Simple Summary
Kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one), a flavonoid, is richly found in fruits and vegetables. Kaempferol has been proven to reduce tumor cell growth by modulating several pathways and molecular mechanisms, such as causing G0/G1 phase arrest in esophageal cancer, reducing G2/M cell cycle proteins in gastric cancer, inducing apoptosis by Akt/mTOR pathway in pancreatic cancer, causing cell cycle arrest (especially HT-29 human colon cancer cells), and suppressing cell growth by PI3K/mTOR/MMP signaling pathways in liver cancer. The application of nanotechnology has been shown to enhance the efficacy of kaempferol against gastrointestinal cancer. Mechanistic studies showed kaempferol-conjugated nanoparticles inducing oxidative stress-mediated apoptosis and cell cycle arrest in liver cancer cells, potentially leading to anti-cancer effects. Notably, kaempferol-conjugated gold nanoclusters have also shown efficacy in reducing tumor volume in vivo. However, there is a lack of research specifically focused on gastrointestinal cancers, highlighting the need for further exploration in this area.
Abstract
In recent years, kaempferol, a natural flavonoid present in various fruits and vegetables, has received significant attention in gastrointestinal cancer research due to its varied therapeutic effects. Kaempferol has been proven to alter several molecular mechanisms and pathways, such as the PI3/Akt, mTOR, and Erk/MAPK pathway involved in cancer progression, showing its inhibitory effects on cell proliferation, survival, angiogenesis, metastasis, and migration. Kaempferol is processed in the liver and small intestine, but limited bioavailability has been a major concern in the clinical implications of kaempferol. Nano formulations have been proven to enhance kaempferol’s efficacy in cancer prevention. The synergy of nanotechnology and kaempferol has shown promising results in in vitro studies, highlighting the importance for more in vivo research and clinical trials to determine safety and efficacy. This review aims to focus on the role of kaempferol in various types of gastrointestinal cancer and how the combination of kaempferol with nanotechnology helps in improving therapeutic efficacy in cancer treatment.
1. Introduction
The second leading cause of cancer-related death worldwide is gastrointestinal cancer (GI) [1]. Affecting the entire gastrointestinal tract and its associated organs, GI cancer encompasses various types, such as esophageal, colon, pancreatic, stomach (gastric), and liver cancer [2]. Symptoms and risk factors differ depending on the type of cancer. Individuals diagnosed with esophageal cancer may encounter challenges in swallowing, while those with stomach cancer might undergo symptoms resembling ulcers, such as indigestion, reduced appetite, bloating, and pain [3,4,5,6,7]. Continuous stomach acid problems, a diet high in salty and smoked foods, smoking, and a family history of the disease are all risk factors for stomach cancer [8]. Early identification is critical for efficient treatment, and gastrointestinal malignancies are identified using a variety of diagnostic procedures.
The type and stage of cancer determine which therapeutic measures are used. Surgical techniques, radiation therapy, chemotherapy, and targeted therapy are among the available treatment approaches, but these comes with numerous side effects, such as the fact that these therapies can damage the surrounding healthy cells [9,10]. Radiation therapy can cause long-term side effects to the brain, spinal cord, and nerves. Surgery may also result in side effects such as tissue scarring, pain, intestinal problems, and chronic diarrhea. Chemotherapy can lead to side effects like neutropenia, lymphedema, hair loss, nausea and vomiting, and blood clots [11]. Phytochemicals, which are bio-active compounds found in several fruits, cereals, and vegetables, such as apples, cherries, berries, kale, broccoli, spinach, tomatoes, and leafy vegetables have been examined for their possible role in disease prevention, particularly in gastrointestinal (GI) cancer [12]. Phytochemicals have fewer and less severe side effects as compared to conventional cancer treatments [13,14]. They have been shown to improve cancer prognosis through a variety of biological processes that increase apoptosis and prevent cancer growth [12]. Furthermore, phytochemicals have been shown to have strong anticancer effects, triggering apoptosis and autophagy, and decreasing tumor cell resistance to chemotherapeutic drugs in gastric cancer [15,16,17,18,19,20,21]. Some phytochemicals have been examined for their potential impact on GI cancer, including quercetin, ellagic acid, allicin, curcumins, and others [12,15,22,23,24].
Kaempferol, a natural flavonoid having various functions (Figure 1), has interestingly shown potential in lowering GI malignancies, particularly colorectal and stomach cancers [25]. It has been shown in research studies that kaempferol reduces cancer cell proliferation, causes apoptosis, and blocks the cell cycle at the G2/M phase in colorectal and gastric cancer cell lines. Furthermore, kaempferol has shown its ability to overcome 5-fluorouracil resistance, indicating its usefulness as a chemotherapeutic drug. It has also been proved to affect several signaling pathways that are related with the progression and initiation of GI cancers [26,27,28]. This review will address the significance of kaempferol in preventing various GI malignancies.
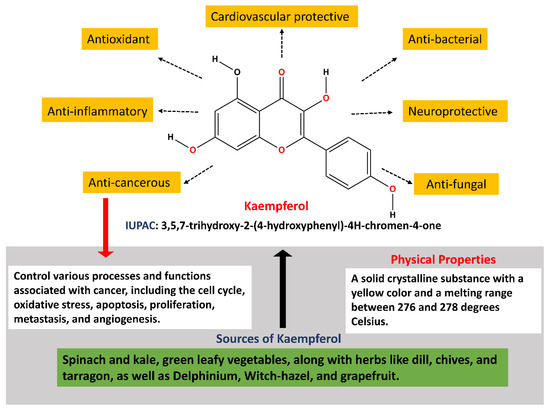
Figure 1.
Diverse functions of kaempferol.
2. Chemistry and Pharmacokinetics of Kaempferol
Kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one) is a polyphenol, a flavonoid that is richly found in fruits and vegetables. Kaempferol possesses a diphenylpropane structure, which contributes to its hydrophobic characteristics. Its synthesis begins with the condensation of 4-coumaroyl-CoA and three malonyl-CoA molecules, catalyzed by chalcone synthase, yielding naringenin chalcone. Subsequently, under the action of chalcone isomerase, naringenin chalcone is converted into naringenin, a flavanone. Then, through the enzymatic activity of flavanone 3-dioxygenase, a hydroxyl group is added to naringenin at the C3 position, resulting in dihydrokaempferol. Finally, the formation of kaempferol is accomplished by the introduction of a double bond at the C2–C3 position in the dihydrokaempferol skeleton, mediated by flavonol synthase (Figure 2) [29,30,31]. Kaempferol is primarily found in its conjugated form in both plasma and urine [32]. Kaempferol is frequently consumed in the form of a glycoside. Glycosides are compounds composed of a sugar molecule attached to a non-sugar molecule, referred to as an aglycone [33,34]. The colon’s flora helps in metabolizing kaempferol glycoside into aglycones, which is further metabolized and absorbed into systemic circulation [35]. This suggests that the metabolism of kaempferol involves its conjugation with other molecules, likely to enhance its solubility and facilitate its excretion from the body. Such conjugation processes play a crucial role in the pharmacokinetics and elimination of kaempferol from the systemic circulation, shaping its bioavailability and potential physiological effects [36].
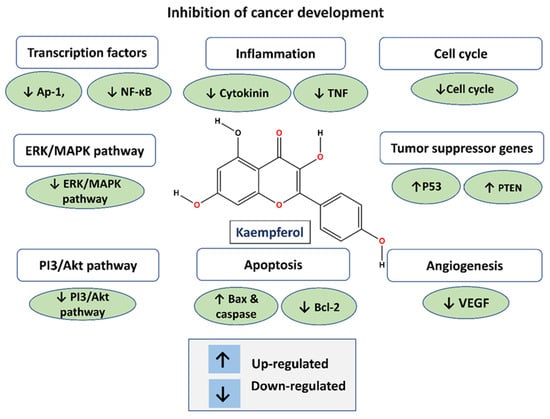
Figure 2.
Anti-cancer function of kaempferol.
