Protective Effects of Melatonin against Carcinogen-Induced Oxidative Damage in the Thyroid
Abstract
Simple Summary
Abstract
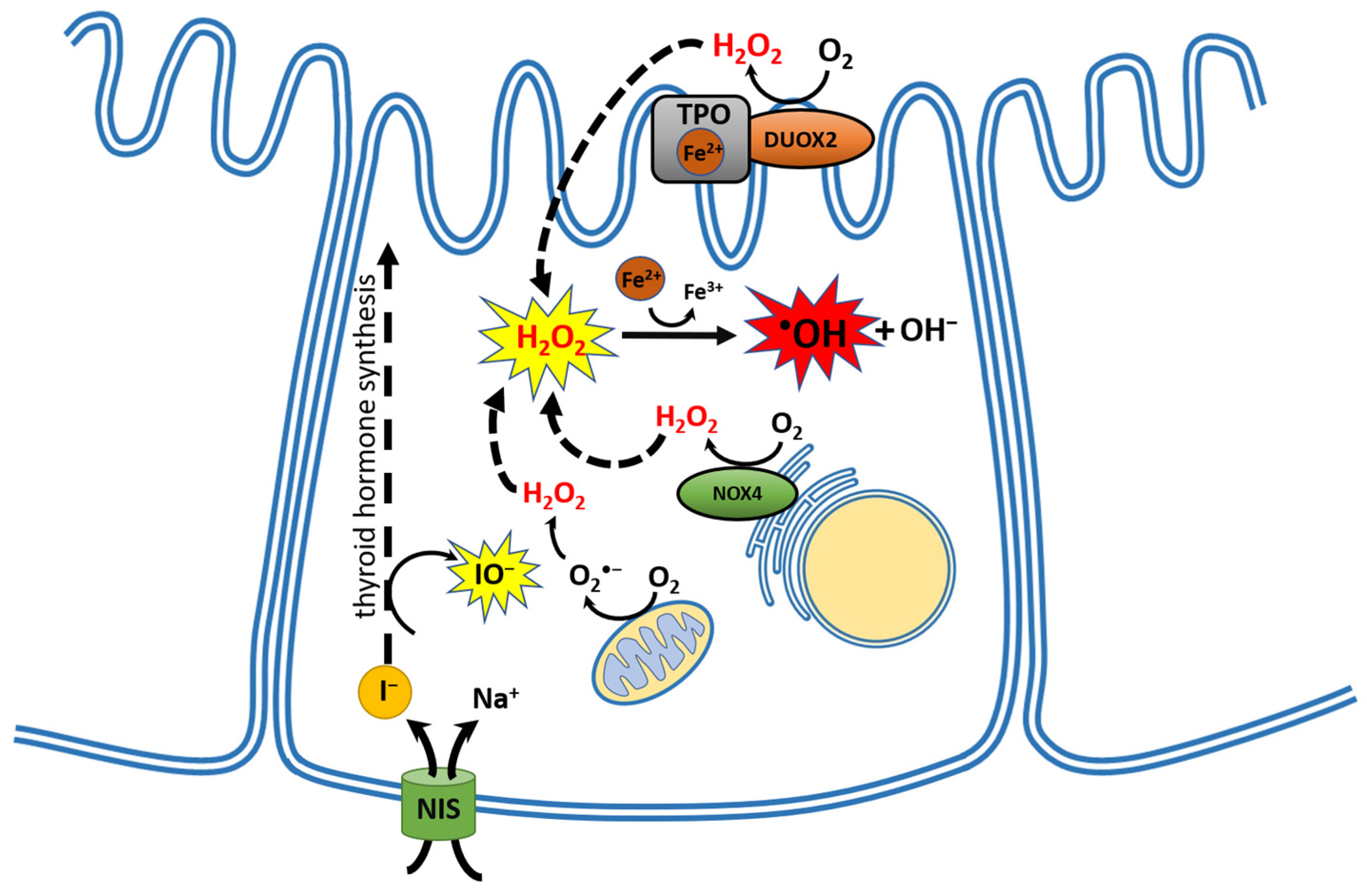
1. The Thyroid Gland as an Organ of Oxidative Nature
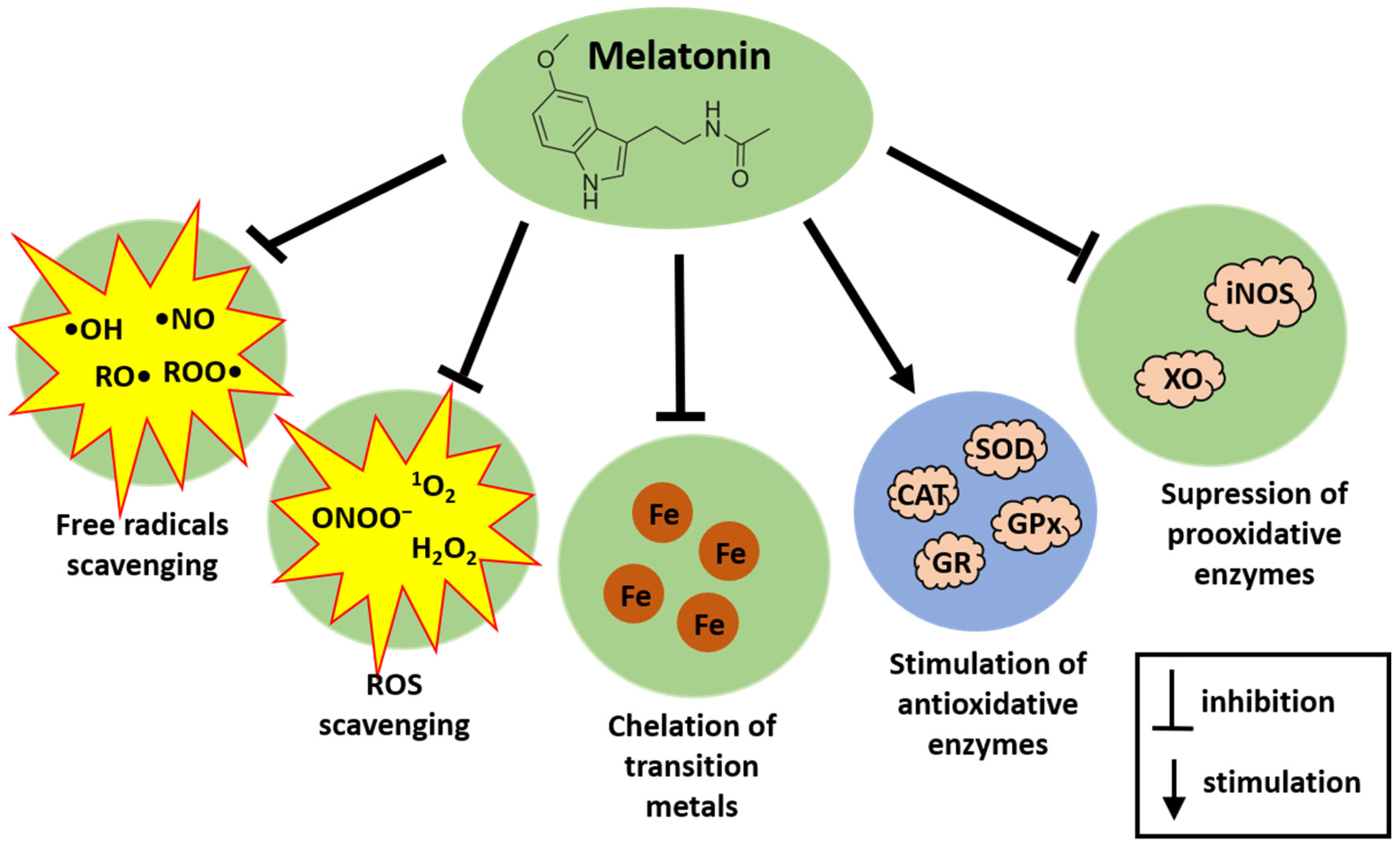
2. Melatonin as an Antioxidant—The Short Overview
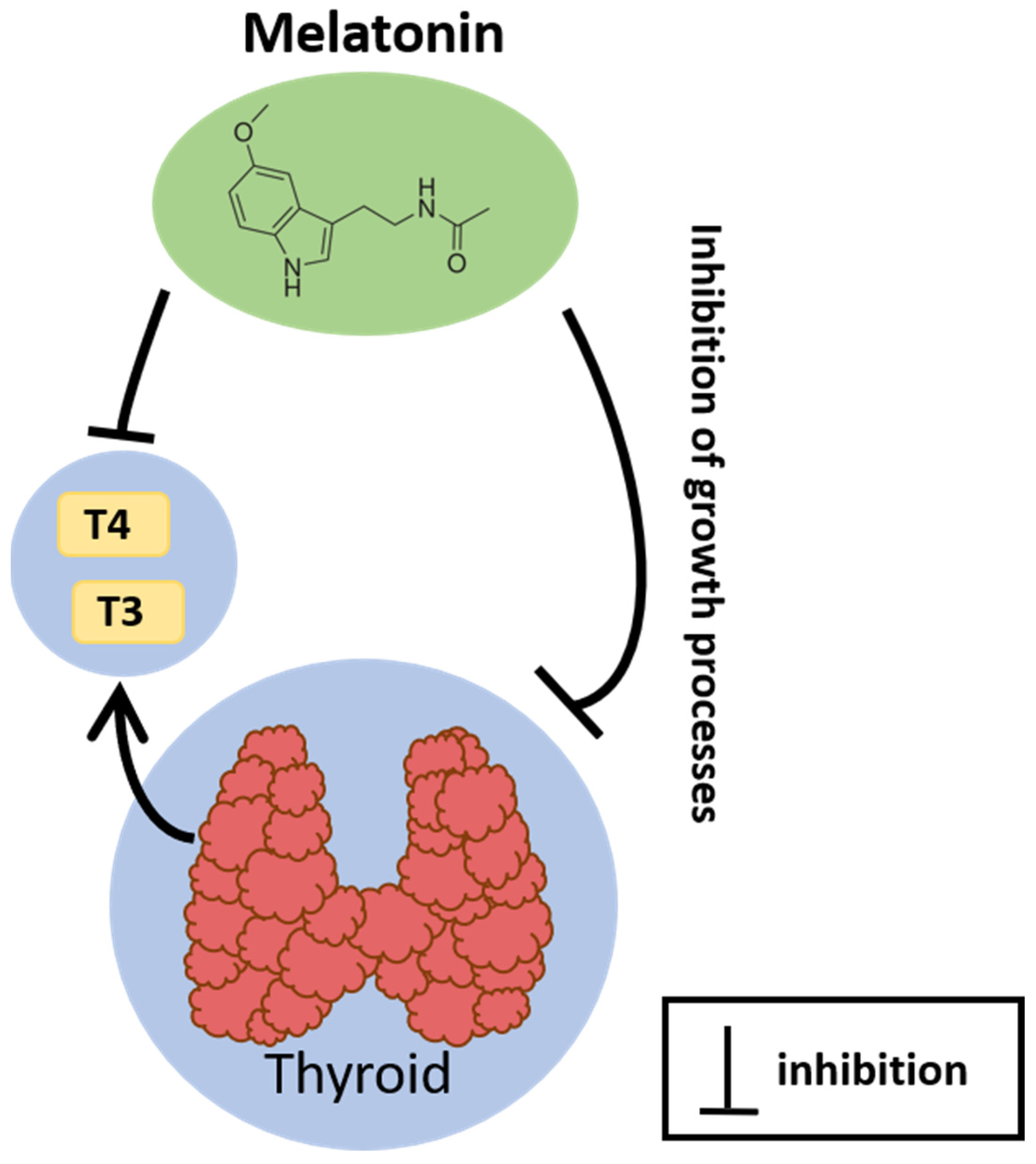
3. Relationship between the Thyroid and Melatonin
4. Potential Protective Effects of Melatonin against Carcinogenesis in Humans, Human Tissues, or Human-like Cell Lines
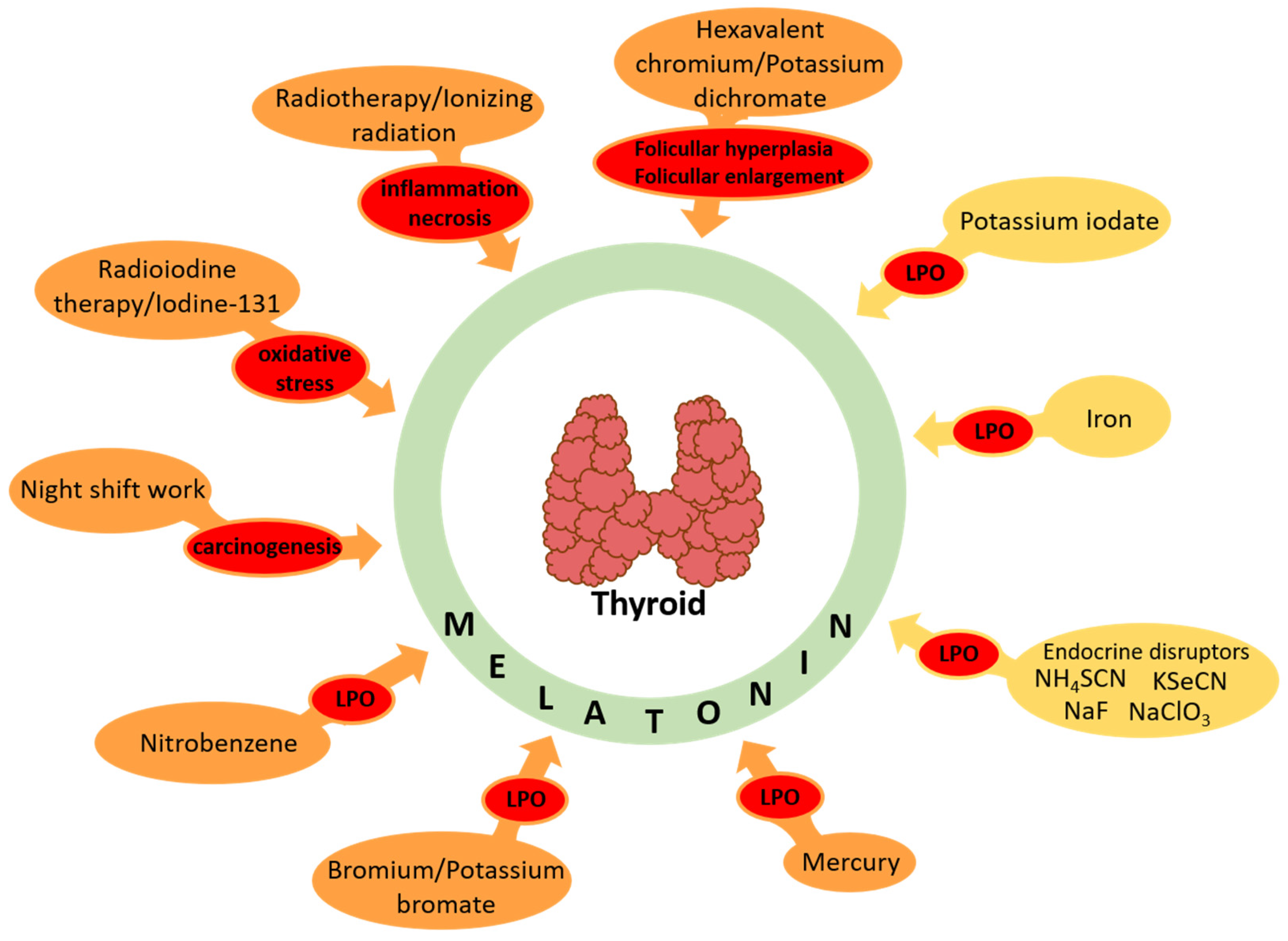
5. Evidence on Protective Effects of Melatonin against Oxidative Damage to Macromolecules Caused by Documented/Potential Carcinogens in the Thyroid
5.1. Melatonin and Oxidative Stress in the Thyroid
5.2. Protective Effects of Melatonin against Oxidative Damage Caused by Documented Carcinogens (Listed in the International Agency for Research on Cancer (IARC) Monographs)
5.2.1. Hexavalent Chromium/Potassium Dichromate
5.2.2. Radiotherapy/Ionizing Radiation
5.2.3. Radioiodinetherapy/Iodine-131
5.2.4. Night-Shift Work
5.2.5. Nitrobenzene
5.2.6. Bromium/Potassium Bromate
5.2.7. Mercury
5.3. Protective Effects of Melatonin against Oxidative Damage Caused by Potential Carcinogens (Not Listed in the International Agency for Research on Cancer Monographs)
5.3.1. Potassium Iodate
5.3.2. Iron-Induced Oxidative Damage
5.3.3. Endocrine Disruptors
| Agent/Dose | Species/Organ/Tissue/ Cellular Compartment | Effect of Agent | Dose of Melatonin, Which Reduced or Prevented the Effect of Agent | Refs. |
|---|---|---|---|---|
| Potassium iodate (KIO3) | Porcine thyroid homogenates | ↑MDA + 4-HDA | 5 mM | [25,84,85] |
| Ferrous ion (Fe2+)/ferrous sulfate (FeSO4) 40 µM or 37.5 µM–4.8 mM | Porcine thyroid homogenates | ↑MDA + 4-HDA | 5 mM | [20,21] |
| Ammonium thiocyanate (NH4SCN) 250–500 mM | Porcine thyroid homogenates | ↑MDA + 4-HDA | 5 mM | [134] |
| Sodium fluoride (NaF) 25–100 mM | Porcine thyroid homogenates | ↑MDA + 4-HDA | 5 mM | [134] |
| Potassium selenocyanate (KSeCN) 500 mM | Porcine thyroid homogenates | ↑MDA + 4-HDA | 5 mM | [134] |
| Sodium chlorate (NaClO3) 0.5–10 mM | Porcine thyroid homogenates | ↑MDA + 4-HDA | No protection | [134] |
6. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Maenhaut, C.; Christophe, D.; Vassart, G.; Dumont, J.; Roger, P.P.; Opitz, R. Ontogeny, Anatomy, Metabolism and Physiology of the Thyroid. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2015. [Google Scholar]
- Szanto, I.; Pusztaszeri, M.; Mavromati, M. H2O2 Metabolism in Normal Thyroid Cells and in Thyroid Tumorigenesis: Focus on NADPH Oxidases. Antioxidants 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Karbownik-Lewińska, M.; Kokoszko-Bilska, A. Oxidative damage to macromolecules in the thyroid–experimental evidence. Thyroid. Res. 2012, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Faria, C.C.; Fortunato, R.S. The role of dual oxidases in physiology and cancer. Genet. Mol. Biol. 2020, 43 (Suppl. S1), e20190096. [Google Scholar] [CrossRef] [PubMed]
- Grasberger, H. Defects of thyroidal hydrogen peroxide generation in congenital hypothyroidism. Mol. Cell. Endocrinol. 2010, 322, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, W.; Zhang, L.; Wang, F.; Wang, F.; Gu, M.; Wang, X.; Liu, S. Identification and analyzes of DUOX2 mutations in two familial congenital hypothyroidism cases. Endocrine 2021, 72, 147–156. [Google Scholar] [CrossRef]
