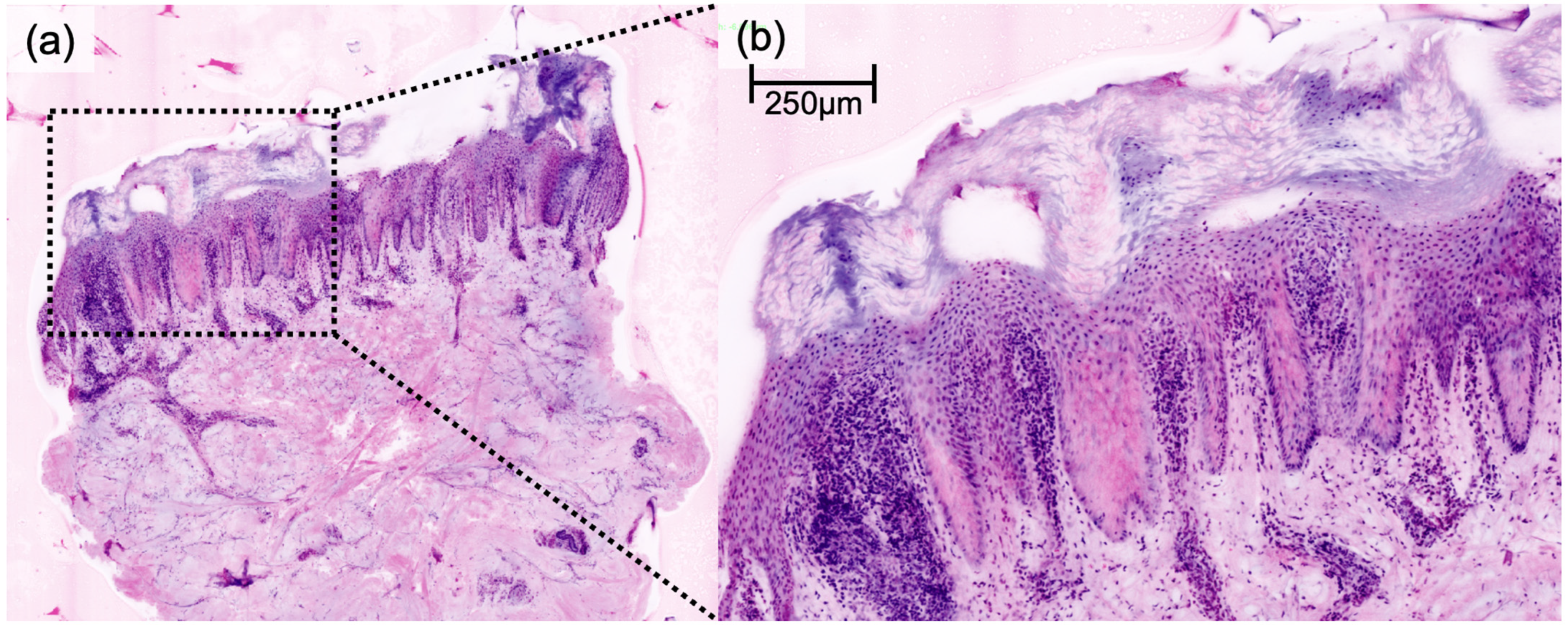
DFSP presented a characteristic honeycomb-like appearance with interlacing spindle cells (
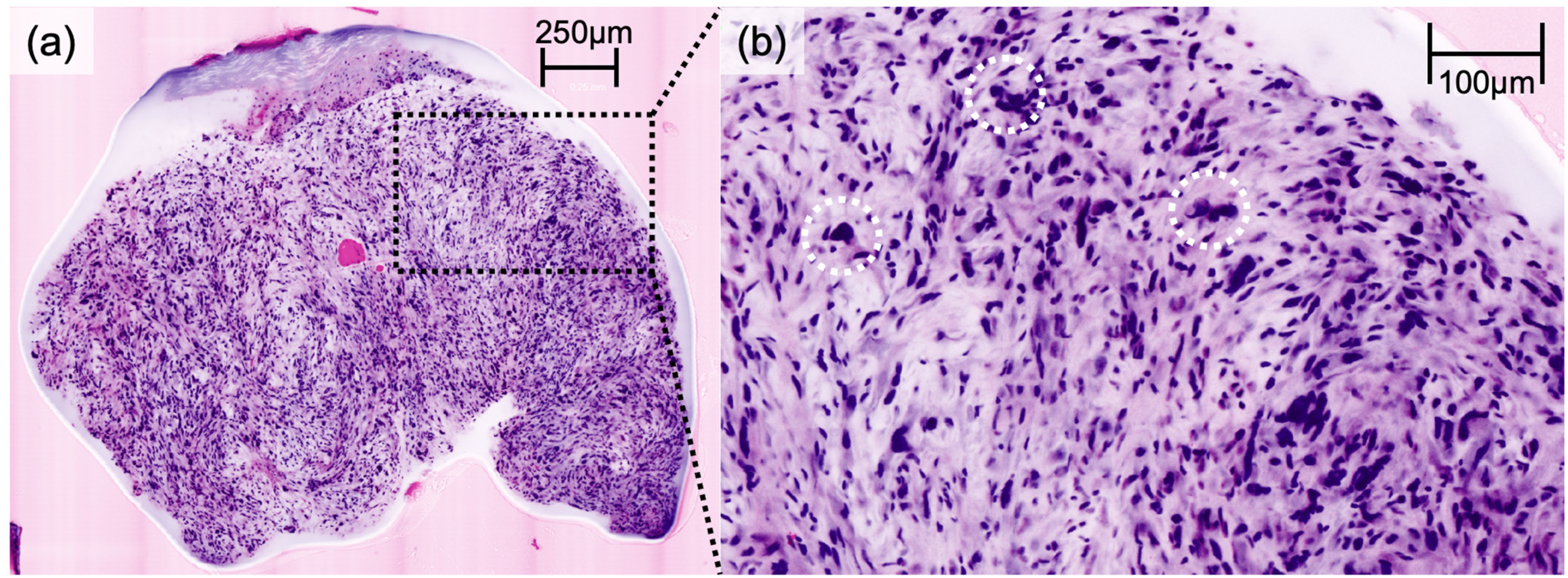
Figure 1), while AFX presented with pleomorphic spindle cells and multinucleated giant cells (
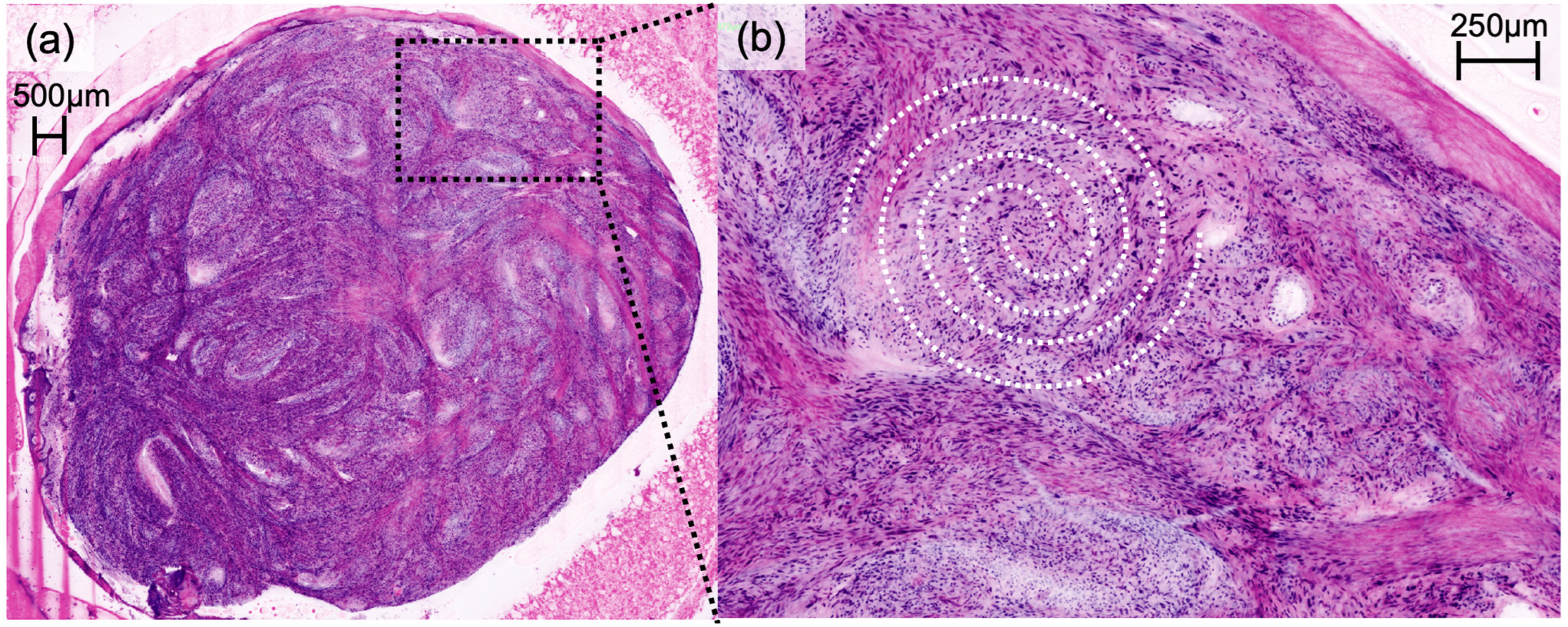
Figure 2). Irregularly shaped and hypercellular stromal elements characterized fibrosarcoma, while lymphoma displayed dense lymphocytic infiltrates out of proportion with disrupted epidermal architecture (so-called epidermotropism). Leiomyoma presented with smooth muscle cells in a whorled pattern surrounded by interlacing fascicles (
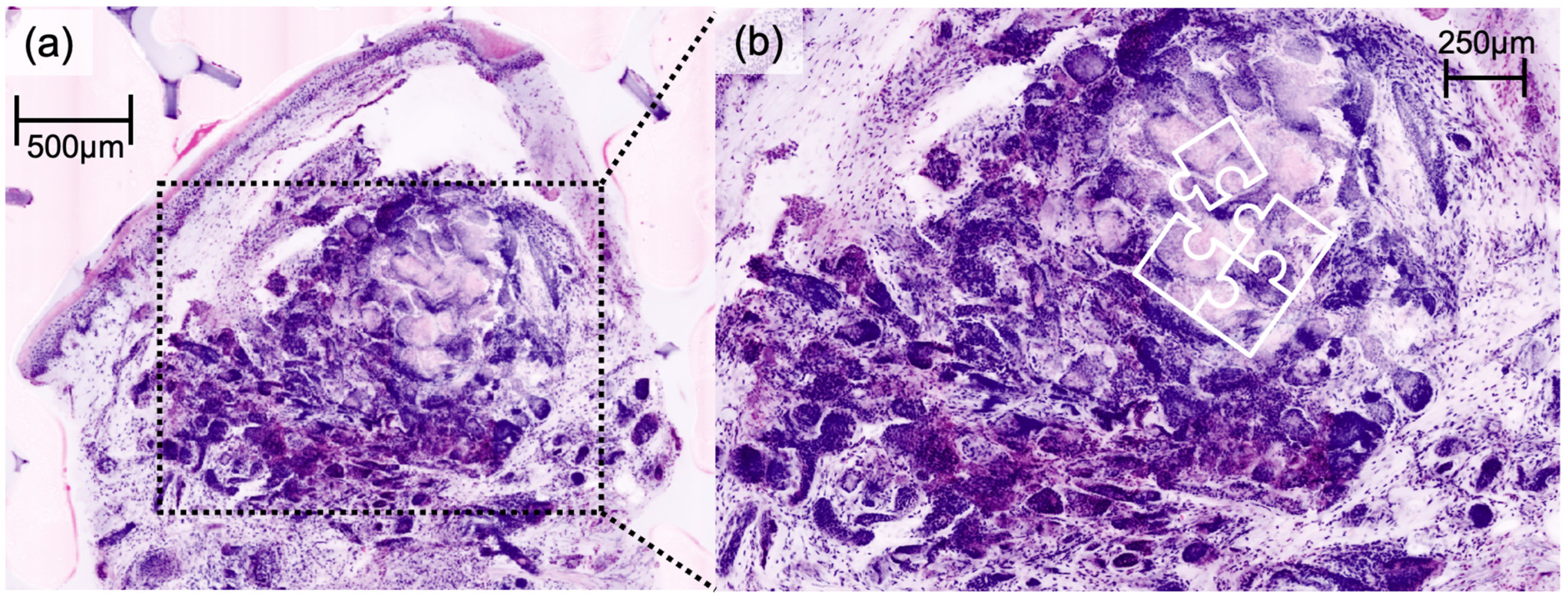
Figure 3). Cylindroma (
Figure 4) showed aggregations of basaloid cells forming characteristic jigsaw puzzle-like patterns, and prurigo nodularis manifested as hyperkeratotic nodules with underlying dermal fibrosis and perivascular inflammatory infiltrate (
