Textbook Neoadjuvant Outcome—Novel Composite Measure of Oncological Outcomes among Gastric Cancer Patients Undergoing Multimodal Treatment
Abstract
Simple Summary
Abstract
1. Introduction
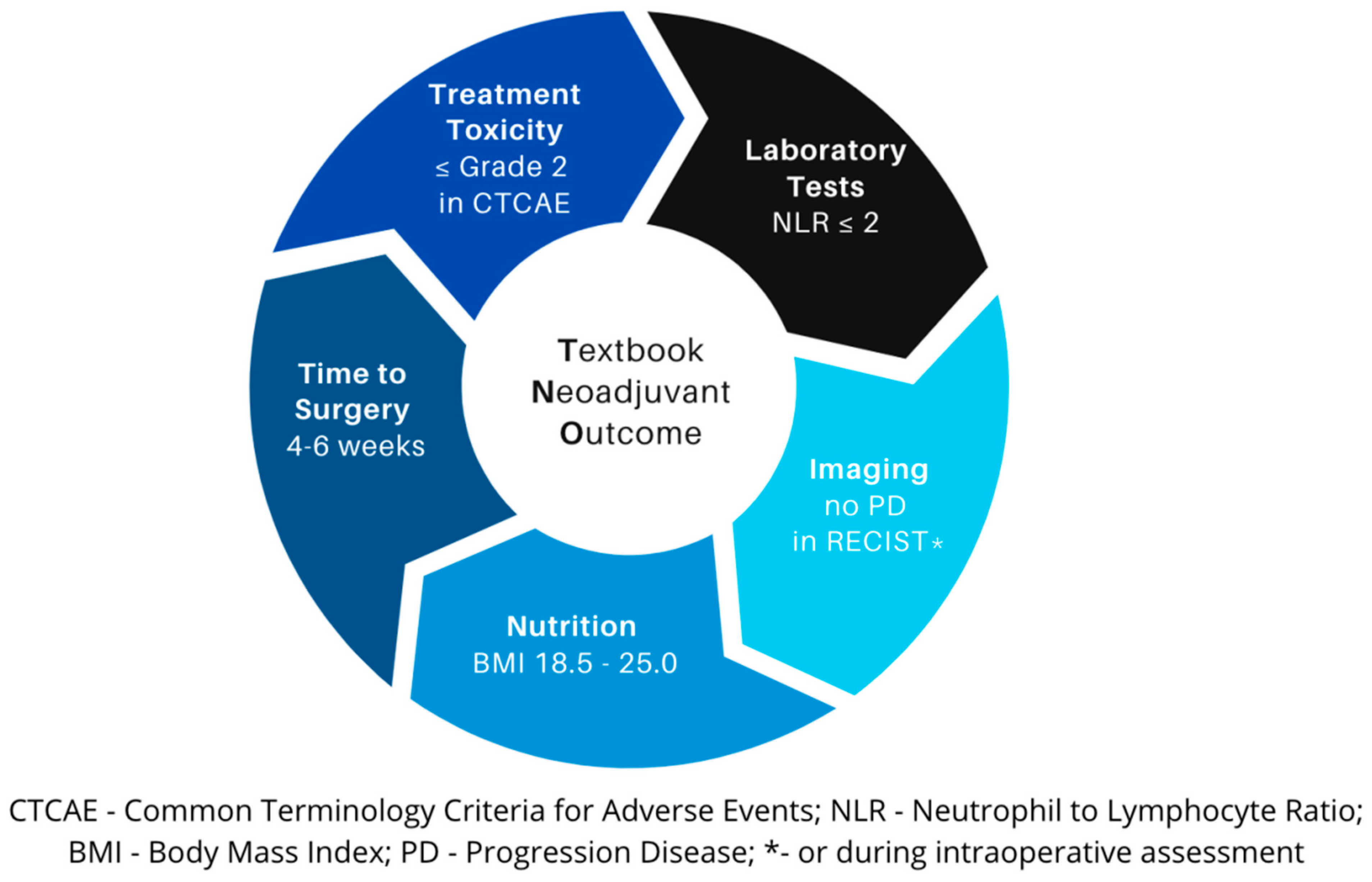
2. Exploring TNO Components
3. Treatment Toxicity
4. Laboratory Tests
| Inflammatory Marker | Study | Population | Results | Distinctive Advantages | Limitations |
|---|---|---|---|---|---|
| NLR | Post-hoc exploratory analysis of REAL-2 RCT [35] Retrospective analysis [39] | 908 advanced AEG cancer patients undergoing multimodal treatment from UK and Australia 106 locally advanced GC patients | High NLR associated with OS (HR = 1.73, 1.50–2.00) High NLR associated with OS (HR = 1.94, 1.02–3.70) | Predictive factor of short- and long-term outcomes [41], peritoneal and/or metastatic disease [40] Independent prognostic factor in multimodal treatment | Underrepresentation of the population with poor performance status due RCT design Relatively small sample size |
| PLR | Meta-analysis of 8 studies [42] | 4513 GC patients undergoing upfront surgery | High PLR not a reliable predictor for OS (HR = 0.99, 95% CI: 0.9–1.1) | High PLR correlated with a higher risk of LN metastasis and serosal invasion | Not a negative predictor for OS |
| LMR | Retrospective analysis [39] | 106 locally advanced GC patients undergoing NAC | High LMR not a reliable predictor for OS (HR = 0.92, 95% CI: 0.47–1.79) | Reassessment of LMR at post-12-month might be helpful in predicting the long-term survival [45] | Lack of prognostic and predictive role in European population undergoing NAC |
| IBI | Retrospective analysis [43] | 6359 cancer patients | High IBI associated with physical condition, malnutrition, cachexia, and short-term outcomes; independent risk factor (HR = 1.114; 95% CI, 1.072–1.157) | Combined value of NLR and CRP | Asian population, little data regarding GC patients [46] |
| GPS, mGPS | Retrospective analysis [37] | 1710 GC patients undergoing curative or palliative surgery | mGPS associated with postoperative mortality (OR, 1.845; 95% CI, 1.184–2.875) | Indicator of nutritional status, different prognostic value of mGPS depending on tumor stage | Japanese population, prognostic significance of GPS in GC has not been fully investigated |
| PNI | Two-institutional retrospective analysis [33] | 206 AEG and UGC patients undergoing curative-intent surgery | Predictive factor of OS (HR = 8.946) and RFS (HR = 6.416) | Indicator of nutritional status | Asian population, cohort limited to upper GC patients, no assessment after NAC |
5. Radiological Evaluation
6. Nutrition
7. Time to Surgery
8. Systemic Therapy
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferlay, J.E.M.; Lam, F. Global Cancer Observatory: Cancer Tomorrow; International Agency for Research on Cancer: Lyon, France, 2018; Available online: https://gco.iarc.fr/tomorrow/en (accessed on 2 February 2024).
- Aquina, C.T.; Ejaz, A.; Tsung, A.; Pawlik, T.M.; Cloyd, J.M. National Trends in the Use of Neoadjuvant Therapy before Cancer Surgery in the US from 2004 to 2016. JAMA Netw. Open 2021, 4, e211031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Hofheinz, R.D.; Pauligk, C.; Kopp, H.G.; Haag, G.M.; Luley, K.B.; Meiler, J.; Homann, N.; Lorenzen, S.; Schmalenberg, H.; et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): Results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016, 17, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lim, D.H.; Kim, S.; Park, S.H.; Park, J.O.; Park, Y.S.; Lim, H.Y.; Choi, M.G.; Sohn, T.S.; Noh, J.H.; et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: The ARTIST trial. J. Clin. Oncol. 2012, 30, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Zang, D.Y.; Han, B.; Ji, J.H.; Kim, T.G.; Oh, S.Y.; Hwang, I.G.; Kim, J.H.; Shin, D.; Lim, D.H.; et al. ARTIST 2: Interim results of a phase III trial involving adjuvant chemotherapy and/or chemoradiotherapy after D2-gastrectomy in stage II/III gastric cancer (GC). J. Clin. Oncol. 2019, 37, 4001. [Google Scholar] [CrossRef]
