Proton Therapy in Non-Rhabdomyosarcoma Soft Tissue Sarcomas of Children and Adolescents
Abstract
Simple Summary
Abstract
1. Introduction
2. Proton Beam Therapy
3. Proton Beam Therapy in Pediatric NRSTS
4. Lessons from Adult Sarcoma Experiences
5. Discussion
- -
- PBT should be recommended for young children (for example, younger than 3 or 6 years of age) in order to minimize exposure to medium-to-low radiation doses and the risk of long-term side effects.
- -
- Since the limited availability of PBT, this technique should be recommended to patients with relatively good prognosis (therefore, it should not be indicated in metastatic patients).
- -
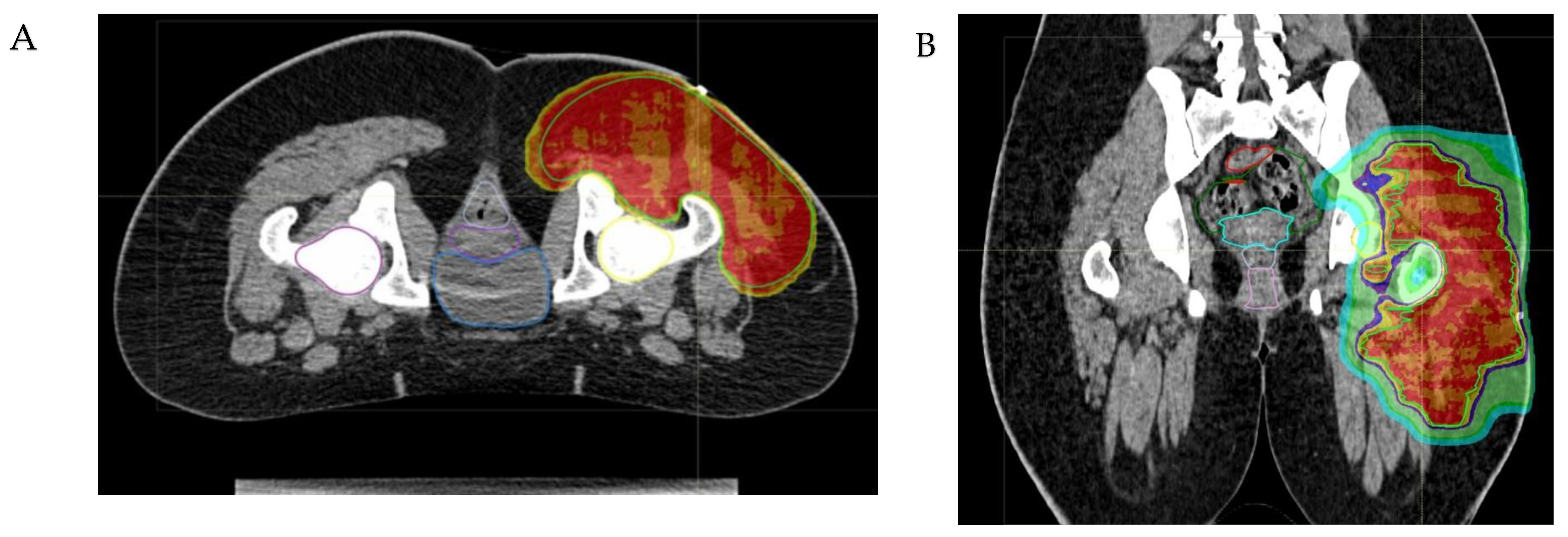
- The anatomical site and the subsequent fragility of the surrounding organs at risk to potential radiation damage is a critical matter. The head–neck, craniofacial, intra-abdominal, pelvic, and paravertebral regions may be considered elective sites, where PBT can minimize radiation exposure to nearby organs (Figure 2). In addition to minimizing the risk of late sequelae, the reduced irradiation of surrounding tissues (mucosae, for example) may also reduce acute toxicity and thus improve compliance with intensive multimodal treatment including concomitant chemotherapy. While PBT may be less indicated for extremity tumors, exceptions should be made for young patients due to the potential for preserving growth plate cartilage and the lymphatic and vascular–nerve pathways present in the limbs.
- -
- The physical properties of protons allow for a significant escalation in dose, potentially up to approximately 60 Gy Relative Biological Effectiveness (RBE), in the treatment of radioresistant histotypes, such as Malignant Peripheral Nerve Sheath Tumors (MPNSTs) (see Figure 3).