Figure 5), among other distinctive features observed in each of these skin diseases.
4. Discussion
Our study illustrates the potential of EVCM in the accurate assessment of not only common but also rare skin diseases. It is imperative to highlight the primary objective of EVCM as a tool for pattern recognition, aiming for rapid diagnoses directly at the bedside or in the operation room. Therefore, the unique advantage of EVCM lies in its near real-time visualization capabilities, allowing for rapid identification of characteristic histologic features and patterns, facilitating the decision-making process during surgical procedures. Our results, as seen in
Figure 1,
Figure 2,
Figure 3 and
Figure 4, affirm the feasibility of this approach, revealing that the distinctive histologic features, cellular arrangements, specific architectural patterns, and unique cellular morphology specific to each rare skin disease may be readily apparent in the EVCM images.
As might be expected, we found a strong correlation between imaging experience and diagnostic accuracy. Although we hoped to observe greater skill transferability between conventional histology and EVCM, the outcomes exhibited variations in distinguishing between healthy, BCC, SCC, and rare skin diseases and identifying specific patterns depending on the examiner’s training. While the imaging inexperienced dermatohistopathologist reached 60% concordance, the imaging-trained dermatologist obtained 88% agreement with dermatohistopathology. Only imaging-trained dermatohistopathologists achieved a concordance of up to 92% with the gold standard dermatohistopathology.
Not all patterns outlined in this study were universally recognized by all examiners. This is not surprising, given the examiners’ lack of prior exposure to these patterns in EVCM. We hypothesize that their proficiency will notably improve after target-oriented training or a comprehensive study of this manuscript.
While we demonstrate that characteristic patterns of rare skin diseases may be identifiable in EVCM, it is essential to note that larger-scale investigations are imperative to determine key parameters, such as the sensitivity and specificity of the aforementioned features and patterns. Furthermore, the current quality of EVCM images may not consistently match the quality of a conventional histological slide, adding complexity to the analysis. Consequently, we propose that EVCM could serve as a supplementary tool in the diagnostic process of rare skin diseases, contingent upon the reliable identification of distinct features and patterns in skin biopsies, allowing for real-time diagnosis.