- Hulur, I.; Hermanns, P.; Nestoris, C.; Heger, S.; Refetoff, S.; Pohlenz, J.; Grasberger, H. A single copy of the recently identified dual oxidase maturation factor (DUOXA) 1 gene produces only mild transient hypothyroidism in a patient with a novel biallelic DUOXA2 mutation and monoallelic DUOXA1 deletion. J. Clin. Endocrinol. Metab. 2011, 96, E841–E845. [Google Scholar] [CrossRef] [PubMed]
- Szanto, I. NADPH Oxidase 4 (NOX4) in Cancer: Linking Redox Signals to Oncogenic Metabolic Adaptation. Int. J. Mol. Sci. 2022, 23, 2702. [Google Scholar] [CrossRef]
- Azouzi, N.; Cailloux, J.; Cazarin, J.M.; Knauf, J.A.; Cracchiolo, J.; Al Ghuzlan, A.; Hartl, D.; Polak, M.; Carré, A.; El Mzibri, M.; et al. NADPH Oxidase NOX4 Is a Critical Mediator of BRAFV600E-Induced Downregulation of the Sodium/Iodide Symporter in Papillary Thyroid Carcinomas. Antioxid. Redox Signal 2017, 26, 864–877. [Google Scholar] [CrossRef]
- Oglio, R.; Salvarredi, L.; Rossich, L.; Copelli, S.; Pisarev, M.; Juvenal, G.; Thomasz, L. Participation of NADPH 4 oxidase in thyroid regulation. Mol. Cell. Endocrinol. 2019, 480, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.F.; Ma, Z.; Liu, Z.; Terada, L.S. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol. Cell. Biol. 2010, 30, 3553–3568. [Google Scholar] [CrossRef]
- Malhotra, J.D.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress: A vicious cycle or a double-edged sword? Antioxid. Redox Signal. 2007, 9, 2277–2293. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemistry of Hydrogen Peroxide Formation and Elimination in Mammalian Cells, and Its Role in Various Pathologies. Stresses 2022, 2, 256–274. [Google Scholar] [CrossRef]
- Garofalo, V.; Condorelli, R.A.; Cannarella, R.; Aversa, A.; Calogero, A.E.; La Vignera, S. Relationship between Iron Deficiency and Thyroid Function: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 4790. [Google Scholar] [CrossRef] [PubMed]
- Abbas, W.; Elmugabil, A.; Hamdan, H.Z.; Rayis, D.A.; Adam, I. Iron deficiency and thyroid dysfunction among sudanese women in first trimester of pregnancy: A cross-sectional study. BMC Endocr. Disord. 2023, 23, 223. [Google Scholar] [CrossRef] [PubMed]
- Krohn, K.; Maier, J.; Paschke, R. Mechanisms of disease: Hydrogen peroxide, DNA damage and mutagenesis in the development of thyroid tumors. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Penna, G.; Rubio, I.G.S.; Brust, E.S.; Cazarin, J.; Hecht, F.; Alkmim, N.R.; Rajão, K.M.A.B.; Ramos, H.E. Congenital hypothyroidism and thyroid cancer. Endocr. Relat. Cancer 2021, 28, R217–R230. [Google Scholar] [CrossRef] [PubMed]
- Rynkowska, A.; Stępniak, J.; Karbownik-Lewińska, M. Fenton Reaction-Induced Oxidative Damage to Membrane Lipids and Protective Effects of 17β-Estradiol in Porcine Ovary and Thyroid Homogenates. Int. J. Environ. Res. Public Health 2020, 17, 6841. [Google Scholar] [CrossRef] [PubMed]
- Rynkowska, A.; Stępniak, J.; Karbownik-Lewińska, M. Melatonin and Indole-3-Propionic Acid Reduce Oxidative Damage to Membrane Lipids Induced by High Iron Concentrations in Porcine Skin. Membranes 2021, 11, 571. [Google Scholar] [CrossRef]
- Stępniak, J.; Rynkowska, A.; Karbownik-Lewińska, M. Membrane Lipids in the Thyroid Comparing to Those in Non-Endocrine Tissues Are Less Sensitive to Pro-Oxidative Effects of Fenton Reaction Substrates. Front. Mol. Biosci. 2022, 9, 901062. [Google Scholar] [CrossRef]