- -
- PBT can have a crucial role in the treatment of pediatric, adolescent and young adult patients with NRSTS associated with genetic syndromes like neurofibromatosis type 1 (NF1), including MPNST, due to the increased risk of radiation-induced carcinogenesis.

6. Conclusions
- -
- Develop shared guidelines for PBT indications;
- -
- Centralize RT in high-level referral centers: on the one hand, it is advisable that PBT techniques may be developed in the context of clinical studies, by a well-trained multidisciplinary team with experience in managing particle beams; on the other side, centralization is of key value in order to optimize the use of limited resources;
- -
- Improve quality assurance program [63];
- -
- Define international protocols to compare photon and proton radiation techniques in terms of local control and toxicity.
Author Contributions
Funding
Conflicts of Interest
References
- Ferrari, A.; Spunt, S.L.; Sparber-Sauer, M.; Walterhouse, D.O.; Pajtler, K.W.; Meyer, W.H.; Orbach, D.; Weiss, A. Controversies and challenges in the management of paediatric non-rhabdomyosarcoma soft tissue sarcomas. Lancet Child Adolesc. Health 2022, 6, 221–223. [Google Scholar] [CrossRef]
- Ferrari, A.; Sultan, I.; Huang, T.T.; Rodriguez-Galindo, C.; Shehadeh, A.; Meazza, C.; Ness, K.K.; Casanova, M.; Spunt, S.L. Soft tissue sarcoma across the age spectrum: A population-based study from the Surveillance Epidemiology and End Results database. Pediatr. Blood Cancer. 2011, 57, 943–949. [Google Scholar] [CrossRef]
- Ferrari, A.; Brennan, B.; Casanova, M.; Corradini, N.; Berlanga, P.; Schoot, R.A.; Ramirez-Villar, G.L.; Safwat, A.; Guillen Burrieza, G.; Dall’Igna, P.; et al. Pediatric Non-Rhabdomyosarcoma Soft Tissue Sarcomas: Standard of Care and Treatment Recommendations from the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG). Cancer Manag. Res. 2022, 14, 2885–2902. [Google Scholar] [CrossRef]
- Ferrari, A.; Orbach, D.; Sparber-Sauer, M.; Walterhouse, D.O.; Pajtler, K.W.; Meyer, W.H.; Spunt, S.L.; Weiss, A.R. The treatment approach to pediatric non-rhabdomyosarcoma soft tissue sarcomas: A critical review from the INternational Soft Tissue SaRcoma ConsorTium. Eur. J. Cancer 2022, 169, 10–19. [Google Scholar] [CrossRef]
- Spunt, S.L.; Million, L.; Chi, Y.Y.; Anderson, J.; Tian, J.; Hibbitts, E.; Coffin, C.; McCarville, M.B.; Randall, R.L.; Parham, D.M.; et al. A risk-based treatment strategy for non-rhabdomyosarcoma soft-tissue sarcomas in patients younger than 30 years (ARST0332): A Children’s Oncology Group prospective study. Lancet Oncol. 2020, 21, 145–161. [Google Scholar] [CrossRef]
- Ferrari, A.; van Noesel, M.M.; Brennan, B.; Zanetti, I.; Corradini, N.; Casanova, M.; Berlanga, P.; Merks, J.H.M.; Alaggio, R.; Schifflers, S.; et al. Paediatric non-rhabdomyosarcoma soft tissue sarcomas: The prospective NRSTS 2005 study by the European Pediatric Soft Tissue Sarcoma Study Group (EpSSG). Lancet Child Adolesc. Health 2021, 5, 546–558. [Google Scholar] [CrossRef]
- Weiss, A.R.; Chen, Y.L.; Scharschmidt, T.J.; Xue, W.; Gao, Z.; Black, J.O.; Choy, E.; Davis, J.L.; Fanburg-Smith, J.C.; Kao, S.C.; et al. Outcomes After Preoperative Chemoradiation With or Without Pazopanib in Non-Rhabdomyosarcoma Soft Tissue Sarcoma: A Report From Children’s Oncology Group and NRG Oncology. J. Clin. Oncol. 2023, 41, 4842–4848. [Google Scholar] [CrossRef]