- Leong, T.; Smithers, B.M.; Michael, M.; Gebski, V.; Boussioutas, A.; Miller, D.; Simes, J.; Zalcberg, J.; Haustermans, K.; Lordick, F.; et al. TOPGEAR: A randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC Cancer 2015, 15, 532. [Google Scholar] [CrossRef] [PubMed]
- Donlon, N.E.; Moran, B.; Kamilli, A.; Davern, M.; Sheppard, A.; King, S.; Donohoe, C.L.; Lowery, M.; Cunningham, M.; Ravi, N.; et al. CROSS Versus FLOT Regimens in Esophageal and Esophagogastric Junction Adenocarcinoma: A Propensity-Matched Comparison. Ann. Surg. 2022, 276, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.V.; Preston, S.R.; O’Neill, B.; Lowery, M.A.; Baeksgaard, L.; Crosby, T.; Cunningham, M.; Cuffe, S.; Griffiths, G.O.; Parker, I.; et al. Trimodality therapy versus perioperative chemotherapy in the management of locally advanced adenocarcinoma of the oesophagus and oesophagogastric junction (Neo-AEGIS): An open-label, randomised, phase 3 trial. Lancet Gastroenterol. Hepatol. 2023, 8, 1015–1027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoeppner, J.; Lordick, F.; Brunner, T.; Glatz, T.; Bronsert, P.; Rothling, N.; Schmoor, C.; Lorenz, D.; Ell, C.; Hopt, U.T.; et al. ESOPEC: Prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer 2016, 16, 503. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiche, L.; Yang, H.K.; Abbassi, F.; Robles-Campos, R.; Stain, S.C.; Ko, C.Y.; Neumayer, L.A.; Pawlik, T.M.; Barkun, J.S.; Clavien, P.A. Quality and Outcome Assessment for Surgery. Ann. Surg. 2023, 278, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Shannon, A.B.; Straker, R.J., 3rd; Keele, L.; Fraker, D.L.; Roses, R.E.; Miura, J.T.; Karakousis, G.C. Lymph Node Evaluation after Neoadjuvant Chemotherapy for Patients with Gastric Cancer. Ann. Surg. Oncol. 2022, 29, 1242–1253. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.H.; Lee, H.J.; Ahn, H.S.; Kim, J.W.; Kim, W.H.; Lee, K.U.; Yang, H.K. Stage migration effect on survival in gastric cancer surgery with extended lymphadenectomy: The reappraisal of positive lymph node ratio as a proper N-staging. Ann. Surg. 2012, 255, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.D.; Schwarz, R.R.; Schwarz, R.E. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: Data from a large US-population database. J. Clin. Oncol. 2005, 23, 7114–7124. [Google Scholar] [CrossRef] [PubMed]
- Baxter, N.N.; Tuttle, T.M. Inadequacy of lymph node staging in gastric cancer patients: A population-based study. Ann. Surg. Oncol. 2005, 12, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Morote, S.; Yang, H.K.; Lacueva, J.; Rubio-Garcia, J.J.; Alacan-Friedrich, L.; Fierley, L.; Villodre, C.; Ramia, J.M. Textbook outcome in oncological gastric surgery: A systematic review and call for an international consensus. World J. Surg. Oncol. 2023, 21, 288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Brien, S.M.; Shahian, D.M.; DeLong, E.R.; Normand, S.L.; Edwards, F.H.; Ferraris, V.A.; Haan, C.K.; Rich, J.B.; Shewan, C.M.; Dokholyan, R.S.; et al. Quality measurement in adult cardiac surgery: Part 2—Statistical considerations in composite measure scoring and provider rating. Ann. Thorac. Surg. 2007, 83, S13–S26. [Google Scholar] [CrossRef] [PubMed]
- van der Kaaij, R.T.; de Rooij, M.V.; van Coevorden, F.; Voncken, F.E.M.; Snaebjornsson, P.; Boot, H.; van Sandick, J.W. Using textbook outcome as a measure of quality of care in oesophagogastric cancer surgery. Br. J. Surg. 2018, 105, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Merath, K.; Chen, Q.; Bagante, F.; Alexandrescu, S.; Marques, H.P.; Aldrighetti, L.; Maithel, S.K.; Pulitano, C.; Weiss, M.J.; Bauer, T.W.; et al. A Multi-institutional International Analysis of Textbook Outcomes among Patients Undergoing Curative-Intent Resection of Intrahepatic Cholangiocarcinoma. JAMA Surg. 2019, 154, e190571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sedlak, K.; Rawicz-Pruszynski, K.; Mlak, R.; Van Sandick, J.; Gisbertz, S.; Pera, M.; Dal Cero, M.; Baiocchi, G.L.; Celotti, A.; Morgagni, P.; et al. Textbook Oncological Outcome in European Gastrodata. Ann. Surg. 2023, 278, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Deal, A.M.; Kris, M.G.; Scher, H.I.; Hudis, C.A.; Sabbatini, P.; Rogak, L.; Bennett, A.V.; Dueck, A.C.; Atkinson, T.M.; et al. Symptom Monitoring with Patient-Reported Outcomes during Routine Cancer Treatment: A Randomized Controlled Trial. J. Clin. Oncol. 