- Karbownik, M.; Lewiński, A. Melatonin reduces Fenton reaction-induced lipid peroxidation in porcine thyroid tissue. J. Cell. Biochem. 2003, 90, 806–811. [Google Scholar] [CrossRef]
- Stępniak, J.; Lewiński, A.; Karbownik-Lewińska, M. Membrane lipids and nuclear DNA are differently susceptive to Fenton reaction substrates in porcine thyroid. Toxicol. Vitr. 2013, 27, 71–78. [Google Scholar] [CrossRef]
- Stepniak, J.; Lewinski, A.; Karbownik-Lewinska, M. Oxidative damage to membrane lipids in the thyroid–no differences between sexes. Drug Chem. Toxicol. 2021, 44, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, P.; Edwards, C. Integrated approach to the mechanisms of thyroid toxins: Electron transfer, reactive oxygen species, oxidative stress, cell signaling, receptors, and antioxidants. J. Recept. Signal Transduct. Res. 2010, 30, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Iwan, P.; Stepniak, J.; Karbownik-Lewinska, M. Pro-Oxidative Effect of KIO3 and Protective Effect of Melatonin in the Thyroid-Comparison to Other Tissues. Life 2021, 11, 592, Erratum in Life 2022, 12, 592. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Stepniak, J.; Lewinski, A.; Karbownik-Lewinska, M. Sexual Dimorphism of NADPH Oxidase/H2O2 System in Rat Thyroid Cells; Effect of Exogenous 17β-Estradiol. Int. J. Mol. Sci. 2018, 19, 4063. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, S.; Baldini, E.; Pironi, D.; Lauro, A.; D’Orazi, V.; Tartaglia, F.; Tripodi, D.; Lori, E.; Gagliardi, F.; Praticò, M.; et al. Iodine: Its Role in Thyroid Hormone Biosynthesis and Beyond. Nutrients 2021, 13, 4469. [Google Scholar] [CrossRef] [PubMed]
- Karbownik-Lewińska, M.; Stępniak, J.; Iwan, P.; Lewiński, A. Iodine as a potential endocrine disruptor-a role of oxidative stress. Endocrine 2022, 78, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Lee, K.; Park, Y.I.; Park, S.; Back, K. Molecular cloning and functional analysis of serotonin N-acetyltransferase from the cyanobacterium Synechocystis sp. PCC 6803. J. Pineal Res. 2013, 55, 371–376. [Google Scholar] [CrossRef]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef]
- Margulis, L. Symbiotic theory of the origin of eukaryotic organelles; criteria for proof. Symp. Soc. Exp. Biol. 1975, 29, 21–38. [Google Scholar]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Paredes, S.D.; Korkmaz, A.; Sainz, R.M.; Mayo, J.C.; Fuentes-Broto, L.; Reiter, R.J. The changing biological roles of melatonin during evolution: From an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. Camb. Philos. Soc. 2010, 85, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Reiter, R.J.; Zimmerman, S.; Hardeland, R. Melatonin: Both a Messenger of Darkness and a Participant in the Cellular Actions of Non-Visible Solar Radiation of Near Infrared Light. Biology 2023, 12, 89. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Galano, A.; Reiter, R.J. Cyclic-3-hydroxymelatonin (C3HOM), a potent antioxidant, scavenges free radicals and suppresses oxidative reactions. Curr. Med. Chem. 2014, 21, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tang, Q.; Zeng, Y. Melatonin: Potential avenue for treating iron overload disorders. Ageing Res. Rev. 2022, 81, 101717. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin: A Versatile Protector against Oxidative DNA Damage. Molecules 2018, 23, 530. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Karbownik, M.; Calvo, J.R. Significance of melatonin in antioxidative defense system: Reactions and products. Biol. Signals Recept. 2000, 9, 137–159. [Google Scholar] [CrossRef]