Kaempferol, like other flavonoids, is recognized for its anti-inflammatory and antioxidant properties. Because of its antioxidant and anti-inflammatory qualities, kaempferol has been intensively researched for its possible significance in the prevention of gastrointestinal malignancies [37,38,39,40,41]. Kaempferol has been demonstrated to have various modes of action that contribute to its potential as a preventative agent against gastrointestinal malignancies, in addition to its antioxidant and anti-inflammatory characteristics [35]. For example, it has been proven to limit cancer cell development, cause apoptosis (programmed cell death), suppress tumor angiogenesis (the construction of blood vessels that nourish a tumor), and inhibit metastasis (the spread of cancer cells to different sections of the body) [41,42] (Figure 2) (Table 1). Flavonols, including kaempferol, are frequently consumed as glycosides. Studies have demonstrated that glycosides can be absorbed without hydrolysis, despite the suggestion that this requires the prior hydrolysis of absorbable aglycones [31,43,44].
Kaempferol undergoes low to moderate absorption, with the oral bioavailability being very low, estimated at around 2% when compared to intravenous dosages. This limited bioavailability is attributed, at least in part, to extensive first-pass metabolism. Both phase I oxidative metabolism and phase II glucuronidation occur in the intestine as well as in the liver, contributing to the efficient clearance of kaempferol from the systemic circulation upon oral administration [36,45]. In the liver, kaempferol undergoes metabolism, leading to the formation of sulfo-conjugates and glucuronides. Subsequently, in the colon, kaempferol conjugates give rise to phenolic compounds. Ultimately, both kaempferol and the derivatives of kaempferol’s glycosides are excreted in the urine [31,35]. The plasma concentration of kaempferol in rats peaked at 1–1.5 h after oral dose and subsequently dropped up to 6 h. After six hours, no free kaempferol was detected [45]. To address the challenge of low bioavailability associated with kaempferol, research indicates that utilizing nanoformulations such as nanoparticles, nanoemulsions, and nanoencapsulation to deliver kaempferol can significantly enhance its bioavailability and subsequent efficacy. Moreover, these nanoformulations have the potential to enhance selectivity for mutated cells while minimizing their impact on normal cells, thereby offering a promising approach for cancer therapy [46].

Table 1.
The biochemical and physiological properties of kaempferol.
Table 1.
The biochemical and physiological properties of kaempferol.
| Properties | Details | References |
|---|---|---|
| Molecular Weight | 286.24 g/mol | |
| Classification | Flavonol | [47] |
| Solubility | Soluble in polar solvents, such as ethanol and methanol | [48] |
| Melting Point | Approximately 276–278 °C | - |
| Boiling Point | Decomposes before boiling | - |
| Color | Yellow crystalline powder | [47] |
| Odor | Odorless | [47] |
| Taste | Bitter taste | [47] |
| UV Absorption | Absorbs UV light at 266 nm and 365 nm | [49] |
| Biological Sources | Found in tea, apples, onions, grapes, broccoli, and more | [49] |
| Bioavailability | Moderate absorption in the human digestive system | [50] |
| Metabolism | Metabolized in the liver, forming various conjugates | [51] |
| Distribution in Body | Distributed in various tissues | [52] |
| Half-life in Body | Variable, influenced by factors like age and health | [53] |
| Excretion | Excreted mainly through urine | [54] |
| Biological Activities | Antioxidant, anti-inflammatory, anti-cancer, anti-microbial, and neuroprotective properties | [49] |
| Cellular Mechanisms | Modulates gene expression and signaling pathways such as Nrf2, (PI3K)/AKT, ERK/p38 MAPK, Wnt/β-Catenin. | [38] |
| Health Benefits | Enhances heart function by reducing myocardial apoptosis, fibrosis, oxidative stress, and inflammation, while maintaining mitochondrial activity and calcium homeostasis. It also provides neuroprotective advantages. | [55,56] |
| Toxicity | In vitro studies have shown that kaempferol is carcinogenic and toxic, while these effects were not reported in in vivo screenings. | [35] |
| Safety | Several in vitro studies indicate that kaempferol’s interaction with essential nutrients such as iron and folate may hinder iron bioavailability, absorption, and cellular folic acid uptake. Furthermore, a few in vitro studies demonstrate that kaempferol possesses antioxidative properties; excessive supplementation might lead to self-oxidation (pro-oxidation). However, animal studies indicate no pro-oxidation effects after oral consumption. Nevertheless, there have been no human trials investigating the potential toxicity or adverse effects of oral kaempferol consumption. | [57] |
| Medical Applications | Kaempferol may be used for the therapy of hormone-regulated cancers such as ovarian cancer, breast cancer, cervical cancer, hepatocellular carcinoma, and leukemia. | [49] |
| Regulatory Status | Considered only as a natural compound, not a regulated drug | - |
3. Implications of Kaempferol in Gastrointestinal Cancers
The most common GI cancer malignancies include gastric cancer, colorectal cancer (CRC), liver cancer, and pancreatic cancer. Colorectal cancer is the third most frequent cancer and is associated with a high death rate [58]. On the other hand, stomach cancer ranks fourth in terms of cancer-related mortality [59]. Pancreatic cancer, ranked seventh in terms of global cancer deaths [60], and liver cancer, ranked third in the same category [61], both contribute significantly to cancer-related mortality globally. The following sections explore the most important characteristics of these gastrointestinal tumor malignancies (Figure 3).
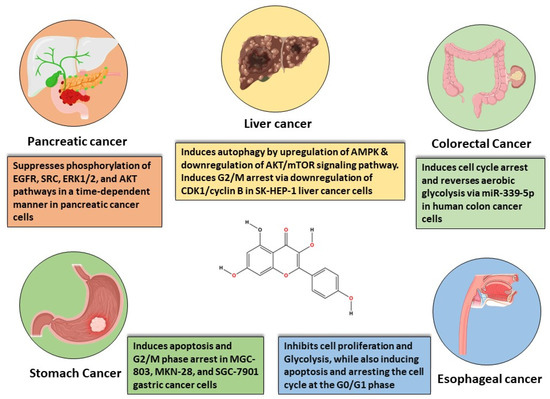
Figure 3.
In vitro-based anti-cancer mechanisms of kaempferol in various gastrointestinal cancers.
3.1. Esophageal Cancer
Esophageal cancer is the world’s tenth most frequently occurring malignancy and typically affects men and those born male who are 60 years or older [62]. Esophageal cancer is frequently misdiagnosed until it has progressed to an advanced stage, at which point treatment may involve surgery, radiation, chemotherapy, and supportive care. Recent trends in treating esophageal cancer with kaempferol have shown promising results, with the compound demonstrating potential in inhibiting cell proliferation, inducing apoptosis, and arresting the cell cycle at various phases [26,63].
Kaempferol has been shown to reduce tumor cell growth and impede in vitro clonal formation. Additionally, it causes G0/G1 phase arrest in tumor cells [64]. Notably, kaempferol significantly inhibits tumor glycolysis, leading to a marked decrease in glucose uptake and lactate production in tumor cells. This effect is attributed to the downregulation of hexokinase-2, a key enzyme involved in glycolysis. Mechanistic studies have revealed that kaempferol directly affects the activity of the epidermal growth factor receptor (EGFR). The inhibition of EGFR by kaempferol results in the suppression of downstream signaling pathways associated with glycolysis. Further investigations have demonstrated that exogenous overexpression of EGFR in tumor cells attenuates the suppression of glycolysis induced by kaempferol. This suggests that EGFR plays a crucial role in mediating the inhibitory effects of kaempferol on glycolysis in tumor cells. Overall, these findings highlight the potential of kaempferol as a therapeutic agent targeting glycolysis in cancer cells, with EGFR serving as a key molecular target for its anticancer effects [65,66,67].
Mechanistically, it may increase the expression of pro-apoptotic genes such as B-cell lymphoma-2-associated X protein (Bax) via the mitochondrial signaling pathway [68]. It suppresses caspase-9 expression while also downregulating the production of the anti-apoptotic protein B-cell lymphoma-2 (Bcl-2). Caspase-9 inhibition activates caspase-3, resulting in a caspase cascade that leads to apoptosis [67,69]
Furthermore, kaempferol has potential in the therapy of human esophageal cancer cell lines. Flavone treatment can cause G2/M arrest by increasing GADD45β and 14-3-3ε levels, while decreasing cyclin B1 mRNA and protein. It can also promote p53-independent mitochondrial apoptosis by upregulating PIG3 and promoting caspase-9 and caspase-3 cleavage [70]. These findings emphasize kaempferol’s therapeutic potential for esophageal cancer. The chemical has been proven to be more effective than docetaxel in reducing primary tumor growth, and it can overcome cisplatin resistance [26]. Even with these encouraging results, more research is required to fully understand the safety and effectiveness of kaempferol in the treatment of esophageal cancer. This research should include clinical trials as well as long-term follow-up studies.