2016, 34, 557–565. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Basch, E.; Deal, A.M.; Dueck, A.C.; Scher, H.I.; Kris, M.G.; Hudis, C.; Schrag, D. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring during Routine Cancer Treatment. JAMA 2017, 318, 197–198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, T.H.; Kim, I.H.; Kang, S.J.; Choi, M.; Kim, B.H.; Eom, B.W.; Kim, B.J.; Min, B.H.; Choi, C.I.; Shin, C.M.; et al. Korean Practice Guidelines for Gastric Cancer 2022: An Evidence-based, Multidisciplinary Approach. J. Gastric Cancer 2023, 23, 3–106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Claassen, Y.H.M.; Hartgrink, H.H.; Dikken, J.L.; de Steur, W.O.; van Sandick, J.W.; van Grieken, N.C.T.; Cats, A.; Trip, A.K.; Jansen, E.P.M.; Meershoek-Klein Kranenbarg, W.M.; et al. Surgical morbidity and mortality after neoadjuvant chemotherapy in the CRITICS gastric cancer trial. Eur. J. Surg. Oncol. 2018, 44, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, N.; Zhou, H.; Wang, T.; Mao, Q.; Zhang, X.; Zhao, D. Effects of Neoadjuvant Chemotherapy Toxicity and Postoperative Complications on Short-term and Long-term Outcomes After Curative Resection of Gastric Cancer. J. Gastrointest. Surg. 2020, 24, 1278–1289. [Google Scholar] [CrossRef] [PubMed]
- Coccolini, F.; Nardi, M.; Montori, G.; Ceresoli, M.; Celotti, A.; Cascinu, S.; Fugazzola, P.; Tomasoni, M.; Glehen, O.; Catena, F.; et al. Neoadjuvant chemotherapy in advanced gastric and esophago-gastric cancer. Meta-analysis of randomized trials. Int. J. Surg. 2018, 51, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C.; on behalf of the ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Services Usdohah. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0; Services Usdohah: Beijing, China, 2017. [Google Scholar]
- Ruol, A.; Portale, G.; Castoro, C.; Merigliano, S.; Cagol, M.; Cavallin, F.; Chiarion Sileni, V.; Corti, L.; Rampado, S.; Costantini, M.; et al. Effects of neoadjuvant therapy on perioperative morbidity in elderly patients undergoing esophagectomy for esophageal cancer. Ann. Surg. Oncol. 2007, 14, 3243–3250. [Google Scholar] [CrossRef] [PubMed]
- Kudou, K.; Nakashima, Y.; Haruta, Y.; Nambara, S.; Tsuda, Y.; Kusumoto, E.; Ando, K.; Kimura, Y.; Hashimoto, K.; Yoshinaga, K.; et al. Comparison of Inflammation-Based Prognostic Scores Associated with the Prognostic Impact of Adenocarcinoma of Esophagogastric Junction and Upper Gastric Cancer. Ann. Surg. Oncol. 2021, 28, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Grenader, T.; Waddell, T.; Peckitt, C.; Oates, J.; Starling, N.; Cunningham, D.; Bridgewater, J. Prognostic value of neutrophil-to-lymphocyte ratio in advanced oesophago-gastric cancer: Exploratory analysis of the REAL-2 trial. Ann. Oncol. 2016, 27, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Skorzewska, M.; Pikula, A.; Geca, K.; Mlak, R.; Rawicz-Pruszynski, K.; Sedlak, K.; Pasnik, I.; Polkowski, W.P. Systemic inflammatory response markers for prediction of response to neoadjuvant chemotherapy in patients with advanced gastric cancer. Cytokine 2023, 172, 156389. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Hiki, N.; Nunobe, S.; Kumagai, K.; Kubota, T.; Aikou, S.; Sano, T.; Yamaguchi, T. Prognostic importance of the inflammation-based Glasgow prognostic score in patients with gastric cancer. Br. J. Cancer 2012, 107, 275–279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakamura, N.; Kinami, S.; Tomita, Y.; Miyata, T.; Fujita, H.; Takamura, H.; Ueda, N.; Kosaka, T. The neutrophil/lymphocyte ratio as a predictor of successful conversion surgery for stage IV gastric cancer: A retrospective study. BMC Cancer 2020, 20, 363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pikula, A.; Skorzewska, M.; Pelc, Z.; Mlak, R.; Geca, K.; Sedlak, K.; Cisel, B.; Kwietniewska, M.; Rawicz-Pruszynski, K.; Polkowski, W.P. Prognostic Value of Systemic Inflammatory Response Markers in Patients Undergoing Neoadjuvant Chemotherapy and Gastrectomy for Advanced Gastric Cancer in the Eastern European Population. Cancers 2022, 14, 1997. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grenader, T.; Plotkin, Y.; Mohammadi, B.; Dawas, K.; Hashemi, M.; Mughal, M.; Bridgewater, J.A. Predictive Value of the Neutrophil/Lymphocyte Ratio in Peritoneal and/or Metastatic Disease at Staging Laparoscopy for Gastric and Esophageal Adenocarcinoma. J. Gastrointest. Cancer 2015, 46, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, R.; Inagawa, S.; Sano, N.; Tadano, S.; Adachi, S.; Yamamoto, M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur. J. Surg. Oncol. 2018, 44, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xu, W.; Cheng, H.; Shen, W.; Ying, J.; Cheng, F.; Xu, W. The Prognostic Role of the Platelet-Lymphocytes Ratio in Gastric Cancer: A Meta-Analysis. PLoS ONE 2016, 11, e0163719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, H.; Ruan, G.; Ge, Y.; Zhang, Q.; Zhang, H.; Lin, S.; Song, M.; Zhang, X.; Liu, X.; Li, X.; et al. Inflammatory burden as a prognostic biomarker for cancer. Clin. Nutr. 2022, 41, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Okamoto, K.; Kawaguchi, T.; Nakamura, F.; Miyamoto, H.; Takayama, T. Treatment Response Predictors of Neoadjuvant Therapy for Locally Advanced Gastric Cancer: Current Status and Future Perspectives. Biomedicines 2022, 10, 1614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, J.X.; Wang, Z.K.; Huang, Y.Q.; Xie, J.W.; Wang, J.B.; Lu, J.; Chen, Q.Y.; Lin, M.; Tu, R.H.; Huang, Z.N.; et al. Dynamic Changes in Pre-and Postoperative Levels of Inflammatory Markers and Their Effects on the Prognosis of Patients with Gastric Cancer. J. Gastrointest. Surg. 2021, 25, 387–396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ding, P.; Wu, H.; Liu, P.; Sun, C.; Yang, P.; Tian, Y.; Guo, H.; Liu, Y.; Zhao, Q. The inflammatory burden index: Apromising prognostic predictor in patients with locally advanced gastric cancer. Clin. Nutr. 2023, 42, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Fournier, L.; de Geus-Oei, L.F.; Regge, D.; Oprea-Lager, D.E.; D’Anastasi, M.; Bidaut, L.; Bauerle, T.; Lopci, E.; Cappello, G.; Lecouvet, F.; et al. Twenty Years on: RECIST as a Biomarker of Response in Solid Tumours an EORTC Imaging Group—ESOI Joint Paper. Front. Oncol. 2021, 11, 800547. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023, 26, 1–25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schwartz, L.H.; Seymour, L.; Litiere, S.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Standardisation and disease-specific adaptations: Perspectives from the RECIST Working Group. Eur. J. Cancer 2016, 62, 138–145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Goebel, J.; Hoischen, J.; Gramsch, C.; Schemuth, H.P.; Hoffmann, A.C.; Umutlu, L.; Nassenstein, K. Tumor response assessment: Comparison between unstructured free text reporting in routine clinical workflow and computer-aided evaluation based on RECIST 1.1 criteria. J. Cancer Res. Clin. Oncol. 2017, 143, 2527–2533. [Google Scholar] [CrossRef] [PubMed]