- Barlow-Walden, L.R.; Reiter, R.J.; Abe, M.; Pablos, M.; Menendez-Pelaez, A.; Chen, L.D.; Poeggeler, B. Melatonin stimulates brain glutathione peroxidase activity. Neurochem. Int. 1995, 26, 497–502. [Google Scholar] [CrossRef]
- Abo Taleb, H.A.; Alghamdi, B.S. Neuroprotective Effects of Melatonin during Demyelination and Remyelination Stages in a Mouse Model of Multiple Sclerosis. J. Mol. Neurosci. 2020, 70, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.W.; Kleszczyński, K.; Hardkop, L.H.; Kruse, N.; Zillikens, D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2’-deoxyguanosine) in ex vivo human skin. J. Pineal Res. 2013, 54, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Laothong, U.; Pinlaor, P.; Hiraku, Y.; Boonsiri, P.; Prakobwong, S.; Khoontawad, J.; Pinlaor, S. Protective effect of melatonin against Opisthorchis viverrini-induced oxidative and nitrosative DNA damage and liver injury in hamsters. J. Pineal Res. 2010, 49, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Sokolovic, D.; Djordjevic, B.; Kocic, G.; Stoimenov, T.J.; Stanojkovic, Z.; Sokolovic, D.M.; Veljkovic, A.; Ristic, G.; Despotovic, M.; Milisavljevic, D.; et al. The Effects of Melatonin on Oxidative Stress Parameters and DNA Fragmentation in Testicular Tissue of Rats Exposed to Microwave Radiation. Adv. Clin. Exp. Med. 2015, 24, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Waldhauser, F.; Weiszenbacher, G.; Frisch, H.; Zeitlhuber, U.; Waldhauser, M.; Wurtman, R.J. Fall in nocturnal serum of melatonin during puberty and pubescence. Lancet 1984, 1, 362–365. [Google Scholar] [CrossRef]
- Sack, R.L.; Lewy, A.J.; Erb, D.L.; Vollmer, W.M.; Singer, C.M. Human melatonin production decreases with age. J. Pineal Res. 1986, 3, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Sae-Teaw, M.; Johns, J.; Johns, N.P.; Subongkot, S. Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. J. Pineal Res. 2013, 55, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Esteban, S.; Nicolaus, C.; Garmundi, A.; Rial, R.V.; Rodríguez, A.B.; Ortega, E.; Ibars, C.B. Effect of orally administered l-tryptophan on serotonin, melatonin, and the innate immune response in the rat. Mol. Cell. Biochem. 2004, 267, 39–46. [Google Scholar] [CrossRef]
- Li, J.; Somers, V.K.; Xu, H.; Lopez-Jimenez, F.; Covassin, N. Trends in Use of Melatonin Supplements Among US Adults, 1999–2018. JAMA 2022, 327, 483–485. [Google Scholar] [CrossRef]
- Morsali, S.; Sabahi, Z.; Kakaei, J.; Hakimzadeh, Z.; Hamidi, S.; Gholipour-Khalili, E.; Sanaie, S.; Talebi, M.; Naseri, A. Clinical efficacy and safety of melatonin supplementation in multiple sclerosis: A systematic review. Inflammopharmacology 2023, 31, 2213–2220. [Google Scholar] [CrossRef]