3.2. Gastric Cancer
As per the GLOBOCAN 2022 statistics, stomach cancer had approximately 1,089,103 incidents and caused 768,793 deaths, which makes it the fourth most common cause of death globally [59]. East Asia, Eastern Europe, and South America are among the regions with higher incidence and fatality rates. Males are noticeably twice as likely than females to have stomach cancer. Ageing populations and better living circumstances have an impact on the prevalence of Helicobacter pylori, a significant cause of stomach cancer, even though incidence and death rates have gradually declined over the past century [71].
Researchers have extensively examined the impact of kaempferol on various cells of gastric cancer. In a 2010 study, kaempferol demonstrated a dose- and time-dependent induction of apoptosis and G2/M phase arrest in MGC-803 cells [61]. Another study revealed that kaempferol induced apoptosis in MKN-28 gastric cancer cell lines, while showing no effect on conventional gastric epithelial cell lines (GSE-1) [28]. Notably, xenograft tumor development was reduced without affecting body weight, the liver, or spleen in kaempferol-treated mice [28]. Additionally, SGC-7901 and MKN-28 cell lines exhibited lower levels of p-ERK, p-Akt, and COX-2 expression [32]. In 2018, researchers investigated the molecular processes and biological activity of kaempferol in stomach cancer therapy. According to the study, kaempferol induced autophagy and cell death, lowered p62 production, and accelerated the transition from LC3-I to LC3-II through an IRE1-JNK1-mediated Bcl-2-Beclin-1 link [72]. The research also uncovered another mechanism involving the HDAC/G9a pathway for kaempferol-mediated epigenetic alterations related to autophagy and cell death [72].
3.3. Colorectal Cancer
CRC stands out as one of the most prominent solid tumors in the Western world. The development of abnormal growths known as polyps in the colon or rectum is a precursor to colorectal cancer, with the potential for these polyps to progress into cancer over time [73]. Symptoms of CRC encompass alterations in bowel habits, the presence of blood in the stool, and abdominal pain [74]. Based on the molecular mechanisms of tumor formation and progression, CRC is generally classified into three classes: sporadic, inflammation-dependent, and familial [75,76]. Of these, sporadic CRC has the highest prevalence, approximately 75% [77]. In patients with inflammatory bowel disease (IBD), chronic inflammation plays a major role in the carcinogenesis of inflammation-dependent CRC [78,79]. Treatment options of CRC are determined on the stage of the disease, the patient’s performance level, and, increasingly, the molecular makeup of the tumor. Because malignancies are diagnosed at earlier stages in countries with monitoring programs, both the incidence and fatality rates have decreased [27,80]. Colorectal cancer therapy options are consistent in the metastatic scenario. Over time, CRC treatment techniques have evolved from using 5-fluorouracil (5-FU) as a single agent to more complex combination regimens that include 5-FU together with oxaliplatin, irinotecan, or a combination of both [27,80]. While traditional treatment approaches including radiation, chemotherapy, and surgery have a significant impact on the management of colorectal cancer, drug resistance and toxicity continue to be major obstacles. As a result, dietary therapy agents, especially natural products, may have been thought of as the safest substitutes for treating CRC, improving the symptoms and quality of life for individuals with the disease [81].
Kaempferol has been recommended as a promising drug for the prevention of colon cancer due to its capacity to cause cell cycle arrest, especially in HT-29 human colon cancer cells [82]. It has been shown to overcome colorectal LS174 cancer cell resistance to 5-Fu via decreasing PKM2-mediated glycolysis, implying that kaempferol may have a chemotherapeutic role, either alone or in conjunction with 5-Fu [27,80]. Kaempferol’s antioxidative and anti-inflammatory properties may help to regulate cancer growth and progression [25]. Additionally, it has been demonstrated to reverse aerobic glycolysis through regulation mediated by miR-339-5p, with potent anti-colon cancer effects [83]. Regarding how it works, kaempferol encourages colon cancer cells to express microRNA-326 (miR-326). Furthermore, miR-326 could obstruct PKM mRNA’s alternative splicing factors indirectly. As a result, this modulation aids in reversing the colorectal cancer cells’ resistance to 5-Fu.
3.4. Pancreatic Cancer
Pancreatic cancer (PC) patients show no noticeable symptoms until the disease reaches an advanced stage characterized by invasive pancreatic metastasis, making early detection challenging. Consequently, pancreatic cancer has emerged as one of the most challenging cancer malignancies [84,85]. A significant proportion of patients experience relapse, and despite potentially aggressive treatments, the 5-year survival rate remains low, ranging from 2% to 9% [70]. Pancreatic ductal adenocarcinoma is the most frequent pancreatic cancer. Family history, pancreatitis, and diabetes are all risk factors for pancreatic cancer [84].
Most PC malignancies are classified as ductal adenocarcinoma and hence represent exocrine pancreatic malignancy, whereas a minority are neuroendocrine tumors. The bulk of pancreatic ductal adenocarcinomas originate from precursor lesions classified as pancreatic intraepithelial neoplasia, which evolve in a stepwise manner through the acquisition of genetic changes to result in the formation of overt pancreatic ductal adenocarcinoma [86].
Kaempferol suppresses PC cell proliferation and migration by modulating the EGFR-related pathway. Its effective, low-dose migratory activity reduction in human pancreatic cancer cells without causing cellular harm is very notable [87]. Kaempferol reduces PC cell viability by promoting apoptosis. Kaempferol’s anti-cancer action is mediated by the suppression of phosphorylation levels in EGFR, ERK1/2, Src, and AKT pathways demonstrated in a time-dependent manner in Miapaca-2 and Panc-1 cells [87,88,89]. Furthermore, in the case of PC, the promotion of apoptosis is mediated by AKT/mTOR signaling and TGM2 pathways (Figure 4).
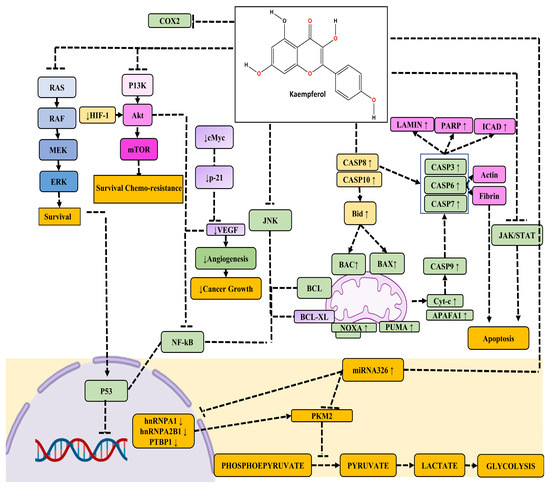
Figure 4.
The figure illustrates the anti-cancer activity of kaempferol across various molecular pathways. Kaempferol exerts its anti-cancer effects through intricate signaling pathways. It inhibits the MAPK, PI3/AKT/mTOR, and JNK pathways, thereby suppressing the NF-κB pathway. Simultaneously, the downregulation of the JNK pathway reduces the expression of anti-apoptotic proteins Bcl-XL and Bcl-2. Moreover, kaempferol enhances the expression of pro-apoptotic factors such as CASP8 and CASP10, leading to an increase in mitochondrial proteins BAX and BAK, and the activation of CASP8, CASP3, CASP6, and CASP7, ultimately resulting in apoptosis. Additionally, kaempferol promotes the expression of key apoptotic regulators including Cytochrome c, Apaf-1, NOXA, and PUMA, which are pivotal in orchestrating the apoptotic cascade. Notably, the inhibition of the JNK pathway by kaempferol has a dual impact on apoptosis, as it dampens P53-mediated cell apoptosis while simultaneously boosting CASP-mediated apoptosis, thereby contributing to the overall anti-cancer properties of kaempferol.
Research on kaempferol indicates that its anti-cancer effects on PC cell development may stem from its cytotoxic and antioxidative activities [90,91]. Kaempferol has been observed to inhibit cell growth by inducing apoptosis in cancer cells, notably through the downregulation of proliferating cell nuclear antigen (PCNA) and activation of cleaved caspase-3 pathways. This effect was dose-dependent, as evidenced by the varied responses observed at different dosages of kaempferol. Interestingly, when pancreatic cancer cells were pre-treated with z-VAD-FMK, an inhibitor of caspase activity, the inhibitory effect of kaempferol on cell growth was significantly reduced, suggesting the involvement of caspase-dependent pathways in its mechanism of action [92]. Additionally, kaempferol treatment suppresses the formation of fatty acids (FAS) and the growth of cells, causing the cells in breast and prostate cancer to undergo apoptosis. This demonstrates that blocking FAS could be linked to apoptosis in a range of cancer cells [93]. These findings show that kaempferol can function as a safe chemotherapeutic reagent in human PC cells, making it a promising option for PC treatment [88].