- Spunt, S.L.; Poquette, C.A.; Hurt, Y.S.; Cain, A.M.; Rao, B.N.; Merchant, T.E.; Jenkins, J.J.; Santana, V.M.; Pratt, C.B.; Pappo, A.S. Prognostic factors for children and adolescents with surgically resected nonrhabdomyosarcoma soft tissue sarcoma: An analysis of 121 patients treated at St Jude Children’s Research Hospital. J. Clin. Oncol. 1999, 17, 3697–3705. [Google Scholar] [CrossRef]
- Spunt, S.L.; Hill, D.A.; Motosue, A.M.; Billups, C.A.; Cain, A.M.; Rao, B.N.; Pratt, C.B.; Merchant, T.E.; Pappo, A.S. Clinical features and outcome of initially unresected nonmetastatic pediatric nonrhabdomyosarcoma soft tissue sarcoma. J. Clin. Oncol. 2002, 20, 3225–3235. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Casanova, M.; Collini, P.; Meazza, C.; Luksch, R.; Massimino, M.; Cefalo, G.; Terenziani, M.; Spreafico, F.; Catania, S.; et al. Adult-type soft tissue sarcomas in pediatric-age patients: Experience at the Istituto Nazionale Tumori in Milan. J. Clin. Oncol. 2005, 23, 4021–4030. [Google Scholar] [CrossRef]
- Ferrari, A.; Miceli, R.; Casanova, M.; Gronchi, A.; Collini, P.; Meazza, C.; Zaffignani, E.; Massimino, M.; Spreafico, F.; Mariani, L. Adult-type soft tissue sarcomas in paediatric age: A nomogram-based prognostic comparison with adult sarcoma. Eur. J. Cancer 2007, 43, 2691–2697. [Google Scholar] [CrossRef] [PubMed]
- Trojani, M.; Contesso, G.; Coindre, J.M.; Rouesse, J.; Bui, N.B.; de Mascarel, A.; Goussot, J.F.; David, M.; Bonichon, F.; Lagarde, C. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int. J. Cancer 1984, 33, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, S.; Gherlinzoni, F.; De Paoli, A.; Bonetti, M.; Azzarelli, A.; Comandone, A.; Olmi, P.; Buonadonna, A.; Pignatti, G.; Barbieri, E.; et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: Results of the Italian randomized cooperative trial. J. Clin. Oncol. 2001, 19, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Palmerini, E.; Quagliuolo, V.; Martin Broto, J.; Lopez Pousa, A.; Grignani, G.; Brunello, A.; Blay, J.Y.; Tendero, O.; Diaz Beveridge, R.; et al. Neoadjuvant Chemotherapy in High-Risk Soft Tissue Sarcomas: Final Results of a Randomized Trial From Italian (ISG), Spanish (GEIS), French (FSG), and Polish (PSG) Sarcoma Groups. J. Clin. Oncol. 2020, 38, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: Meta-analysis of individual data. Lancet 1997, 350, 1647–1654. [Google Scholar] [CrossRef]
- Pervaiz, N.; Colterjohn, N.; Farrokhyar, F.; Tozer, R.; Figueredo, A.; Ghert, M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008, 113, 573–581. [Google Scholar] [CrossRef]
- Pasquali, S.; Pizzamiglio, S.; Touati, N.; Litiere, S.; Marreaud, S.; Kasper, B.; Gelderblom, H.; Stacchiotti, S.; Judson, I.; Dei Tos, A.P.; et al. EORTC—Soft Tissue and Bone Sarcoma Group. The impact of chemotherapy on survival of patients with extremity and trunk wall soft tissue sarcoma: Revisiting the results of the EORTC-STBSG 62931 randomised trial. Eur. J. Cancer 2019, 109, 51–60. [Google Scholar] [CrossRef]
- Ferrari, A.; Miceli, R.; Rey, A.; Oberlin, O.; Orbach, D.; Brennan, B.; Mariani, L.; Carli, M.; Bisogno, G.; Cecchetto, G.; et al. Non-metastatic unresected paediatric non-rhabdomyosarcoma soft tissue sarcomas: Results of a pooled analysis from United States and European groups. Eur. J. Cancer 2011, 47, 724–731. [Google Scholar] [CrossRef]