- Minich, D.M.; Henning, M.; Darley, C.; Fahoum, M.; Schuler, C.B.; Frame, J. Is Melatonin the “Next Vitamin D”?: A Review of Emerging Science, Clinical Uses, Safety, and Dietary Supplements. Nutrients 2022, 14, 3934. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.P.; Gögenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Zetner, D.; Andersen, L.P.K.; Alder, R.; Jessen, M.L.; Tolstrup, A.; Rosenberg, J. Pharmacokinetics and Safety of Intravenous, Intravesical, Rectal, Transdermal, and Vaginal Melatonin in Healthy Female Volunteers: A Cross-Over Study. Pharmacology 2021, 106, 169–176. [Google Scholar] [CrossRef]
- Lewinski, A.; Karbownik, M. REVIEW. Melatonin and the thyroid gland. Neuro Endocrinol. Lett. 2002, 23 (Suppl. S1), 73–78. [Google Scholar] [PubMed]
- Wood, S.; Loudon, A. Clocks for all seasons: Unwinding the roles and mechanisms of circadian and interval timers in the hypothalamus and pituitary. J. Endocrinol. 2014, 222, R39–R59. [Google Scholar] [CrossRef] [PubMed]
- Yasuo, S.; Nakao, N.; Ohkura, S.; Iigo, M.; Hagiwara, S.; Goto, A.; Ando, H.; Yamamura, T.; Watanabe, M.; Watanabe, T.; et al. Long-day suppressed expression of type 2 deiodinase gene in the mediobasal hypothalamus of the Saanen goat, a short-day breeder: Implication for seasonal window of thyroid hormone action on reproductive neuroendocrine axis. Endocrinology 2006, 147, 432–440. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ertek, S. Molecular economy of nature with two thyrotropins from different parts of the pituitary: Pars tuberalis thyroid-stimulating hormone and pars distalis thyroid-stimulating hormone. Arch. Med. Sci. 2021, 17, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.A.; Teubner, B.J.; Smith, C.D.; Prendergast, B.J. Exogenous T3 mimics long day lengths in Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R2368–R2372. [Google Scholar] [CrossRef] [PubMed]
- Hanon, E.A.; Lincoln, G.A.; Fustin, J.M.; Dardente, H.; Masson-Pévet, M.; Morgan, P.J.; Hazlerigg, D.G. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr. Biol. 2008, 18, 1147–1152. [Google Scholar] [CrossRef]
- Garcia-Marin, R.; de Miguel, M.; Fernández-Santos, J.M.; Carrillo-Vico, A.; Utrilla, J.C.; Morillo-Bernal, J.; Díaz-Parrado, E.; Rodríguez-Prieto, I.; Guerrero, J.M.; Martín-Lacave, I. Melatonin-synthesizing enzymes and melatonin receptor in rat thyroid cells. Histol. Histopathol. 2012, 27, 1429–1438. [Google Scholar] [CrossRef]
- Garcia-Marin, R.; Fernandez-Santos, J.M.; Morillo-Bernal, J.; Gordillo-Martinez, F.; Vazquez-Roman, V.; Utrilla, J.C.; Carrillo-Vico, A.; Guerrero, J.M.; Martin-Lacave, I. Melatonin in the thyroid gland: Regulation by thyroid-stimulating hormone and role in thyroglobulin gene expression. J. Physiol. Pharmacol. 2015, 66, 643–652. [Google Scholar]
- Taheri, P.; Mogheiseh, A.; Shojaee Tabrizi, A.; Nazifi, S.; Salavati, S.; Koohi, F. Changes in thyroid hormones, leptin, ghrelin and, galanin following oral melatonin administration in intact and castrated dogs: A preliminary study. BMC Vet. Res. 2019, 15, 145. [Google Scholar] [CrossRef] [PubMed]
- Mogulkoc, R.; Baltaci, A.K. The effect of intraperitoneal melatonin supplementation on the release of thyroid hormones and testosterone in rats with hyperthyroid. Neuro Endocrinol. Lett. 2003, 24, 345–347. [Google Scholar] [PubMed]