- Pelc, Z.; Skorzewska, M.; Rawicz-Pruszynski, K.; Polkowski, W.P. Lymph Node Involvement in Advanced Gastric Cancer in the Era of Multimodal Treatment-Oncological and Surgical Perspective. Cancers 2021, 13, 2509. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.J.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berbis, M.A.; Aneiros-Fernandez, J.; Mendoza Olivares, F.J.; Nava, E.; Luna, A. Role of artificial intelligence in multidisciplinary imaging diagnosis of gastrointestinal diseases. World J. Gastroenterol. 2021, 27, 4395–4412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rawicz-Pruszynski, K.; Erodotou, M.; Pelc, Z.; Sedlak, K.; Polkowski, W.; Pawlik, T.M.; Wijnhoven, B.P.L. Techniques of staging laparoscopy and peritoneal fluid assessment in gastric cancer: A systematic review. Int. J. Surg. 2023, 109, 3578–3589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ikoma, N.; Blum, M.; Chiang, Y.J.; Estrella, J.S.; Roy-Chowdhuri, S.; Fournier, K.; Mansfield, P.; Ajani, J.A.; Badgwell, B.D. Yield of Staging Laparoscopy and Lavage Cytology for Radiologically Occult Peritoneal Carcinomatosis of Gastric Cancer. Ann. Surg. Oncol. 2016, 23, 4332–4337. [Google Scholar] [CrossRef] [PubMed]
- Rawicz-Pruszynski, K.; Mielko, J.; Pudlo, K.; Lisiecki, R.; Skoczylas, T.; Murawa, D.; Polkowski, W.P. Yield of staging laparoscopy in gastric cancer is influenced by Lauren histologic subtype. J. Surg. Oncol. 2019, 120, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Sando, A.D.; Fougner, R.; Royset, E.S.; Dai, H.Y.; Gronbech, J.E.; Bringeland, E.A. Response Evaluation after Neoadjuvant Chemotherapy for Resectable Gastric Cancer. Cancers 2023, 15, 2318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pressoir, M.; Desne, S.; Berchery, D.; Rossignol, G.; Poiree, B.; Meslier, M.; Traversier, S.; Vittot, M.; Simon, M.; Gekiere, J.P.; et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br. J. Cancer 2010, 102, 966–971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Attar, A.; Malka, D.; Sabate, J.M.; Bonnetain, F.; Lecomte, T.; Aparicio, T.; Locher, C.; Laharie, D.; Ezenfis, J.; Taieb, J. Malnutrition is high and underestimated during chemotherapy in gastrointestinal cancer: An AGEO prospective cross-sectional multicenter study. Nutr. Cancer 2012, 64, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Schiessel, D.L.; Orrutea, A.K.G.; Tramontt, C.; Cavagnari, M.A.V.; Novello, D.; Macedo, D.S. Clinical and nutritional characteristics on overall survival impact in patients with gastrointestinal cancer. Clin Nutr. ESPEN 2022, 48, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Gioulbasanis, I.; Martin, L.; Baracos, V.E.; Thezenas, S.; Koinis, F.; Senesse, P. Nutritional assessment in overweight and obese patients with metastatic cancer: Does it make sense? Ann. Oncol. 2015, 26, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA 2013, 309, 71–82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Solanki, S.; Chakinala, R.C.; Haq, K.F.; Khan, M.A.; Kifayat, A.; Linder, K.; Khan, Z.; Mansuri, U.; Haq, K.S.; Nabors, C.; et al. Inpatient burden of gastric cancer in the United States. Ann. Transl. Med. 2019, 7, 772. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ida, S.; Kumagai, K.; Nunobe, S. Current status of perioperative nutritional intervention and exercise in gastric cancer surgery: A review. Ann. Gastroenterol. Surg. 2022, 6, 197–203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kovoor, J.G.; Nann, S.D.; Barot, D.D.; Garg, D.; Hains, L.; Stretton, B.; Ovenden, C.D.; Bacchi, S.; Chan, E.; Gupta, A.K.; et al. Prehabilitation for general surgery: A systematic review of randomized controlled trials. ANZ J. Surg. 2023, 93, 2411–2425. [Google Scholar] [CrossRef] [PubMed]
- Tsoulfas, G. The Critical Evolution of the Concept of Frailty in Surgery. Ann. Surg. Oncol. 2024, 31, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Kwon, J.; Han, J.; Fan, G.H.; Hastie, D.J.; Lee, K.J.; Karagozian, R. The clinical impact of frailty on the postoperative outcomes of patients undergoing gastrectomy for gastric cancer: A propensity-score matched database study. Gastric Cancer 2022, 25, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.; Varley, P.; Youk, A.; Borrebach, J.D.; Perez, S.; Massarweh, N.N.; Johanning, J.M.; Hall, D.E. Recalibration and External Validation of the Risk Analysis Index: A Surgical Frailty Assessment Tool. Ann. Surg. 2020, 272, 996–1005. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ljungqvist, O.; Scott, M.; Fearon, K.C. Enhanced Recovery after Surgery: A Review. JAMA Surg. 2017, 152, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, U.O.; Oppelstrup, H.; Thorell, A.; Nygren, J.; Ljungqvist, O. Adherence to the ERAS protocol is Associated with 5-Year Survival after Colorectal Cancer Surgery: A Retrospective Cohort Study. World J. Surg. 2016, 40, 1741–1747. [Google Scholar] [CrossRef] [PubMed]