- Ferrari, A.; Merks, J.H.M.; Chisholm, J.C.; Orbach, D.; Brennan, B.; Gallego, S.; van Noesel, M.M.; McHugh, K.; van Rijn, R.R.; Gaze, M.N.; et al. Outcomes of metastatic non-rhabdomyosarcoma soft tissue sarcomas (NRSTS) treated within the BERNIE study: A randomised, phase II study evaluating the addition of bevacizumab to chemotherapy. Eur. J. Cancer 2020, 130, 72–80. [Google Scholar] [CrossRef]
- Ferrari, A.; Orbach, D.; Casanova, M.; van Noesel, M.M.; Berlanga, P.; Brennan, B.; Corradini, N.; Schoot, R.A.; Ramirez-Villar, G.L.; Hjalgrim, L.L.; et al. Metastatic adult-type non-rhabdomyosarcoma soft tissue sarcomas in children and adolescents: A cohort study from the European paediatric Soft tissue sarcoma Study Group. Cancer 2023, 129, 2542–2552. [Google Scholar] [CrossRef]
- Pratt, C.B.; Maurer, H.M.; Gieser, P.; Salzberg, A.; Rao, B.N.; Parham, D.; Thomas, P.R.; Marcus, R.B.; Cantor, A.; Pick, T.; et al. Treatment of unresectable or metastatic pediatric soft tissue sarcomas with surgery, irradiation, and chemotherapy: A Pediatric Oncology Group study. Med. Pediatr. Oncol. 1998, 30, 201–209. [Google Scholar] [CrossRef]
- Pappo, A.S.; Devidas, M.; Jenkins, J.; Rao, B.; Marcus, R.; Thomas, P.; Gebhardt, M.; Pratt, C.; Grier, H.E. Phase II trial of neoadjuvant vincristine, ifosfamide, and doxorubicin with granulocyte colony-stimulating factor support in children and adolescents with advanced-stage nonrhabdomyosarcomatous soft tissue sarcomas: A Pediatric Oncology Group Study. J. Clin. Oncol. 2005, 23, 4031–4038. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Vennarini, S.; Fiore, M.; Bergamaschi, L.; Chiaravalli, S.; Morosi, C.; Colombo, C.; Pecori, E.; Puma, N.; Luksch, R.; et al. Local treatment in initially unresected non-rhabdomyosarcoma soft-tissue sarcomas of children and adolescents: A retrospective single-center experience. Pediatr. Blood Cancer 2024, 71, e30901. [Google Scholar] [CrossRef]
- Ferrari, A.; Brecht, I.B.; Koscielniak, E.; Casanova, M.; Scagnellato, A.; Bisogno, G.; Alaggio, R.; Cecchetto, G.; Catania, S.; Meazza, C.; et al. The role of adjuvant chemotherapy in children and adolescents with surgically resected, high-risk adult-type soft tissue sarcomas. Pediatr. Blood Cancer 2005, 45, 128–134. [Google Scholar] [CrossRef]
- Milgrom, S.A.; Million, L.; Mandeville, H.; Safwat, A.; Ermoian, R.P.; Terezakis, S. Non-rhabdomyosarcoma soft-tissue sarcoma. Pediatr. Blood Cancer 2021, 68 (Suppl. S2), e28279. [Google Scholar] [CrossRef] [PubMed]
- Sparber-Sauer, M.; Ferrari, A.; Spunt, S.L.; Vokuhl, C.; Casey, D.; Lautz, T.B.; Meyer, W.H.; Walterhouse, D.O.; Pajtler, K.W.; Alaggio, R.; et al. The significance of margins in pediatric Non-Rhabdomyosarcoma soft tissue sarcomas: Consensus on surgical margin definition harmonization from the INternational Soft Tissue SaRcoma ConsorTium (INSTRuCT). Cancer Med. 2023, 12, 11719–11730. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Tepper, J.; Glatstein, E.; Costa, J.; Baker, A.; Brennan, M.; DeMoss, E.V.; Seipp, C.; Sindelar, W.F.; Sugarbaker, P.; et al. The treatment of soft-tissue sarcomas of the extremities: Prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann. Surg. 1982, 196, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Million, L.; Hayes-Jordan, A.; Chi, Y.Y.; Donaldson, S.S.; Wolden, S.; Morris, C.; Terezakis, S.; Laurie, F.; Morano, K.; Fitzgerald, T.J.; et al. Local Control For High-Grade Nonrhabdomyosarcoma Soft Tissue