- Belviranli, M.; Baltaci, A.K. The relation between reduced serum melatonin levels and zinc in rats with induced hypothyroidism. Cell Biochem. Funct. 2008, 26, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Cini, G.; Neri, B.; Pacini, A.; Cesati, V.; Sassoli, C.; Quattrone, S.; D’Apolito, M.; Fazio, A.; Scapagnini, G.; Provenzani, A.; et al. Antiproliferative activity of melatonin by transcriptional inhibition of cyclin D1 expression: A molecular basis for melatonin-induced oncostatic effects. J. Pineal Res. 2005, 39, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Liu, D.; Di, S.; Zhang, Z.; Li, W.; Zhang, J.; Xu, L.; Guo, K.; Zhu, Y.; Li, X.; et al. Histone deacetylase 9 downregulation decreases tumor growth and promotes apoptosis in non-small cell lung cancer after melatonin treatment. J. Pineal Res. 2019, 67, e12587. [Google Scholar] [CrossRef] [PubMed]
- Carbajo-Pescador, S.; Ordoñez, R.; Benet, M.; Jover, R.; García-Palomo, A.; Mauriz, J.L.; González-Gallego, J. Inhibition of VEGF expression through blockade of Hif1α and STAT3 signalling mediates the anti-angiogenic effect of melatonin in HepG2 liver cancer cells. Br. J. Cancer 2013, 109, 83–91. [Google Scholar] [CrossRef] [PubMed]
- El-Sokkary, G.H.; Ismail, I.A.; Saber, S.H. Melatonin inhibits breast cancer cell invasion through modulating DJ-1/KLF17/ID-1 signaling pathway. J. Cell. Biochem. 2019, 120, 3945–3957. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.C.; Chen, P.C.; Chiou, P.C.; Hsu, C.J.; Liu, P.I.; Yang, Y.C.; Reiter, R.J.; Yang, S.F.; Tang, C.H. Melatonin suppresses lung cancer metastasis by inhibition of epithelial-mesenchymal transition through targeting to Twist. Clin. Sci. 2019, 133, 709–722. [Google Scholar] [CrossRef]
- Gurunathan, S.; Qasim, M.; Kang, M.H.; Kim, J.H. Role and Therapeutic Potential of Melatonin in Various Type of Cancers. OncoTargets Ther. 2021, 14, 2019–2052. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.A.; Tan, D.X.; Acuna-Castroviejo, D.; Qin, L.; Yang, S.F.; Xu, K. Melatonin, a Full Service Anti-Cancer Agent: Inhibition of Initiation, Progression and Metastasis. Int. J. Mol. Sci. 2017, 18, 843. [Google Scholar] [CrossRef] [PubMed]
- Mafi, A.; Rezaee, M.; Hedayati, N.; Hogan, S.D.; Reiter, R.J.; Aarabi, M.H.; Asemi, Z. Melatonin and 5-fluorouracil combination chemotherapy: Opportunities and efficacy in cancer therapy. Cell Commun. Signal. 2023, 21, 33. [Google Scholar] [CrossRef]
- Lissoni, P. Biochemotherapy with standard chemotherapies plus the pineal hormone melatonin in the treatment of advanced solid neoplasms. Pathol. Biol. 2007, 55, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Kartini, D.; Taher, A.; Panigoro, S.S.; Setiabudy, R.; Jusman, S.W.; Haryana, S.M.; Murdani, A.; Rustamadji, P.; Karisyah, A.; Rasyid, S.H. Melatonin effect on hypoxia inducible factor-1α and clinical response in patients with oral squamous cell carcinoma receiving neoadjuvant chemotherapy: A randomized controlled trial. J. Carcinog. 2021, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.C.S.; Zortea, M.; Souza, A.; Santos, V.; Biazús, J.V.; Torres, I.L.S.; Fregni, F.; Caumo, W. Clinical impact of melatonin on breast cancer patients undergoing chemotherapy; effects on cognition, sleep and depressive symptoms: A randomized, double-blind, placebo-controlled trial. PLoS ONE 2020, 15, e0231379. [Google Scholar] [CrossRef] [PubMed]