3.5. Liver Cancer
The most common form of liver cancer is called hepatocellular carcinoma (HCC), which starts in the main liver cell type called hepatocytes [61]. Liver cancer may be classified into two main categories: primary liver cancer, which starts inside the liver, and secondary liver cancer, which develops when cancer cells migrate to the liver from another area of the body. A hard mass just below the rib cage on the right side, upper abdominal pain, swollen belly, pain in the right shoulder blade or back, and jaundice are typical signs of liver cancer [94,95].
Mylonis et al. discovered that in hypoxic environments, kaempferol reduces the survival of hepatoma (Huh-7) cancer cells, hypoxia-inducible factor 1 (HIF-1), and mitogen-activated protein kinase (MAPK) [96]. The mitochondrial tricarboxylic acid (TCA) cycle, membrane-bound ATPase (Ca2+, ATPase, Na+/K+ ATPase, Mg2+, and ATPase), nucleic acids, and multiple classes of enzymes like G-6-P, F-1,6-diphosphatase and hexokinase, which includes carbohydrate-metabolizing enzyme classes, were significantly modulated by kaempferol in an in vivo study. This modification was seen in HCC caused by aflatoxin B1 (AFB1), demonstrating strong anti-carcinogenic qualities [97]. Another investigation identified kaempferol as an anti-inflammatory agent, demonstrating its capacity to dose-dependently inhibit the downregulation of tumor necrosis factor-alpha (TNF-α) on liver-X-receptor alpha (LXR-α) [98]. Kaempferol has been shown to exert hepatoprotective effects by modulating key enzymes and signaling pathways in the liver. In pre-clinical studies, kaempferol demonstrated hepatoprotective effects by the activation of SIRT1 and downregulation of CYP2E1and PARP1. However, the exact mechanism by which kaempferol inhibits CYP2E1 after treatment still needs to be explored [99].
Kaempferol has been found to induce apoptosis and provoke G2 m stage cell cycle arrest, consequently impeding the invasion and migration of cancerous cells. This effect is mediated through various mechanisms. Kaempferol triggers the release of cytochrome-c by generating ROS, which in turn initiates mitochondria swelling and loss of mitochondrial membrane potential (MtMP), ultimately leading to increased levels of caspase 3. Furthermore, Kaempferol has been observed to upregulate the expression of several key proteins involved in cellular signaling pathways. These include non-receptor tyrosine-protein kinase (TYK-2), Janus kinase-1 (JAK-1), microtubule-associated protein-1A-1B light chain-3 (MAPILC3), STAT1-2, and autophagy genes (Atg 5, Atg 7, and Atg 12), along with beclin-1 and phosphatase and tensin homolog (PTEN). Kaempferol downregulates the expression of cytokine signaling-3 (SOCS-3), PI3K-AKT-mTOR, miRNA-21, signal transducer and activator of transcription-3 (STAT-3), phosphorylated-mTOR signaling pathways, and HIF-1 in HCC. Overall, these findings highlight the multifaceted effects of kaempferol in modulating cellular pathways involved in cancer progression, underscoring its potential as a therapeutic agent in the treatment of HCC [96,100,101].
Additionally, kaempferol has been shown to decrease TNF-α production by inhibiting IκB kinase (IKK) and MAPK, thereby suppressing NF-κB activity and its associated pathway. However, when faced with the inflammatory conditions induced by TNF-α stimulation, kaempferol did not fully restore ABCA1 expression back to untreated levels, although it did mitigate the extent of expression. Interestingly, ABCA1 mRNA expression was significantly increased with a high dose of kaempferol alone, indicating a positive correlation between ABCA1 and kaempferol. This implies that kaempferol may be more beneficial as a preventive agent [102]. Kaempferol has been shown in several studies to protect the liver against a variety of oxidative stressors [103,104,105]. Furthermore, data demonstrated that kaempferol had no substantial toxicity on normal cells. kaempferol’s multi-targeting action can minimize medication resistance throughout treatment [106].
4. Synergistic Effect and the Significance of Nanotechnology in Kaempferol’s Therapeutic Efficacy
Nanotechnology has emerged as a crucial tool in both the diagnosis and therapy of gastrointestinal (GI) cancers, which rank among the most prevalent and lethal cancers worldwide. Nanoparticles offer numerous advantages over conventional cancer treatments, including heightened specificity in detection, reduced drug toxicity, and enhanced capabilities in therapy for GI cancers [107]. Various types of nanoparticles are employed in the treatment of GI cancers, encompassing quantum dots (QDs), carbon nanotubes (CNTs), metallic nanoparticles (MNPs), and dendrimers. These nanoparticles exhibit distinct optical properties, excellent biocompatibility, surface effects, and small size effects, rendering them well suited for both diagnostic and therapeutic purposes [108]. Among these, metallic nanoparticles, such as gold and iron oxide nanoparticles, have garnered significant attention due to their promising properties and therapeutic potential in cancer treatment. Gold nanoparticles (GNPs), for instance, are established nanostructures known for their strong light absorption, enabling them to generate thermal energy that facilitates the photothermal destruction of cancerous tissue. This photothermal ablation can be achieved through various means, including GNPs, selenium nanoparticles, or copper sulfide nanoparticles [109]. Additionally, nanogels represent another type of nanoparticle utilized in GI cancer treatment. These nanoscale networks, formed through either noncovalent interactions or covalent crosslinking of polymer chains, offer distinct advantages. Nanogels are particularly noteworthy as oral drug delivery systems due to their heightened sensitivity to external stimuli compared to other delivery systems, making them promising candidates for effective GI cancer therapy [107].
Nanotechnology has been useful in the chemotherapy of malignant GI tumors because of its ability to target specific enzymes and the tumor’s unique microenvironment. The pH changes between tissue sites, as well as the particular hypoxic conditions found in malignant GI tumors, provide guidance for the development of responsive nanocarriers. Enzyme reactions can be exploited in nanodrug delivery systems, such Gal-Dox, which targets β-galactosidase and has substantial anticancer effects [89]. Another application of nanotechnology in GI cancers is drug or gene delivery systems. Nanodevices can load drugs at a high concentration, which are efficiently delivered to specific sites with fewer side effects. Due to their high sensitivity, specificity, and permeability, nanotechnologies have mostly been used in MRI-based clinical applications for the imaging of the GI system and tumor detection. Drug delivery based on nanotechnology will be important for future medical care, particularly cancer treatment. Nanomaterials exhibit remarkable use for enhancing therapeutic effectiveness due to their high biocompatibility [107,110].
Research investigating the potential synergy between kaempferol and nanotechnology in the treatment of GI cancer has received attention. Kaempferol’s limited bioavailability has been a concern, but studies using nanoformulations have suggested an enhancement in its efficacy. For instance, kaempferol-coated silver nanoparticles (AgNPs) exhibited a synergistic impact on apoptosis in HepG2 cells. A reduction in Bcl-2 levels, a rise in Bax and Cyc-c levels, the activation of caspase-3 (due to mitochondrial membrane rupture), and an improvement in p53-mediated cell cycle arrest were the indicators of this impact. According to the study, kaempferol-coated AgNPs may induce oxidative stress-mediated apoptosis and cell cycle arrest in liver cancer (HepG2) cells, hence having an anti-cancer impact [111]. It has been demonstrated that kaempferol-conjugated gold nanoclusters can target and eliminate the nucleus of cancer cells. Nanoformulations incorporating kaempferol have shown significant cancer cell inhibition activities by reducing cell viability [112,113], inducing apoptosis [114], damaging cancer cell nuclei [112], and increasing LDH leakage percentage [111]. PEGylated AuNPs-DOX@ kaempferol showed significant anticancer efficacy in vivo, leading to a decrease in tumor volume [94]. These findings suggest that kaempferol-conjugated gold nanoclusters hold potential for the development of effective cancer treatments.
Kaempferol conjugation with nanoparticles has been extensively studied for combating cancer, showing promising results in various cancer types (Table 2). However, there is a lack of research specifically focused on GI cancers, highlighting the need for further exploration in this area.