Regarding the use of EVCM modes, Vladimirova et al. showed that a variety of dermatohistopathological features could be identified using all four EVCM modes [
3]. Each mode specializes in accentuating specific structures or characteristics; FM particularly emphasizes cell nuclei and enhances the contrast of cell structures, while RM is particularly useful for the analysis of matrix structures such as elastic and collagen fibers [
3]. Although OM entails the strength of the FM and RM, its appearance differs significantly from conventional histological images, unlike the DHE images [
3]. While the relatively new integration of FM, RM, OM, and DHE modes is a powerful diagnostic tool, it requires significant EVCM training for dermatohistopathologists to be proficient in all modes. In our study, we, therefore, focused only on the DHE since the DHE images are easier for EVCM-unexperienced dermatohistopathologists to interpret. However, our results suggest that some EVCM training is necessary for H&E-trained dermatohistopathologists to achieve adequate results. The EVCM-trained dermatohistopathologist and the EVCM-trained dermatologist with no experience in dermatohistopathology successfully identified BCC and SCC, achieving a high level of accuracy in distinguishing them from both healthy samples and rare skin diseases. However, The EVCM-unexperienced dermatohistopathologist did not perform as well despite a significant training in dermatohistopathology. These findings may explain different levels in accuracy using EVCM in the literature [
29,
30]. Nonetheless, it appears that the DHE mode may be sufficient to identify BCC, SCC, rare skin diseases and healthy skin. Consequently, dermatologists in a clinical setting may be able to identify more complex skin samples that may require a more detailed analysis using all four modes by an EVCM-trained dermatohistopathologist.
A primary limitation of our study, which may have influenced the generalizability of the findings, was the limited sample size due to the rarity of the investigated diseases. Therefore, rare skin diseases warrant further investigation to determine the extent of skin diseases that can be accurately diagnosed using EVCM. Nonetheless, we demonstrated that the few mentions of the studied rare diseases in EVCM literature did not prevent EVCM-trained dermatohistopathologists and EVCM-trained dermatologists from correctly differentiating between rare skin diseases, BCC, SCC, and healthy skin. The next steps should be the study of histological patterns of rare skin diseases in EVCM in a larger setting and the determination of the time needed to train an EVCM-unexperienced dermatohistopathologist. The use of fluorescent-labeled antibodies may further increase the scope [
9].
5. Conclusions
To conclude, our comprehensive analysis of uncommon and rare skin diseases using EVCM has highlighted its promising role as a rapid and reliable imaging modality in the realm of dermatohistopathology. The high diagnostic accuracy and concordance between the EVCM findings and conventional dermatohistopathological assessments underline the utility of EVCM as an effective supplementary diagnostic tool for distinguishing rare skin diseases from more prevalent conditions. We show that even though EVCM has primarily been designed for fast intraoperative decisions in Mohs surgery, it offers the possibility to recognize and differentiate less common diagnoses in order to maximize patient safety. Nevertheless, it is crucial to ascertain specific training for EVCM-unexperienced dermatohistopathologists and dermatologists to attain proficiency in its use.
Author Contributions
Conceptualization, M.D. and D.H.; methodology, L.M. and M.D.; validation, D.H.; formal analysis, L.M. and M.D.; investigation, L.M., M.D. and M.M.; data curation, L.B. and L.S.; writing—original draft preparation, L.M. and M.D.; writing—review and editing, M.M., L.B., L.S., L.E.F. and D.H.; supervision, L.E.F. and D.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and obtained by the votum of the ethics committee of LMU Munich (Protocol Nr. 19-150).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethnical and privacy restrictions.