Sarcoma Assigned to Radiation Therapy on ARST0332: A Report From the Childrens Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Chi, Y.Y.; De Salvo, G.L.; Orbach, D.; Brennan, B.; Randall, R.L.; McCarville, M.B.; Black, J.O.; Alaggio, R.; Hawkins, D.S.; et al. Surgery alone is sufficient therapy for children and adolescents with low-risk synovial sarcoma: A joint analysis from the European paediatric soft tissue sarcoma Study Group and the Children’s Oncology Group. Eur. J. Cancer 2017, 78, 1–6. [Google Scholar] [CrossRef]
- Tinkle, C.L.; Fernandez-Pineda, I.; Sykes, A.; Lu, Z.; Hua, C.H.; Neel, M.D.; Bahrami, A.; Shulkin, B.L.; Kaste, S.C.; Pappo, A.; et al. Nonrhabdomyosarcoma soft tissue sarcoma (NRSTS) in pediatric and young adult patients: Results from a prospective study using limited-margin radiotherapy. Cancer 2017, 123, 4419–4429. [Google Scholar] [CrossRef]
- Al Yami, A.; Griffin, A.M.; Ferguson, P.C.; Catton, C.N.; Chung, P.W.; Bell, R.S.; Wunder, J.S.; O’Sullivan, B. Positive surgical margins in soft tissue sarcoma treated with preoperative radiation: Is a postoperative boost necessary? Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Davis, A.M.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Kandel, R.; Goddard, K.; Sadura, A.; et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet 2002, 359, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Kraybill, W.G.; Harris, J.; Spiro, I.J.; Ettinger, D.S.; DeLaney, T.F.; Blum, R.H.; Lucas, D.R.; Harmon, D.C.; Letson, G.D.; Eisenberg, B. Phase II study of neoadjuvant chemotherapy and radiation therapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. J. Clin. Oncol. 2006, 24, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Haas, R.L. Preoperative radiotherapy in soft tissue sarcoma: From general guidelines to personalized medicine. Chin. Clin. Oncol. 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.D.; Chen, Q.; Million, L.; Spunt, S.L.; Fitzgerald, T.J.; Hu, C.; Rao, S.S.; Laurie, F.; Kessel, S.; Morano, K.; et al. Preoperative Intensity Modulated Radiation Therapy Compared to Three-Dimensional Conformal Radiation Therapy for High-Grade Extremity Sarcomas in Children: Analysis of the Children’s Oncology Group Study ARST0332. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.R. Radiological use of fast protons. Radiology 1946, 47, 487–491. [Google Scholar] [CrossRef]
- Particle Therapy Co-Operative Group. Particle Therapy Facilities in Clinical Operation. Available online: https://www.ptcog.ch/index.php/facilities-in-operation (accessed on 5 October 2023).
- Held, K.D.; Lomax, A.J.; Troost, E.G.C. Proton therapy special feature: Introductory editorial. Br. J. Radiol. 2020, 93, 20209004. [Google Scholar] [CrossRef] [PubMed]
- Newhauser, W.D.; Zhang, R. The physics of proton therapy. Phys. Med. Biol. 2015, 60, R155–R209. [Google Scholar] [CrossRef]
- Paganetti, H. Relative Biological Effectiveness (RBE) Values for Proton Beam Therapy. Variations as a Function of Biological Endpoint, Dose, and Linear Energy Transfer. Phys. Med. Biol. 2014, 59, R419–R472. [Google Scholar] [CrossRef]
- Mohan, R.; Grosshans, D. Proton therapy—Present and future. Adv. Drug Deliv. Rev. 2017, 109, 26–44. [Google Scholar] [CrossRef]