- Stępniak, J.; Krawczyk-Lipiec, J.; Lewiński, A.; Karbownik-Lewińska, M. Sorafenib versus Lenvatinib Causes Stronger Oxidative Damage to Membrane Lipids in Noncancerous Tissues of the Thyroid, Liver, and Kidney: Effective Protection by Melatonin and Indole-3-Propionic Acid. Biomedicines 2022, 10, 2890. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Liu, M.; Liang, F.; Zhao, L.; Gao, C.; Jiang, X.; Zhang, X.; Zhan, H.; Hu, H.; Zhao, Z. Cardiotoxicity of sorafenib is mediated through elevation of ROS level and CaMKII activity and dysregulation of calcium homoeostasis. Basic. Clin. Pharmacol. Toxicol. 2020, 126, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Aras, S.; Tanzer, I.H.O.; Karaçavuş, S.; Sayir, N.; Erdem, E.; Hacımustafaoğlu, F.; Erdoğan, C.E.; Sapmaz, T.; İkizceli, T.; Pençe, H.H.; et al. Effect of melatonin on low and high dose radiotherapy induced thyroid injury. Biotech. Histochem. 2023, 98, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Arıcıgil, M.; Dündar, M.A.; Yücel, A.; Eryılmaz, M.A.; Aktan, M.; Alan, M.A.; Fındık, S.; Kılınç, İ. Melatonin prevents possible radiotherapy-induced thyroid injury. Int. J. Radiat. Biol. 2017, 93, 1350–1356. [Google Scholar] [CrossRef]
- Zou, Z.W.; Liu, T.; Li, Y.; Chen, P.; Peng, X.; Ma, C.; Zhang, W.J.; Li, P.D. Melatonin suppresses thyroid cancer growth and overcomes radioresistance via inhibition of p65 phosphorylation and induction of ROS. Redox Biol. 2018, 16, 226–236. [Google Scholar] [CrossRef]
- Ghorbani-Anarkooli, M.; Dabirian, S.; Zendedel, A.; Moladoust, H.; Bahadori, M.H. Effects of melatonin on the toxicity and proliferation of human anaplastic thyroid cancer cell line. Acta Histochem. 2021, 123, 151700. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Sun, W.; Li, X.; Xu, W. Melatonin promotes apoptosis of thyroid cancer cells via regulating the signaling of microRNA-21 (miR-21) and microRNA-30e (miR-30e). Bioengineered 2022, 13, 9588–9601. [Google Scholar] [CrossRef] [PubMed]
- Iwan, P.; Stepniak, J.; Karbownik-Lewinska, M. Cumulative Protective Effect of Melatonin and Indole-3-Propionic Acid against KIO3-Induced Lipid Peroxidation in Porcine Thyroid. Toxics 2021, 9, 89. [Google Scholar] [CrossRef] [PubMed]
- Iwan, P.; Stepniak, J.; Karbownik-Lewinska, M. Melatonin reduces high levels of lipid peroxidation induced by potassium iodate in porcine thyroid. Int. J. Vitam. Nutr. Res. 2021, 91, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, M.; Zasada, K.; Lewinski, A.; Karbownik-Lewinska, M. Melatonin restores the basal level of lipid peroxidation in rat tissues exposed to potassium bromate in vitro. Neuro Endocrinol. Lett. 2010, 31, 363–369. [Google Scholar] [PubMed]
- Karbownik, M.; Stasiak, M.; Zygmunt, A.; Zasada, K.; Lewiński, A. Protective effects of melatonin and indole-3-propionic acid against lipid peroxidation, caused by potassium bromate in the rat kidney. Cell Biochem. Funct. 2006, 24, 483–489. [Google Scholar] [CrossRef] [PubMed]
- IARC. Arsenic, Metals, Fibres, and Dusts. In Volume 100C: A Review of Human Carcinogens; IARC: Lyon, France, 2012. [Google Scholar]
- Deng, Y.; Wang, M.; Tian, T.; Lin, S.; Xu, P.; Zhou, L.; Dai, C.; Hao, Q.; Wu, Y.; Zhai, Z.; et al. The Effect of Hexavalent Chromium on the Incidence and Mortality of Human Cancers: A Meta-Analysis Based on Published Epidemiological Cohort Studies. Front. Oncol. 2019, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Islam, R.; Wang, Y.; Zhang, X.; Liu, L.Z. Epigenetic Regulation in Chromium-, Nickel- and Cadmium-Induced Carcinogenesis. Cancers 2022, 14, 5768. [Google Scholar] [CrossRef] [PubMed]