Acknowledgments
We express our gratitude to Katrin Kerl-French and Michael Flaig for gold standard dermatohistopathological assessments. We thank Vivascope GmbH, Germany for making Vivascope 2500 G-4 device available for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Richard, M.A.; Paul, C.; Nijsten, T.; Gisondi, P.; Salavastru, C.; Taieb, C.; Trakatelli, M.; Puig, L.; Stratigos, A. Prevalence of most common skin diseases in Europe: A population-based study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Garbe, C.; Peris, K.; Soura, E.; Forsea, A.M.; Hauschild, A.; Arenbergerova, M.; Bylaite, M.; Del Marmol, V.; Bataille, V.; Samimi, M.; et al. The evolving field of Dermato-oncology and the role of dermatologists: Position Paper of the EADO, EADV and Task Forces, EDF, IDS, EBDV–UEMS and EORTC Cutaneous Lymphoma Task Force. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2183–2197. [Google Scholar] [CrossRef]
- Vladimirova, G.; Ruini, C.; Kapp, F.; Kendziora, B.; Ergün, E.Z.; Bağcı, I.S.; Krammer, S.; Jastaneyah, J.; Sattler, E.C.; Flaig, M.J.; et al. Ex vivo confocal laser scanning microscopy: A diagnostic technique for easy real-time evaluation of benign and malignant skin tumours. J. Biophotonics 2022, 15, e202100372. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; Perrot, J.L.; Labeille, B.; Cambazard, F.; Rubegni, P. Ex vivo confocal microscopy: An emerging technique in dermatology. Dermatol. Pract. Concept. 2018, 8, 109–119. [Google Scholar] [CrossRef]
- Bennàssar, A.; Vilata, A.; Puig, S.; Malvehy, J. Ex vivo fluorescence confocal microscopy for fast evaluation of tumour margins during Mohs surgery. Br. J. Dermatol. 2014, 170, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Malvehy, J.; Perez-Anker, J.; Toll, A.; Pigem, R.; Garcia, A.; Alos, L.L.; Puig, S. Ex vivo confocal microscopy: Revolution in fast pathology in dermatology. Br J Dermatol 2020, 183, 1011–1025. [Google Scholar] [CrossRef]
- Sinem Bağcı, I.; Aoki, R.; Vladimirova, G.; Ergün, E.; Ruzicka, T.; Sárdy, M.; French, L.E.; Hartmann, D. New-generation diagnostics in inflammatory skin diseases: Immunofluorescence and histopathological assessment using ex vivo confocal laser scanning microscopy in cutaneous lupus erythematosus. Exp. Dermatol. 2021, 30, 684–690. [Google Scholar] [CrossRef]
- Krammer, S.; Krammer, C.; Vladimirova, G.; Salzer, S.; Ruini, C.; Sattler, E.; French, L.E.; Hartmann, D. Ex vivo Confocal Laser Scanning Microscopy: A Potential New Diagnostic Imaging Tool in Onychomycosis Comparable with Gold Standard Techniques. Front. Med. 2020, 7, 586648. [Google Scholar] [CrossRef]
- Hartmann, D.; Krammer, S.; Vural, S.; Bachmann, M.R.; Ruini, C.; Sárdy, M.; Ruzicka, T.; Berking, C.; von Braunmühl, T. Immunofluorescence and confocal microscopy for ex-vivo diagnosis of melanocytic and non-melanocytic skin tumors: A pilot study. J. Biophotonics 2018, 11, e201700211. [Google Scholar] [CrossRef]
- Lamberti, A.; Cinotti, E.; Habougit, C.; Labeille, B.; Rubegni, P.; Perrot, J.-L. Ex vivo confocal microscopy for dermatofibrosarcoma protuberans. Skin Res. Technol. 2019, 25, 589–591. [Google Scholar] [CrossRef]
- Pampena, R.; Peccerillo, F.; Piana, S.; Paolino, G.; Mercuri, S.R.; Pellacani, G.; Longo, C. Atypical fibroxanthoma: In-vivo and ex-vivo confocal features. Ital. J. Dermatol. Venerol. 2021, 156, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef]
- Waldman, A.; Schmults, C. Cutaneous Squamous Cell Carcinoma. Hematol. Oncol. Clin. N. Am. 2019, 33, 1–12. [Google Scholar] [CrossRef]
- Kreicher, K.L.; Kurlander, D.E.; Gittleman, H.R.; Barnholtz-Sloan, J.S.; Bordeaux, J.S. Incidence and Survival of Primary Dermatofibrosarcoma Protuberans in the United States. Dermatol. Surg. 2016, 42 (Suppl. S1), S24–S31. [Google Scholar] [CrossRef]