- Mitin, T.; Zietman, A.L. Promise and pitfalls of heavy-particle therapy. J. Clin. Oncol. 2014, 32, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Vennarini, S.; Del Baldo, G.; Lorentini, S.; Pertile, R.; Fabozzi, F.; Merli, P.; Megaro, G.; Scartoni, D.; Carai, A.; Tornesello, A.; et al. Acute Hematological Toxicity during Cranio-Spinal Proton Therapy in Pediatric Brain Embryonal Tumors. Cancers 2022, 14, 1653. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H. Range uncertainties in proton therapy and the role of Monte Carlo simulations. Phys. Med. Biol. 2012, 57, R99–R117. [Google Scholar] [CrossRef] [PubMed]
- Unkelbach, J.; Paganetti, H. Robust Proton Treatment Planning: Physical and Biological Optimization. Semin. Radiat. Oncol. 2018, 28, 88–96. [Google Scholar] [CrossRef]
- Janopaul-Naylor, J.; Kanter, K.R.; Flampouri, S.; Nguyen, V.; Olson, T.A.; Eaton, B.R. Adjuvant chemoradiation for high-grade cardiac leiomyosarcoma in a child: Case report and review of literature. Pediatr. Blood Cancer. 2021, 68, e29241. [Google Scholar] [CrossRef] [PubMed]
- Dunn, R.; Marget, M.J.; Momin, S.; Garcia-Rodriguez, L. Recurrent malignant peripheral nerve sheath tumor of the parietal scalp. Am. J. Otolaryngol. 2021, 42, 102812. [Google Scholar] [CrossRef]
- Ye, C.; Guo, J.; Liu, X.; Jiang, G.; Wang, W. Surgery and Proton Beam Therapy for Tracheal Synovial Sarcoma. Ann. Thorac. Surg. 2020, 110, e265–e267. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, B.S.; Stoker, J.; Vern-Gross, T. Proton Beam Therapy for Unresectable Mediastinal and Pericardial Spindle Cell Sarcoma: A Case Report. Int. J. Part. Ther. 2023, 10, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, N.; Leiser, D.; Pica, A.; Bachtiary, B.; Weber, D.C. Clinical Outcome After Pencil Beam Scanning Proton Therapy of Patients With Non-Metastatic Malignant and Benign Peripheral Nerve Sheath Tumors. Front. Oncol. 2022, 12, 881665. [Google Scholar] [CrossRef]
- Vogel, J.; Both, S.; Kirk, M.; Chao, H.H.; Bagatell, R.; Li, Y.; Womer, R.; Balamuth, N.; Reilly, A.; Kurtz, G.; et al. Proton therapy for pediatric head and neck malignancies. Pediatr. Blood Cancer 2018, 65, e26858. [Google Scholar] [CrossRef]
- Vázquez, M.; Baust, K.; Ilundain, A.; Leiser, D.; Bachtiary, B.; Pica, A.; Kliebsch, U.L.; Calaminus, G.; Weber, D.C. Pencil Beam Scanning Proton Therapy for Adolescents and Young Adults with Head and Neck Sarcomas. Int. J. Part. Ther. 2023, 10, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Hug, E.B.; Sweeney, R.A.; Nurre, P.M.; Holloway, K.C.; Slater, J.D.; Munzenrider, J.E. Proton radiotherapy in management of pediatric base of skull tumors. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Gaito, S.; France, A.; Crellin, A.M.; Thwaites, D.I.; Ahern, V.; Indelicato, D.; Timmermann, B.; Smith, E. Outcomes of Patients Treated in the UK Proton Overseas Programme: Non-central Nervous System Group. Clin. Oncol. 2023, 35, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.R.; Chen, Y.L.; Scharschmidt, T.J.; Chi, Y.Y.; Tian, J.; Black, J.O.; Davis, J.L.; Fanburg-Smith, J.C.; Zambrano, E.; Anderson, J.; et al. Pathological response in children and adults with large unresected intermediate-grade or high-grade soft tissue sarcoma receiving preoperative chemoradiotherapy with or without pazopanib (ARST1321): A multicentre, randomised, open-label, phase 2 trial. Lancet Oncol. 2020, 21, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Soft Tissue Sarcoma Version 3. 2023. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1464 (accessed on 13 March 2023).