- Romario, U.F.; Ascari, F.; De Pascale, S.; GIRCG. Implementation of the ERAS program in gastric surgery: A nationwide survey in Italy. Updates Surg. 2023, 75, 141–148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vitaloni, M.; Caccialanza, R.; Ravasco, P.; Carrato, A.; Kapala, A.; de van der Schueren, M.; Constantinides, D.; Backman, E.; Chuter, D.; Santangelo, C.; et al. The impact of nutrition on the lives of patients with digestive cancers: A position paper. Support. Care Cancer 2022, 30, 7991–7996. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caccialanza, R.; Goldwasser, F.; Marschal, O.; Ottery, F.; Schiefke, I.; Tilleul, P.; Zalcman, G.; Pedrazzoli, P. Unmet needs in clinical nutrition in oncology: A multinational analysis of real-world evidence. Ther. Adv. Med. Oncol. 2020, 12, 1758835919899852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Team, Working Group I. The SuRF Report 2. In Surveillance of Chronic Disease Risk Factors: Country-Level Data and Comparable Estimates; WHO: Geneva, Switzerland, 2005; p. 22. [Google Scholar]
- Francois, Y.; Nemoz, C.J.; Baulieux, J.; Vignal, J.; Grandjean, J.P.; Partensky, C.; Souquet, J.C.; Adeleine, P.; Gerard, J.P. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: The Lyon R90-01 randomized trial. J. Clin. Oncol. 1999, 17, 2396. [Google Scholar] [CrossRef] [PubMed]
- Wolthuis, A.M.; Penninckx, F.; Haustermans, K.; De Hertogh, G.; Fieuws, S.; Van Cutsem, E.; D’Hoore, A. Impact of interval between neoadjuvant chemoradiotherapy and TME for locally advanced rectal cancer on pathologic response and oncologic outcome. Ann. Surg. Oncol. 2012, 19, 2833–2841. [Google Scholar] [CrossRef] [PubMed]
- Omarini, C.; Guaitoli, G.; Noventa, S.; Andreotti, A.; Gambini, A.; Palma, E.; Papi, S.; Tazzioli, G.; Balduzzi, S.; Dominici, M.; et al. Impact of time to surgery after neoadjuvant chemotherapy in operable breast cancer patients. Eur. J. Surg. Oncol. 2017, 43, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, K.; Klevebro, F.; Sunde, B.; Rouvelas, I.; Lindblad, M.; Szabo, E.; Halldestam, I.; Smedh, U.; Wallner, B.; Johansson, J.; et al. Oncological outcomes of standard versus prolonged time to surgery after neoadjuvant chemoradiotherapy for oesophageal cancer in the multicentre, randomised, controlled NeoRes II trial. Ann. Oncol. 2023, 34, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zheng, Z.; Deng, W.; Yin, J.; Bai, Z.; Liu, X.; Zhang, J.; Zhang, Z. Interval time between neoadjuvant chemotherapy and surgery in advanced gastric cancer doesn’t affect outcome: A meta analysis. Front. Surg. 2022, 9, 1047456. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Z.; Zhang, Z.; Liu, H.; Chen, J. Time to surgery does not affect oncologic outcomes in locally advanced gastric cancer after neoadjuvant chemotherapy: A meta-analysis. Future Oncol. 2023, 19, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Reinsoo, A.; Bausys, A.; Umarik, T.; Strupas, K. ASO Author Reflections: Gastrectomy within 30 Days after Neoadjuvant Chemotherapy is Associated with the Highest Rate of Major Pathologic Response in Advanced Gastric Cancer. Ann. Surg. Oncol. 2021, 28, 4456–4457. [Google Scholar] [CrossRef] [PubMed]