Table 2.
List of nanoparticles conjugated with kaempferol in cancer.
5. Safety Aspects of Kaempferol
Kaempferol has been proven to have low toxicity in various investigations, including in bladder cancer, where it was discovered to be a strong inhibitor with good safety [26]. Kaempferol inhibits malignant cells while not affecting healthy ones, potentially contributing to its safety profile [122]. Its effects on cancer cells are dose-dependent. Higher doses may have a greater impact on cancer cells while limiting the impact on healthy cells. Synergistic effects of kaempferol with other anti-cancer medications can improve safety by reducing individual doses [83,122]. Nano-based formulations of kaempferol have been produced to overpower the quick degradation and decrease in toxicity, which may further improve its safety profile [115]. Indeed, even if the results are encouraging, more investigation is required to fully comprehend the safety concerns associated with kaempferol use in the prevention of GI malignancies. Comprehensive in vivo investigations and well-planned clinical trials are needed to evaluate the safety and effectiveness of kaempferol in cancer prevention.
6. Conclusions
Kaempferol is a bioflavonoid molecule that helps to reduce the risk of hormone-related malignancies. This compound’s principal actions include oncogene-induced apoptosis and growth inhibition, but it is also thought to increase the host’s immune response. When kaempferol is present in larger amounts, it has an anti-cancer effect, but when it is present in smaller amounts, it has a pro-cancer function. Kaempferol has various problems in health management, including quick metabolism, low water solubility, lysosomal breakdown, and efficient elimination from the body. Not much study has been undertaken on kaempferol’s possible involvement in cancer treatment due to its limited bioavailability. Nanotechnology-based formulations have recently been produced, and their promise for cancer treatment has been established through in vitro testing. However, more in vivo study is needed to improve kaempferol’s bioavailability, allowing for the investigation of its involvement in cancer management with a specific focus on tumor cells. Furthermore, kaempferol has synergistic effects with anti-cancer drugs, increasing efficacy by inhibiting and activating gene activity. While a few human-based studies have been undertaken to assess its relevance in health management, there is a need to move the importance of kaempferol from preclinical to clinical cancer therapy approaches. This transition will provide a better understanding of kaempferol’s potential in cancer treatment and health management.
Author Contributions
Conceptualization, T.S., D.S. and R.S.; methodology; writing—original draft preparation, T.S., D.S., R.S., H.S.T., S.H., S.R. and D.M.M.; writing—review and editing, H.S.T., S.H., S.R. and V.Y.; visualization; supervision, T.S., H.S.T., V.Y. and D.M.M.; project administration, T.S., H.S.T., V.Y. and S.H. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number ISP23-101.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during this current study.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Derakhshan, M.; Yazdanbod, A.; Sadjadi, A.; Shokoohi, B.; McColl, K.; Malekzadeh, R. High incidence of adenocarcinoma arising from the right side of the gastric cardia in NW Iran. Gut 2004, 53, 1262–1266. [Google Scholar] [CrossRef]
- Sitarz, R.; Skierucha, M.; Mielko, J.; Offerhaus, G.J.A.; Maciejewski, R.; Polkowski, W.P. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag. Res. 2018, 10, 239–248. [Google Scholar] [CrossRef]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef]
- Khan, M.M.; Mohsen, M.T.; Malik, M.Z.; Bagabir, S.A.; Alkhanani, M.F.; Haque, S.; Serajuddin, M.; Bharadwaj, M. Identification of potential key genes in prostate cancer with gene expression, pivotal pathways and regulatory networks analysis using integrated bioinformatics methods. Genes 2022, 13, 655. [Google Scholar] [CrossRef]
- Chirom, K.; Malik, M.Z.; Mangangcha, I.R.; Somvanshi, P.; Singh, R.B. Network medicine in ovarian cancer: Topological properties to drug discovery. Brief. Bioinform. 2022, 23, bbac085. [Google Scholar] [CrossRef]
- Ali, S.; Malik, M.Z.; Singh, S.S.; Chirom, K.; Ishrat, R.; Singh, R.B. Exploring novel key regulators in breast cancer network. PLoS ONE 2018, 13, e0198525. [Google Scholar] [CrossRef]
- Malik, M.Z.; Chirom, K.; Ali, S.; Ishrat, R.; Somvanshi, P.; Singh, R.B. Methodology of predicting novel key regulators in ovarian cancer network: A network theoretical approach. BMC Cancer 2019, 19, 1129. [Google Scholar] [CrossRef]
- Hansson, L.-E.; Nyrén, O.; Hsing, A.W.; Bergström, R.; Josefsson, S.; Chow, W.-H.; Fraumeni, J.F., Jr.; Adami, H.-O. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N. Engl. J. Med. 1996, 335, 242–249. [Google Scholar] [CrossRef]
- Shah, M.A.; Kelsen, D.P. Gastric cancer: A primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J. Natl. Compr. Cancer Netw. 2010, 8, 437–447. [Google Scholar] [CrossRef]
- Guggenheim, D.E.; Shah, M.A. Gastric cancer epidemiology and risk factors. J. Surg. Oncol. 2013, 107, 230–236. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rahman, T. The difficulties in cancer treatment. Ecancermedicalscience 2012, 6, ed16. [Google Scholar]
- Al-Ishaq, R.K.; Overy, A.J.; Büsselberg, D. Phytochemicals and gastrointestinal cancer: Cellular mechanisms and effects to change cancer progression. Biomolecules 2020, 10, 105. [Google Scholar] [CrossRef]
- Majrashi, T.A.; Alshehri, S.A.; Alsayari, A.; Muhsinah, A.B.; Alrouji, M.; Alshahrani, A.M.; Shamsi, A.; Atiya, A. Insight into the biological roles and mechanisms of phytochemicals in different types of cancer: Targeting cancer therapeutics. Nutrients 2023, 15, 1704. [Google Scholar] [CrossRef]
- Bharti, A.C.; Singh, T.; Bhat, A.; Pande, D.; Jadli, M. Therapeutic startegies for human papillomavirus infection and associated cancers. Front. Biosci.-Elite 2018, 10, 15–73. [Google Scholar] [CrossRef]
- Liang, Z.; Xu, Y.; Zhang, Y.; Zhang, X.; Song, J.; Jin, J.; Qian, H. Anticancer applications of phytochemicals in gastric cancer: Effects and molecular mechanism. Front. Pharmacol. 2023, 13, 1078090. [Google Scholar] [CrossRef]
- Aggarwal, N.; Yadav, J.; Chhakara, S.; Janjua, D.; Tripathi, T.; Chaudhary, A.; Chhokar, A.; Thakur, K.; Singh, T.; Bharti, A.C. Phytochemicals as potential chemopreventive and chemotherapeutic agents for emerging human papillomavirus–driven head and neck cancer: Current evidence and future prospects. Front. Pharmacol. 2021, 12, 699044. [Google Scholar] [CrossRef]
- Singh Tuli, H.; Rath, P.; Chauhan, A.; Sak, K.; Aggarwal, D.; Choudhary, R.; Sharma, U.; Vashishth, K.; Sharma, S.; Kumar, M. Luteolin, a potent anticancer compound: From chemistry to cellular interactions and synergetic perspectives. Cancers 2022, 14, 5373. [Google Scholar] [CrossRef]
- Kumar, A.; Prajapati, S.; Sharma, M.; Singh, T.; Choudhary, N.; Bharti, A.C.; Sharma, R.; Gupta, P. Quantitative assessment of antioxidant potential of selected homeopathic preparations in clinical practice. Drug Metab. Pers. Ther. 2022, 38, 179–190. [Google Scholar] [CrossRef]
- Tuli, H.S.; Garg, V.K.; Kumar, A.; Aggarwal, D.; Anand, U.; Parashar, N.C.; Saini, A.K.; Mohapatra, R.K.; Dhama, K.; Kumar, M. Anticancer potential of oroxylin A: From mechanistic insight to synergistic perspectives. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 191–212. [Google Scholar] [CrossRef]