- Laughlin, B.S.; Golafshar, M.; Prince, M.; Liu, W.; Kutyreff, C.J.; Ahmed, S.K.; Vern Gross, T.Z.; Haddock, M.; Petersen, I.; DeWees, T.A.; et al. Dosimetric comparison between proton beam therapy, intensity modulated radiation therapy, and 3D conformal therapy for soft tissue extremity sarcoma. Acta Oncol. 2023, 62, 473–479. [Google Scholar] [CrossRef]
- Fogliata, A.; Scorsetti, M.; Navarria, P.; Catalano, M.; Clivio, A.; Cozzi, L.; Lobefalo, F.; Nicolini, G.; Palumbo, V.; Pellegrini, C.; et al. Dosimetric comparison between VMAT with different dose calculation algorithms and protons for soft-tissue sarcoma radiotherapy. Acta Oncol. 2013, 52, 545–552. [Google Scholar] [CrossRef]
- Swanson, E.L.; Indelicato, D.J.; Louis, D.; Flampouri, S.; Li, Z.; Morris, C.G.; Paryani, N.; Slopsema, R. Comparison of three-dimensional (3D) conformal proton radiotherapy (RT), 3D conformal photon, R.T.; and intensity-modulated RT for retroperitoneal and intra-abdominal sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Vennarini, S.; Colombo, F.; Mirandola, A.; Chiaravalli, S.; Orlandi, E.; Massimino, M.; Casanova, M.; Ferrari, A. Clinical Insight on Proton Therapy for Paediatric Rhabdomyosarcoma. Cancer Manag. Res. 2023, 15, 1125–1139. [Google Scholar] [CrossRef]
- Liu, J.; Ng, D.; Lee, J.; Stalley, P.; Hong, A. Chest wall desmoid tumours treated with definitive radiotherapy: A plan comparison of 3D conformal radiotherapy, intensity-modulated radiotherapy and volumetric-modulated arc radiotherapy. Radiat. Oncol. 2016, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.J.; Hu, C.; Ladra, M.M.; Narang, A.K.; Pollack, C.E.; Terezakis, S.A. Socioeconomic factors affect the selection of proton radiation therapy for children. Cancer 2017, 123, 4048–4056. [Google Scholar] [CrossRef]
- Kelly, S.M.; Effeney, R.; Gaze, M.N.; Bernier-Chastagner, V.; Blondeel, A.; Clementel, E.; Corning, C.; Dieckmann, K.; Essiaf, S.; Gandola, L.; et al. QUARTET: A SIOP Europe project for quality and excellence in radiotherapy and imaging for children and adolescents with cancer. Eur. J. Cancer. 2022, 172, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, D.S.; Bisogno, G.; Koscielniak, E. Introducing INSTRuCT: An international effort to promote cooperation and data sharing. Pediatr. Blood Cancer 2020, 70, e28701. [Google Scholar] [CrossRef] [PubMed]

| Authors (Publication Year) | Type of Study | N° of Patients | Median Age, Sex | Histology | Tumor Site | RT Dose | Aim of RT | Outcome | Toxicity |
|---|---|---|---|---|---|---|---|---|---|
| Janopaul-Naylor J et al. (2021) [46] | Case report | 1 pt | 13 yr, F | Leiomyosarcoma (high-grade) | Heart | 66 GyRBE (surgical bed) 52.8 GyRBE (preoperative tumor extent) | Adjuvant | 2-year follow-up: no evidence of disease | Acute Toxicity: no ≥ G3 Late toxicity: mildly prolapsed mitral valve with mild mitral valve regurgitation, intermittent palpitations |
| Dunn R. et al. (2021) [47] | Case report | 1 pt | 16 yr, M | MPNST | H&N | NA | Adjuvant | NA | NA |
| Ye C et al. (2020) [48] | Case report | 1 pt | 19 yr, M | Synovial sarcoma | Trachea | 63 GyRBE (3.5 Gy/fr) | Adjuvant | 18 months follow-up: no evidence of disease | NA |
| Laughlin BS et al. (2023) [49] | Case report | 1 pt | 17 yr, M | Spindle cell sarcoma (high grade) | Mediastinum | 64.8 GyRBE | Definitive | 6.5 years: no evidence of disease | Late toxicity: Stage D Class III/IV Constrictive/Restrictive cardiomyopathy, with chronic pericarditis |