- Riascos, M.C.; Greenberg, J.A.; Palacardo, F.; Edelmuth, R.; Lewis, V.C.; An, A.; Najah, H.; Al Asadi, H.; Safe, P.; Finnerty, B.M.; et al. Timing to Surgery and Lymph Node Upstaging in Gastric Cancer: An NCDB Analysis. Ann. Surg. Oncol. 2023, 31, 1714–1724. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Visser, K.E.; Jonkers, J. Towards understanding the role of cancer-associated inflammation in chemoresistance. Curr. Pharm. Des. 2009, 15, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Zaanan, A.; Bouche, O.; de la Fouchardiere, C.; Samalin-Scalzi, E.; Le Malicot, K.; Pernot, S.; Artru, P.; Ly Lebrun, V.; Aldabbagh, K.; Khemissa Akouz, F.; et al. LBA77 5-fluorouracil and oxaliplatin with or without docetaxel in the first-line treatment of HER2 negative locally advanced (LA) unresectable or metastatic gastric or gastro-esophageal junction (GEJ) adenocarcinoma (GASTFOX-PRODIGE 51): A randomized phase III trial sponsored by the FFCD. Ann. Oncol. 2023, 34, S1318. [Google Scholar] [CrossRef]
- Shitara, K.; Rha, S.Y.; Wyrwicz, L.S.; Oshima, T.; Karaseva, N.; Osipov, M.; Yasui, H.; Yabusaki, H.; Afanasyev, S.; Park, Y.-K.; et al. LBA74 Pembrolizumab plus chemotherapy vs chemotherapy as neoadjuvant and adjuvant therapy in locally-advanced gastric and gastroesophageal junction cancer: The phase 3 KEYNOTE-585 study. Ann. Oncol. 2023, 34, S1316. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Al-Batran, S.-E.; Wainberg, Z.A.; Van Cutsem, E.; Molena, D.; Muro, K.; Hyung, W.J.; Wyrwicz, L.S.; Oh, D.-Y.; Omori, T.; et al. LBA73 Pathological complete response (pCR) to durvalumab plus 5-fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) in resectable gastric and gastroesophageal junction cancer (GC/GEJC): Interim results of the global, phase 3 MATTERHORN study. Ann. Oncol. 2023, 34, S1315–S1316. [Google Scholar] [CrossRef]
- Rijken, A.; Lurvink, R.J.; Luyer, M.D.P.; Nieuwenhuijzen, G.A.P.; van Erning, F.N.; van Sandick, J.W.; de Hingh, I. The Burden of Peritoneal Metastases from Gastric Cancer: A Systematic Review on the Incidence, Risk Factors and Survival. J. Clin. Med. 2021, 10, 4882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thomassen, I.; van Gestel, Y.R.; van Ramshorst, B.; Luyer, M.D.; Bosscha, K.; Nienhuijs, S.W.; Lemmens, V.E.; de Hingh, I.H. Peritoneal carcinomatosis of gastric origin: A population-based study on incidence, survival and risk factors. Int. J. Cancer 2014, 134, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H.; Cunliffe, W.J.; Belliveau, J.; de Bruijn, E.A.; Graves, T.; Mullins, R.E.; Schlag, P. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin. Oncol. 1989, 16 (Suppl. S6), 83–97. [Google Scholar] [PubMed]
- Rau, B.; Lang, H.; Koenigsrainer, A.; Gockel, I.; Rau, H.G.; Seeliger, H.; Lerchenmueller, C.; Reim, D.; Wahba, R.; Angele, M.; et al. Effect of Hyperthermic Intraperitoneal Chemotherapy on Cytoreductive Surgery in Gastric Cancer with Synchronous Peritoneal Metastases: The Phase III GASTRIPEC-I Trial. J. Clin. Oncol. 2023, 42, JCO2202867. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Passot, G.; Villeneuve, L.; Vaudoyer, D.; Bin-Dorel, S.; Boschetti, G.; Piaton, E.; Garofalo, A. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: A randomized and multicenter phase III study. BMC Cancer 2014, 14, 183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Badgwell, B.D. Don’t Call It a Comeback-HIPEC for Gastric Cancer. Ann. Surg. Oncol. 2022, 29, 7244–7245. [Google Scholar] [CrossRef] [PubMed]
- van der Kaaij, R.T.; Wassenaar, E.C.E.; Koemans, W.J.; Sikorska, K.; Grootscholten, C.; Los, M.; Huitema, A.; Schellens, J.H.M.; Veenhof, A.; Hartemink, K.J.; et al. Treatment of PERItoneal disease in Stomach Cancer with cytOreductive surgery and hyperthermic intraPEritoneal chemotherapy: PERISCOPE I initial results. Br. J. Surg. 2020, 107, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Gotze, T.O.; Piso, P.; Lorenzen, S.; Bankstahl, U.S.; Pauligk, C.; Elshafei, M.; Amato, G.; Reim, D.; Bechstein, W.O.; Konigsrainer, A.; et al. Preventive HIPEC in combination with perioperative FLOT versus FLOT alone for resectable diffuse type gastric and gastroesophageal junction type II/III adenocarcinoma—The phase III “PREVENT”—(FLOT9) trial of the AIO/CAOGI/ACO. BMC Cancer 2021, 21, 1158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gervaso, L.; Pellicori, S.; Cella, C.A.; Bagnardi, V.; Lordick, F.; Fazio, N. Biomarker evaluation in radically resectable locally advanced gastric cancer treated with neoadjuvant chemotherapy: An evidence reappraisal. Ther. Adv. Med. Oncol. 2021, 13, 17588359211029559. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| TNO Category | Description | ||