- Singh, T.; Aggarwal, N.; Thakur, K.; Chhokar, A.; Yadav, J.; Tripathi, T.; Jadli, M.; Bhat, A.; Kumar, A.; Narula, R.H. Evaluation of therapeutic potential of selected plant-derived homeopathic medicines for their action against cervical cancer. Homeopathy 2023, 112, 262–274. [Google Scholar] [CrossRef]
- Tuli, H.S.; Sak, K.; Garg, V.K.; Kumar, A.; Adhikary, S.; Kaur, G.; Parashar, N.C.; Parashar, G.; Mukherjee, T.K.; Sharma, U. Ampelopsin targets in cellular processes of cancer: Recent trends and advances. Toxicol. Rep. 2022, 9, 1614–1623. [Google Scholar] [CrossRef]
- Bhat, A.; Yadav, J.; Thakur, K.; Aggarwal, N.; Tripathi, T.; Chhokar, A.; Singh, T.; Jadli, M.; Bharti, A.C. Exosomes from cervical cancer cells facilitate pro-angiogenic endothelial reconditioning through transfer of Hedgehog–GLI signaling components. Cancer Cell Int. 2021, 21, 319. [Google Scholar] [CrossRef]
- Singh, T.; Chhokar, A.; Thakur, K.; Aggarwal, N.; Pragya, P.; Yadav, J.; Tripathi, T.; Jadli, M.; Bhat, A.; Gupta, P. Targeting aberrant expression of STAT3 and AP-1 oncogenic transcription factors and HPV oncoproteins in cervical cancer by Berberis aquifolium. Front. Pharmacol. 2021, 12, 757414. [Google Scholar] [CrossRef]
- Vijh, D.; Imam, M.A.; Haque, M.M.U.; Das, S.; Islam, A.; Malik, M.Z. Network pharmacology and bioinformatics approach reveals the therapeutic mechanism of action of curcumin in Alzheimer disease. Metab. Brain Dis. 2023, 38, 1205–1220. [Google Scholar] [CrossRef]
- Nejabati, H.R.; Roshangar, L. Kaempferol: A potential agent in the prevention of colorectal cancer. Physiol. Rep. 2022, 10, e15488. [Google Scholar] [CrossRef]
- Amjad, E.; Sokouti, B.; Asnaashari, S. A systematic review of anti-cancer roles and mechanisms of kaempferol as a natural compound. Cancer Cell Int. 2022, 22, 260. [Google Scholar] [CrossRef]
- Riahi-Chebbi, I.; Souid, S.; Othman, H.; Haoues, M.; Karoui, H.; Morel, A.; Srairi-Abid, N.; Essafi, M.; Essafi-Benkhadir, K. The Phenolic compound Kaempferol overcomes 5-fluorouracil resistance in human resistant LS174 colon cancer cells. Sci. Rep. 2019, 9, 195. [Google Scholar] [CrossRef]
- Song, H.; Bao, J.; Wei, Y.; Chen, Y.; Mao, X.; Li, J.; Yang, Z.; Xue, Y. Kaempferol inhibits gastric cancer tumor growth: An in vitro and in vivo study. Oncol. Rep. 2015, 33, 868–874. [Google Scholar] [CrossRef]
- Kovalev, V.; Seraya, L. Flavonoids of Glycine hispida. Chem. Nat. Compd. 1984, 20, 626–627. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- M Calderon-Montano, J.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef] [PubMed]
- DuPont, M.; Day, A.; Bennett, R.; Mellon, F.; Kroon, P. Absorption of kaempferol from endive, a source of kaempferol-3-glucuronide, in humans. Eur. J. Clin. Nutr. 2004, 58, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Mizokami, T.; Ito, H.; Ikeda, Y. A randomized, placebo-controlled trial evaluating the safety of excessive administration of kaempferol aglycone. Food Sci. Nutr. 2023, 11, 5427–5437. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a dietary anti-inflammatory agent: Current therapeutic standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef] [PubMed]
- Barve, A.; Chen, C.; Hebbar, V.; Desiderio, J.; Saw, C.L.L.; Kong, A.N. Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharm. Drug Dispos. 2009, 30, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Almatroudi, A.; Allemailem, K.S.; Alwanian, W.M.; Alharbi, B.F.; Alrumaihi, F.; Khan, A.A.; Almatroodi, S.A.; Rahmani, A.H. Effects and Mechanisms of Kaempferol in the Management of Cancers through Modulation of Inflammation and Signal Transduction Pathways. Int. J. Mol. Sci. 2023, 24, 8630. [Google Scholar] [CrossRef]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Rho, H.S.; Ghimeray, A.K.; Yoo, D.S.; Ahn, S.M.; Kwon, S.S.; Lee, K.H.; Cho, D.H.; Cho, J.Y. Kaempferol and kaempferol rhamnosides with depigmenting and anti-inflammatory properties. Molecules 2011, 16, 3338–3344. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed]
- Senrung, A.; Tripathi, T.; Aggarwal, N.; Janjua, D.; Chhokar, A.; Yadav, J.; Chaudhary, A.; Thakur, K.; Singh, T.; Bharti, A.C. Anti-angiogenic Potential of Trans-chalcone in an In Vivo Chick Chorioallantoic Membrane Model: An ATP Antagonist to VEGFR with Predicted Blood-brain Barrier Permeability. Cardiovasc. Hematol. Agents Med. Chem. 2024, 22, 187–211. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.; Watson, D.; Grant, M. Metabolism of quercetin and kaempferol by rat hepatocytes and the identification of flavonoid glycosides in human plasma. Xenobiotica 2002, 32, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Zabela, V.; Sampath, C.; Oufir, M.; Moradi-Afrapoli, F.; Butterweck, V.; Hamburger, M. Pharmacokinetics of dietary kaempferol and its metabolite 4-hydroxyphenylacetic acid in rats. Fitoterapia 2016, 115, 189–197. [Google Scholar] [CrossRef]
- López-Lázaro, M. A new view of carcinogenesis and an alternative approach to cancer therapy. Mol. Med. 2010, 16, 144–153. [Google Scholar] [CrossRef]
- Silva dos Santos, J.; Goncalves Cirino, J.P.; de Oliveira Carvalho, P.; Ortega, M.M. The pharmacological action of kaempferol in central nervous system diseases: A review. Front. Pharmacol. 2021, 11, 565700. [Google Scholar] [CrossRef] [PubMed]
- Bohm, B.A. Introduction to Flavonoids; Harwood Academic Publishers: New York, NY, USA, 1998. [Google Scholar]
- Singh, D.; Kumari, K.; Ahmed, S. Natural herbal products for cancer therapy. In Understanding Cancer; Elsevier: Amsterdam, The Netherlands, 2022; pp. 257–268. [Google Scholar]
- Ma, Y.; Liu, Y.; Sun, A.; Du, Y.; Ye, M.; Pu, X.; Qi, X. Intestinal absorption and neuroprotective effects of kaempferol-3-O-rutinoside. RSC Adv. 2017, 7, 31408–31416. [Google Scholar] [CrossRef]
- Mullen, W.; Edwards, C.A.; Crozier, A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl-and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br. J. Nutr. 2006, 96, 107–116. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, X.; Xu, H.; Liu, G.; Wang, Y.; Sun, H.; Geng, F.; Zhang, N. Tissue Distribution of Total Flavonoids Extracts of Drynariae Rhizoma in Young and Old Rats by UPLC–MS/MS Determination. J. Anal. Methods Chem. 2022, 2022, 2447495. [Google Scholar] [CrossRef]
- Al-Tannak, N.F.; Al-Hasawi, N.A.; Novotny, L. UHPLC-UV analysis of morin and structurally related flavonoids with potential anticancer activity. Curr. Pharm. Anal. 2019, 15, 295–304. [Google Scholar] [CrossRef]
- O’Leary, K.A.; Day, A.J.; Needs, P.W.; Sly, W.S.; O’Brien, N.M.; Williamson, G. Flavonoid glucuronides are substrates for human liver β-glucuronidase. FEBS Lett. 2001, 503, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Kamisah, Y.; Jalil, J.; Yunos, N.M.; Zainalabidin, S. Cardioprotective Properties of Kaempferol: A Review. Plants 2023, 12, 2096. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, C.; Wang, L.-F.; Kuang, X.; Liu, K.; Zhang, H.; Du, J.-R. Neuroprotective effect of kaempferol glycosides against brain injury and neuroinflammation by inhibiting the activation of NF-κB and STAT3 in transient focal stroke. PLoS ONE 2013, 8, e55839. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Mármol, I.; Sánchez-de-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M.J. Colorectal carcinoma: A general overview and future perspectives in colorectal cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-A.; Chen, K.-W.; Hsu, C.-Y. Prediction model for pancreatic cancer—A population-based study from NHIRD. Cancers 2022, 14, 882. [Google Scholar] [CrossRef] [PubMed]
- Liver Cancer: Statistics. Available online: https://www.cancer.net/cancer-types/liver-cancer/statistics (accessed on 25 February 2024).