| Bachmann N. et al. (2022) [50] | Retrospective | 36 pts | 32 yr (3–75) <18 yr (9) 18–39 yr (15) 11 M, 25 F | 31 MPNST 5 PNST | - Trunk (20) - Extremities (5) - H&N (11) | 64 GyRBE (range, 50–74) | Neoadjuvant (28) Adjuvant (5) Definitive (3) Primary treatment (28) Recurrence (8) | 2-year OS, LC, and DC were 75.5%, 73.5%, and 61.2% | Acute toxicity: five G3 (dermatitis, mucositis, and pain) Late Toxicity: four G3 (cataract, osteonecrosis) |
| Vogel J. et al. (2018) [51] | Retrospective | 69 pts with H&N tumors 24 NRSTS | 14 yr (1–21) for the 24 NRSTS 15 M, 9 F | NRSTS | H&N | 63.0 GyRBE (range 36.0–81.0) | NA | 1- and 3-yr OS 93% and 90%. 1- and 3-yr freedom from: - LR: 92% and 85%, - RR: 94% and 86%, - DR: 86% and 78% | Acute toxicity: G3 (oral mucositis, anorexia, dysphagia, dehydration, and dermatitis) |
| Vazquez M. et al. (2023) [52] | Retrospective | 28 pts with H&N tumors, four NRSTS | 23.7 yr (15–37.9) 14 M, 14 F | Four NRSTS: three synovial sarcoma, one fibrosarcoma | H&N | 63 GyRBE (range 45–74) | NA | 5-yr LC, DC and OS were 71.8%, 80.5% and 90.7% | Acute toxicity: 7 G3 (dermatitis, mucositis) Late toxicity: 11 (cataracts, otite media, hearing impairment, sinusitis, osteoradionecrosis, retinopathy) |
| Hug EB. et al. (2002) [53] | Retrospective | 29 pts with skull base tumors, three NRSTS | 12 yr (1–19) 14 M, 15 F | 3 NRSTS | Skull base | 70 GyRBE (range 45–78.6) NRSTS: 50.4 GyRBE (range 50.4–59.6) | Adjuvant | 5-yr LC and OS were 72% and 56% | Late toxicity: two motor weakness and sensory deficits |
| Hwang E. et al. (2023) [54] | Retrospective | 495 pts 37 pts adult-type sarcoma | 11 yr (0–69) - 348 pts < 16 yrs - 111 pts (16–25 yr) | Non-central nervous system tumor | H&N, abdomen, pelvis, thorax, other | 51 GyRBE (range 50.4-55.8) | NA | 2-year and 5-year OS for all patients were 88.3% and 82.1%. 2-year and 5-year LC for all patients were 90.3% and 82.9%. LC for adult-type sarcoma 84.4%. | Late toxicity: - 59 G3 (cataracts, musculoskeletal deformity, premature, menopause and hearing impairment) - Seven G4 Three treatment-related secondary malignancy |
| Million L. et al. (2021) [28] | Sub-analysis of ARST0332 Trial | 193 pts 6 pts had PBT | 148 pts < 18 yr 45 pts 18–30 yr | Synovial sarcoma (75), MPNST (43), Undifferentiated (30), Other (45) | Body wall, extremity, H&N, visceral | Range 55.8–64.8 GyRBE | Adjuvant, neoadjuvant | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vennarini, S.; Colombo, F.; Mirandola, A.; Orlandi, E.; Pecori, E.; Chiaravalli, S.; Massimino, M.; Casanova, M.; Ferrari, A. Proton Therapy in Non-Rhabdomyosarcoma Soft Tissue Sarcomas of Children and Adolescents. Cancers 2024, 16, 1694. https://doi.org/10.3390/cancers16091694
Vennarini S, Colombo F, Mirandola A, Orlandi E, Pecori E, Chiaravalli S, Massimino M, Casanova M, Ferrari A. Proton Therapy in Non-Rhabdomyosarcoma Soft Tissue Sarcomas of Children and Adolescents. Cancers. 2024; 16(9):1694. https://doi.org/10.3390/cancers16091694
Chicago/Turabian StyleVennarini, Sabina, Francesca Colombo, Alfredo Mirandola, Ester Orlandi, Emilia Pecori, Stefano Chiaravalli, Maura Massimino, Michela Casanova, and Andrea Ferrari. 2024. "Proton Therapy in Non-Rhabdomyosarcoma Soft Tissue Sarcomas of Children and Adolescents" Cancers 16, no. 9: 1694. https://doi.org/10.3390/cancers16091694
APA StyleVennarini, S., Colombo, F., Mirandola, A., Orlandi, E., Pecori, E., Chiaravalli, S., Massimino, M., Casanova, M., & Ferrari, A. (2024). Proton Therapy in Non-Rhabdomyosarcoma Soft Tissue Sarcomas of Children and Adolescents. Cancers, 16(9), 1694. https://doi.org/10.3390/cancers16091694