- Koch, M.; Freundl, A.J.; Agaimy, A.; Kiesewetter, F.; Künzel, J.; Cicha, I.; Alexiou, C. Atypical Fibroxanthoma—Histological Diagnosis, Immunohistochemical Markers and Concepts of Therapy. Anticancer Res. 2015, 35, 5717–5735. [Google Scholar]
- Stiller, C.A.; Trama, A.; Serraino, D.; Rossi, S.; Navarro, C.; Chirlaque, M.D.; Casali, P.G. Descriptive epidemiology of sarcomas in Europe: Report from the RARECARE project. Eur. J. Cancer 2013, 49, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Kanitakis, J.; Carbonnel, E.; Chouvet, B.; Labeille, B.; Claudy, A. Cutaneous leiomyomas (piloleiomyomas) in adult patients with human immunodeficiency virus infection. Br. J. Dermatol. 2000, 143, 1338–1340. [Google Scholar] [CrossRef]
- Criscione, V.D.; Weinstock, M.A. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol 2007, 143, 854–859. [Google Scholar] [CrossRef]
- Nihal, M.; Mikkola, D.; Horvath, N.; Gilliam, A.C.; Stevens, S.R.; Spiro, T.P.; Cooper, K.D.; Wood, G.S. Cutaneous lymphoid hyperplasia: A lymphoproliferative continuum with lymphomatous potential. Hum. Pathol. 2003, 34, 617–622. [Google Scholar] [CrossRef]
- Huang, A.H.; Williams, K.A.; Kwatra, S.G. Prurigo nodularis: Epidemiology and clinical features. J. Am. Acad. Dermatol. 2020, 83, 1559–1565. [Google Scholar] [CrossRef]
- Jordão, C.; de Magalhães, T.C.; Cuzzi, T.; Ramos-e-Silva, M. Cylindroma: An update. Int. J. Dermatol. 2015, 54, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Gupta, S. Clinicoepidemiological study of pigmented purpuric dermatoses. Indian Dermatol. Online J. 2012, 3, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Ørholt, M.; Abebe, K.; Rasmussen, L.E.; Aaberg, F.L.; Lindskov, L.J.; Schmidt, G.; Wagenblast, A.L.; Petersen, M.M.; Loya, A.C.; Daugaard, S.; et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma: Local recurrence and metastasis in a nationwide population-based cohort of 1118 patients. .J Am. Acad. Dermatol. 2023, 89, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Folpe, A.L. Adult-type fibrosarcoma: A reevaluation of 163 putative cases diagnosed at a single institution over a 48-year period. Am. J. Surg. Pathol. 2010, 34, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch Institute. Infektionsepidemiologischse Jahrbuch Meldepflichtiger Krankheiten für 2020; Robert Koch Institute: Berlin, Germany, 2021; Volume 212. [Google Scholar]
- Servitje, O.; Muniesa, C.; Benavente, Y.; Monsálvez, V.; Garcia-Muret, M.P.; Gallardo, F.; Domingo-Domenech, E.; Lucas, A.; Climent, F.; Rodriguez-Peralto, J.L.; et al. Primary cutaneous marginal zone B-cell lymphoma: Response to treatment and disease-free survival in a series of 137 patients. J. Am. Acad. Dermatol. 2013, 69, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Anker, J.; Toll, A.; Puig, S.; Malvehy, J. Six steps to reach optimal scanning in ex vivo confocal microscopy. J. Am. Acad. Dermatol. 2022, 86, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Anker, J.; Puig, S.; Malvehy, J. A fast and effective option for tissue flattening: Optimizing time and efficacy in ex vivo confocal microscopy. J Am Acad Dermatol 2020, 82, e157–e158. [Google Scholar] [CrossRef]
- Forchhammer, S.; Grunewald, S.; Möhrle, M.; Metzler, G.; Eigentler, T.; Münch, A.K.; Ogrzewalla, H. Diagnosis of Basal Cell Carcinoma with Ex-vivo Confocal Laser Scanning Microscopy in a Real-life Setting. Acta Derm. Venereol. 2023, 103, adv4859. [Google Scholar] [CrossRef]
- Ogrzewalla, H.; Möhrle, M.; Metzler, G.; Eigentler, T.; Münch, A.K.; Forchhammer, S. A Feasibility Study for Immediate Histological Assessment of Various Skin Biopsies Using Ex Vivo Confocal Laser Scanning Microscopy. Diagnostics 2022, 12, 3030. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).