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent progress regarding kaempferol for the treatment of various diseases. Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, F.; Guo, Y.; Chen, H.; Qian, J.; Wu, L.; Xie, D.; Chen, G. Clinical-Pathological Characteristics of Adenosquamous Esophageal Carcinoma: A Propensity-Score-Matching Study. J. Pers. Med. 2023, 13, 468. [Google Scholar] [CrossRef]
- Tu, L.Y.; Bai, H.H.; Cai, J.Y.; Deng, S.P. The mechanism of kaempferol induced apoptosis and inhibited proliferation in human cervical cancer SiHa cell: From macro to nano. Scanning 2016, 38, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Aichler, M.; Motschmann, M.; Jütting, U.; Luber, B.; Becker, K.; Ott, K.; Lordick, F.; Langer, R.; Feith, M.; Siewert, J.R. Epidermal growth factor receptor (EGFR) is an independent adverse prognostic factor in esophageal adenocarcinoma patients treated with cisplatin-based neoadjuvant chemotherapy. Oncotarget 2014, 5, 6620. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gates, M.A.; Tworoger, S.S.; Hecht, J.L.; De Vivo, I.; Rosner, B.; Hankinson, S.E. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int. J. Cancer 2007, 121, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Wang, X.; Li, C.; Zhao, T.; Jin, H.; Fang, W. Kaempferol inhibits cell proliferation and glycolysis in esophagus squamous cell carcinoma via targeting EGFR signaling pathway. Tumor Biol. 2016, 37, 10247–10256. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Si, L.; Jia, Y.; Jian, W.; Yu, Q.; Wang, M.; Lin, R. Kaempferol exerts anti-proliferative effects on human ovarian cancer cells by inducing apoptosis, G0/G1 cell cycle arrest and modulation of MEK/ERK and STAT3 pathways. J. Buon 2019, 24, 975–981. [Google Scholar] [PubMed]
- Hu, G.; Liu, H.; Wang, M.; Peng, W. IQ motif containing GTPase-activating protein 3 (IQGAP3) inhibits kaempferol-induced apoptosis in breast cancer cells by extracellular signal-regulated kinases 1/2 (ERK1/2) signaling activation. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 7666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, X.-H.; Wang, Z.-J. Flavones and flavonols exert cytotoxic effects on a human oesophageal adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing apoptosis. Food Chem. Toxicol. 2008, 46, 2042–2053. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.; Nilsson, M.; Grabsch, H.; van Grieken, N.T.; Lordick, F. Gastric Cancer. Lancet 2020, 396, 635–648. [Google Scholar]
- Kim, T.W.; Lee, S.Y.; Kim, M.; Cheon, C.; Ko, S.-G. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 2018, 9, 875. [Google Scholar] [CrossRef]
- Alzahrani, S.M.; Al Doghaither, H.A.; Al-Ghafari, A.B. General insight into cancer: An overview of colorectal cancer. Mol. Clin. Oncol. 2021, 15, 271. [Google Scholar] [CrossRef]
- Majumdar, S.R.; Fletcher, R.H.; Evans, A.T. How does colorectal cancer present? Symptoms, duration, and clues to location. Am. J. Gastroenterol. 1999, 94, 3039–3045. [Google Scholar] [CrossRef]
- Narayan, S.; Roy, D. Role of APC and DNA mismatch repair genes in the development of colorectal cancers. Mol. Cancer 2003, 2, 41. [Google Scholar] [CrossRef] [PubMed]
- Roncucci, L.; Mariani, F. Prevention of colorectal cancer: How many tools do we have in our basket? Eur. J. Intern. Med. 2015, 26, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, H.; Kuroda, H.; Imai, Y.; Hiraishi, H. Molecular pathogenesis of sporadic colorectal cancers. Chin. J. Cancer 2016, 35, 4. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Cominelli, F. Colitis-associated and sporadic colon cancers: Different diseases, different mutations? Gastroenterology 2016, 150, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Issa, I.A.; Noureddine, M. Colorectal cancer screening: An updated review of the available options. World J. Gastroenterol. 2017, 23, 5086. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Du, J.e.; Li, C.; Li, H.; Guo, H.; Li, Z. Kaempferol can reverse the 5-Fu resistance of colorectal cancer cells by inhibiting PKM2-mediated glycolysis. Int. J. Mol. Sci. 2022, 23, 3544. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shalom, R.; Bergman, M.; Grossman, S.; Azzam, N.; Sharvit, L.; Fares, F. Inula viscosa extract inhibits growth of colorectal cancer cells in vitro and in vivo through induction of apoptosis. Front. Oncol. 2019, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Park, J.H.Y. Kaempferol induces cell cycle arrest in HT-29 human colon cancer cells. J. Cancer Prev. 2013, 18, 257. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Cui, M.; Li, C.; Li, H.; Dai, Y.; Cui, K.; Li, Z. Kaempferol reverses aerobic glycolysis via miR-339-5p-mediated PKM alternative splicing in colon cancer cells. J. Agric. Food Chem. 2021, 69, 3060–3068. [Google Scholar] [CrossRef]
- Goral, V. Pancreatic cancer: Pathogenesis and diagnosis. Asian Pac. J. Cancer Prev. 2015, 16, 5619–5624. [Google Scholar] [CrossRef]
- Gillen, S.; Schuster, T.; Meyer zum Büschenfelde, C.; Friess, H.; Kleeff, J. Preoperative/neoadjuvant therapy in pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010, 7, e1000267. [Google Scholar] [CrossRef] [PubMed]
- Doulabi, M.S.H.; Ghaedi, K.; Ranji, N.; Koohpar, Z.K. rs5745676 of HGF 3’UTR associates with hsa-miR-340-5p binding potential in breast cancer and gastric cancer in Isfahan population. Hum. Gene 2022, 33, 201075. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.H. Kaempferol inhibits pancreatic cancer cell growth and migration through the blockade of EGFR-related pathway in vitro. PLoS ONE 2016, 11, e0155264. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, L.; Qu, C.; Chen, L.; Geng, Y.; Cheng, C.; Yu, S.; Wang, D.; Yang, L.; Meng, Z. Kaempferol induces ROS-dependent apoptosis in pancreatic cancer cells via TGM2-mediated Akt/mTOR signaling. BMC Cancer 2021, 21, 396. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Vishnoi, K.; Tyagi, A.; Jadli, M.; Singh, T.; Goel, A.; Sharma, A.; Agarwal, K.; Prasad, S.C.; Pandey, D. Characterization of key transcription factors as molecular signatures of HPV-positive and HPV-negative oral cancers. Cancer Med. 2017, 6, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Khanduja, K.; Bhardwaj, A. Stable free radical scavenging and antiperoxidative properties of resveratrol compared in vitro with some other bioflavonoids. Indian J. Biochem. Biophys. 2003, 40, 416–422. [Google Scholar] [PubMed]
- Tatsimo, S.J.N.; Tamokou, J.d.D.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.-R.; Tane, P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res. Notes 2012, 5, 158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, A.Y.; Li, M.; Chen, C.; Yao, Q. Ginkgo biloba extract kaempferol inhibits cell proliferation and induces apoptosis in pancreatic cancer cells. J. Surg. Res. 2008, 148, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.; Besselink, E.; Henning, S.; Go, V.; Heber, D. Phytoestrogens induce differential estrogen receptor alpha-or beta-mediated responses in transfected breast cancer cells. Exp. Biol. Med. 2005, 230, 558–568. [Google Scholar] [CrossRef]
- Ogunwobi, O.O.; Harricharran, T.; Huaman, J.; Galuza, A.; Odumuwagun, O.; Tan, Y.; Ma, G.X.; Nguyen, M.T. Mechanisms of hepatocellular carcinoma progression. World J. Gastroenterol. 2019, 25, 2279. [Google Scholar] [CrossRef]