|---|---|---|---|
| Imaging ** | |||
| Complete Response * | Disappearance of all target lesions, with any pathological lymph nodes diameter < 10 mm in short axis. | ||
| Partial Response * | At least a 30% decrease in the sum of diameters of target lesions, taking as reference the baseline sum diameters. | ||
| Progressive Disease | At least a 20% increase in the sum of diameters of target lesions, taking as reference the smallest sum on study, as well as appearance of one or more new lesions. | ||
| Stable Disease * | Neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the smallest sum diameter. | ||
| Timing to Surgery | |||
| ≤4 weeks | 32% of mPR in European cohort [32]; twofold higher odds for achievement of mPR (OR 2.09; 95% CI 1.01–4.34, p = 0.047). | ||
| 4–6 weeks * | Highest rate of ypT3-4 tumors (67.5%) and any postoperative complications (44.9%). | ||
| >6 weeks | Highest rate of lymphovascular invasion and ypN+ (62.5%), lowest rate of NAC completion (84.7%). | ||
| Nutrition BMI < 18.5 kg/m2 BMI 18.5–25 kg/m2 * BMI > 25 kg/m2 25–29.9 kg/m2 >30 kg/m2 | Underweight Normal weight Overweight Pre-obesity Obesity |  | Among ESPEN criteria, BMI is the only one associated with prognosis; nutritional status deterioration may occur independently of body weight thus it should be assessed at early stage of oncologic treatment. |
| Laboratory Tests NLR ≤ 2 * NLR > 2 | Low NLR; favorable prognosis, increased OS. High NLR; decreased OS and PFS. | ||
| Treatment Toxicity CTCAE v.5 | |||
| Grade 1 * | Mild; no intervention needed, asymptomatic or mild symptoms. | ||
| Grade 2 * | Moderate; requires minimal intervention; affects age-appropriate instrumental ADL. | ||
| Grade 3 | Severe or medically significant; not immediately life-threatening; requires hospitalization; affects self-care ADL. | ||
| Grade 4 | Life-threatening consequences; requires urgent intervention. | ||
| Grade 5 | Death related to adverse event. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelc, Z.; Sędłak, K.; Leśniewska, M.; Mielniczek, K.; Chawrylak, K.; Skórzewska, M.; Ciszewski, T.; Czechowska, J.; Kiszczyńska, A.; Wijnhoven, B.P.L.; et al. Textbook Neoadjuvant Outcome—Novel Composite Measure of Oncological Outcomes among Gastric Cancer Patients Undergoing Multimodal Treatment. Cancers 2024, 16, 1721. https://doi.org/10.3390/cancers16091721
Pelc Z, Sędłak K, Leśniewska M, Mielniczek K, Chawrylak K, Skórzewska M, Ciszewski T, Czechowska J, Kiszczyńska A, Wijnhoven BPL, et al. Textbook Neoadjuvant Outcome—Novel Composite Measure of Oncological Outcomes among Gastric Cancer Patients Undergoing Multimodal Treatment. Cancers. 2024; 16(9):1721. https://doi.org/10.3390/cancers16091721
Chicago/Turabian StylePelc, Zuzanna, Katarzyna Sędłak, Magdalena Leśniewska, Katarzyna Mielniczek, Katarzyna Chawrylak, Magdalena Skórzewska, Tomasz Ciszewski, Joanna Czechowska, Agata Kiszczyńska, Bas P. L. Wijnhoven, and et al. 2024. "Textbook Neoadjuvant Outcome—Novel Composite Measure of Oncological Outcomes among Gastric Cancer Patients Undergoing Multimodal Treatment" Cancers 16, no. 9: 1721. https://doi.org/10.3390/cancers16091721
APA StylePelc, Z., Sędłak, K., Leśniewska, M., Mielniczek, K., Chawrylak, K., Skórzewska, M., Ciszewski, T., Czechowska, J., Kiszczyńska, A., Wijnhoven, B. P. L., Van Sandick, J. W., Gockel, I., Gisbertz, S. S., Piessen, G., Eveno, C., Bencivenga, M., De Manzoni, G., Baiocchi, G. L., Morgagni, P., ... Rawicz-Pruszyński, K. (2024). Textbook Neoadjuvant Outcome—Novel Composite Measure of Oncological Outcomes among Gastric Cancer Patients Undergoing Multimodal Treatment. Cancers, 16(9), 1721. https://doi.org/10.3390/cancers16091721