- Alqahtani, A.; Khan, Z.; Alloghbi, A.S.; Said Ahmed, T.; Ashraf, M.; M. Hammouda, D. Hepatocellular carcinoma: Molecular mechanisms and targeted therapies. Medicina 2019, 55, 526. [Google Scholar] [CrossRef] [PubMed]
- Mylonis, I.; Lakka, A.; Tsakalof, A.; Simos, G. The dietary flavonoid kaempferol effectively inhibits HIF-1 activity and hepatoma cancer cell viability under hypoxic conditions. Biochem. Biophys. Res. Commun. 2010, 398, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Langeswaran, K.; Revathy, R.; Kumar, S.G.; Vijayaprakash, S.; Balasubramanian, M.P. Kaempferol ameliorates aflatoxin B1 (AFB1) induced hepatocellular carcinoma through modifying metabolizing enzymes, membrane bound ATPases and mitochondrial TCA cycle enzymes. Asian Pac. J. Trop. Biomed. 2012, 2, S1653–S1659. [Google Scholar] [CrossRef]
- Ee, Y.Y.; Hoong, C.C. Downregulation in the mRNA expression of nuclear hormone receptor liver-X-receptor alpha (LXR-α) by TNF-α is abolished by the antioxidant kaempferol, but not ascorbic acid, in human hepatocarcinoma HepG2 cells. Asian Biomed. 2012, 6, 585–589. [Google Scholar]
- BinMowyna, M.N.; AlFaris, N.A. Kaempferol suppresses acetaminophen-induced liver damage by upregulation/activation of SIRT1. Pharm. Biol. 2021, 59, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Wonganan, O.; He, Y.-j.; Shen, X.-f.; Wongkrajang, K.; Suksamrarn, A.; Zhang, G.-l.; Wang, F. 6-Hydroxy-3-O-methyl-kaempferol 6-O-glucopyranoside potentiates the anti-proliferative effect of interferon α/β by promoting activation of the JAK/STAT signaling by inhibiting SOCS3 in hepatocellular carcinoma cells. Toxicol. Appl. Pharmacol. 2017, 336, 31–39. [Google Scholar] [CrossRef]
- Huang, W.-W.; Tsai, S.-C.; Peng, S.-F.; Lin, M.-W.; Chiang, J.-H.; Chiu, Y.-J.; Fushiya, S.; Tseng, M.T.; Yang, J.-S. Kaempferol induces autophagy through AMPK and AKT signaling molecules and causes G2/M arrest via downregulation of CDK1/cyclin B in SK-HEP-1 human hepatic cancer cells. Int. J. Oncol. 2013, 42, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Cheah, H.-Y.; Wong, Y.-Y.; Wong, H.-K.; Lim, W.-S.; Chew, C.-H. Nicotinic acid, lauric acid and kaempferol abolish ATP-binding cassette transporter subfamily A member 1 (ABCA1) down-regulation by TNF-α in hepatocarcinoma HepG2 cell line. Biomed. Res. 2014, 25, 419–425. [Google Scholar]
- Shakya, G.; Manjini, S.; Hoda, M.; Rajagopalan, R. Hepatoprotective role of kaempferol during alcohol-and ΔPUFA-induced oxidative stress. J. Basic Clin. Physiol. Pharmacol. 2014, 25, 73–79. [Google Scholar] [CrossRef]
- Tie, F.; Ding, J.; Hu, N.; Dong, Q.; Chen, Z.; Wang, H. Kaempferol and kaempferide attenuate oleic acid-induced lipid accumulation and oxidative stress in HepG2 cells. Int. J. Mol. Sci. 2021, 22, 8847. [Google Scholar] [CrossRef]
- Malik, M.Z.; Ali, S.; Singh, S.S.; Ishrat, R.; Singh, R.B. Dynamical states, possibilities and propagation of stress signal. Sci. Rep. 2017, 7, 40596. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xing, J.; Aikemu, B.; Sun, J.; Zheng, M. Kaempferol exhibits a synergistic effect with doxorubicin to inhibit proliferation, migration, and invasion of liver cancer. Oncol. Rep. 2021, 45, 32. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Li, L.-D.; Li, L.; Li, S. Nanotechnology in diagnosis and therapy of gastrointestinal cancer. World J. Clin. Cases 2022, 10, 5146. [Google Scholar] [CrossRef] [PubMed]
- Kanaoujiya, R.; Porwal, D.; Srivastava, S. Applications of nanomaterials for gastrointestinal tumors: A review. Front. Med. Technol. 2022, 4, 997123. [Google Scholar] [CrossRef]
- Salapa, J.; Bushman, A.; Lowe, K.; Irudayaraj, J. Nano drug delivery systems in upper gastrointestinal cancer therapy. Nano Converg. 2020, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Nikezić, A.V.V.; Bondžić, A.M.; Vasić, V.M. Drug delivery systems based on nanoparticles and related nanostructures. Eur. J. Pharm. Sci. 2020, 151, 105412. [Google Scholar] [CrossRef] [PubMed]
- Alyami, N.M.; Alyami, H.M.; Almeer, R. Using green biosynthesized kaempferol-coated sliver nanoparticles to inhibit cancer cells growth: An in vitro study using hepatocellular carcinoma (HepG2). Cancer Nanotechnol. 2022, 13, 26. [Google Scholar] [CrossRef]
- Govindaraju, S.; Roshini, A.; Lee, M.-H.; Yun, K. Kaempferol conjugated gold nanoclusters enabled efficient for anticancer therapeutics to A549 lung cancer cells. Int. J. Nanomed. 2019, 14, 5147–5157. [Google Scholar] [CrossRef]
- Raghavan, B.S.; Kondath, S.; Anantanarayanan, R.; Rajaram, R. Kaempferol mediated synthesis of gold nanoparticles and their cytotoxic effects on MCF-7 cancer cell line. Process Biochem. 2015, 50, 1966–1976. [Google Scholar] [CrossRef]
- Aghazadeh, T.; Bakhtiari, N.; Rad, I.A.; Ramezani, F. Formulation of kaempferol in nanostructured lipid carriers (NLCs): A delivery platform to sensitization of MDA-MB468 breast cancer cells to paclitaxel. Biointerface Res. Appl. Chem. 2021, 11, 14591–14601. [Google Scholar]
- Luo, H.; Jiang, B.; Li, B.; Li, Z.; Jiang, B.-H.; Chen, Y.C. Kaempferol nanoparticles achieve strong and selective inhibition of ovarian cancer cell viability. Int. J. Nanomed. 2012, 7, 3951–3959. [Google Scholar]
- Nicoleti, L.R.; Di Filippo, L.D.; Duarte, J.L.; Luiz, M.T.; Sábio, R.M.; Chorilli, M. Development, characterization and in vitro cytotoxicity of kaempferol-loaded nanostructured lipid carriers in glioblastoma multiforme cells. Colloids Surf. B Biointerfaces 2023, 226, 113309. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, D.; Ranjithkumar, R.; Nandagopal, J.G.T.; Djearamane, S.; Lee, J.; Wong, L.S. Green synthesis of chitosan/silver nanocomposite using kaempferol for triple negative breast cancer therapy and antibacterial activity. Environ. Res. 2023, 238, 117109. [Google Scholar] [CrossRef] [PubMed]
- Meena, D.; Vimala, K.; Kannan, S. Combined delivery of DOX and Kaempferol using PEGylated gold nanoparticles to target colon cancer. J. Clust. Sci. 2022, 33, 173–187. [Google Scholar] [CrossRef]
- Qanash, H.; Bazaid, A.S.; Aldarhami, A.; Alharbi, B.; Almashjary, M.N.; Hazzazi, M.S.; Felemban, H.R.; Abdelghany, T.M. Phytochemical Characterization and Efficacy of Artemisia judaica Extract Loaded Chitosan Nanoparticles as Inhibitors of Cancer Proliferation and Microbial Growth. Polymers 2023, 15, 391. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, S.; Song, H.; Yu, T.; Zheng, X.; Chu, Q. CaCO3 nanoparticles incorporated with KAE to enable amplified calcium overload cancer therapy. Biomaterials 2021, 277, 121080. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Figueiró, F.; de Fraga Dias, A.; Teixeira, H.F.; Battastini, A.M.O.; Koester, L.S. Kaempferol-loaded mucoadhesive nanoemulsion for intranasal administration reduces glioma growth in vitro. Int. J. Pharm. 2018, 543, 214–223. [Google Scholar] [CrossRef]
- Shahbaz, M.; Imran, M.; Alsagaby, S.A.; Naeem, H.; Al Abdulmonem, W.; Hussain, M.; Abdelgawad, M.A.; El-Ghorab, A.H.; Ghoneim, M.M.; El-Sherbiny, M. Anticancer, antioxidant, ameliorative and therapeutic properties of kaempferol. Int. J. Food Prop. 2023, 26, 1140–1166. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).