- DesMarais, T.L.; Costa, M. Mechanisms of Chromium-Induced Toxicity. Curr. Opin. Toxicol. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Saeed, E.; El-Mansy, A.A.; Mazroa, S.A.; Moustafa, A.M. The possible protective role of vitamin C versus melatonin on potassium dichromate induced changes in thyroid gland: Light and electron microscopic study. Ultrastruct. Pathol. 2023, 47, 73–89. [Google Scholar] [CrossRef]
- IARC. Ionizing Radiation. In Part 1: X- and Gamma (γ)-Radiation, and Neutrons Volume 75: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2000. [Google Scholar]
- Rooney, M.K.; Andring, L.M.; Corrigan, K.L.; Bernard, V.; Williamson, T.D.; Fuller, C.D.; Garden, A.S.; Gunn, B.; Lee, A.; Moreno, A.C.; et al. Hypothyroidism following Radiotherapy for Head and Neck Cancer: A Systematic Review of the Literature and Opportunities to Improve the Therapeutic Ratio. Cancers 2023, 15, 4321. [Google Scholar] [CrossRef]
- Brook, I. Late side effects of radiation treatment for head and neck cancer. Radiat. Oncol. J. 2020, 38, 84–92. [Google Scholar] [CrossRef]
- Karbownik, M.; Reiter, R.J. Antioxidative effects of melatonin in protection against cellular damage caused by ionizing radiation. Proc. Soc. Exp. Biol. Med. 2000, 225, 9–22. [Google Scholar] [CrossRef]
- IARC. Ionizing Radiation. In Part 2: Some Internally Deposited Radionuclides Volume 78: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2001. [Google Scholar]
- Shim, S.R.; Kitahara, C.M.; Cha, E.S.; Kim, S.J.; Bang, Y.J.; Lee, W.J. Cancer Risk After Radioactive Iodine Treatment for Hyperthyroidism: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2125072. [Google Scholar] [CrossRef]
- Pasqual, E.; Schonfeld, S.; Morton, L.M.; Villoing, D.; Lee, C.; Berrington de Gonzalez, A.; Kitahara, C.M. Association Between Radioactive Iodine Treatment for Pediatric and Young Adulthood Differentiated Thyroid Cancer and Risk of Second Primary Malignancies. J. Clin. Oncol. 2022, 40, 1439–1449. [Google Scholar] [CrossRef]
- Cebi Sen, C.; Yumusak, N.; Atilgan, H.I.; Sadic, M.; Koca, G.; Korkmaz, M. The protective effect of melatonin on sperm quality in rat after radioiodine treatment. Andrologia 2018, 50, e12962. [Google Scholar] [CrossRef]
- Barlas, A.M.; Sadic, M.; Atilgan, H.I.; Bag, Y.M.; Onalan, A.K.; Yumusak, N.; Senes, M.; Fidanci, V.; Pekcici, M.R.; Korkmaz, M.; et al. Melatonin: A hepatoprotective agent against radioiodine toxicity in rats. Bratisl. Med. J. 2017, 118, 95–100. [Google Scholar] [CrossRef]
- Alidadi, S.; Shabestani Monfared, A.; Amiri, M.; Zabihi, E.; Assadollahi, E.; Gholami, A.; Moazezi, Z.; Abedian, Z. The efficacy of melatonin against radiotoxicity of iodine-131 and its response to treatment in hyperthyroid patients: A randomized controlled trial. Nucl. Med. Rev. Cent. East. Eur. 2022, 25, 31–36. [Google Scholar] [CrossRef]
- IARC Monographs Vol 124 Group. Carcinogenicity of night shift work. Lancet Oncol. 2019, 20, 1058–1059. [Google Scholar] [CrossRef]
- Erren, T.C.; Morfeld, P.; Groß, J.V.; Wild, U.; Lewis, P. IARC 2019: “Night shift work” is probably carcinogenic: What about disturbed chronobiology in all walks of life? J. Occup. Med. Toxicol. 2019, 14, 29. [Google Scholar] [CrossRef]
- Papantoniou, K.; Konrad, P.; Haghayegh, S.; Strohmaier, S.; Eliassen, A.H.; Schernhammer, E. Rotating Night Shift Work, Sleep, and Thyroid Cancer Risk in the Nurses’ Health Study 2. Cancers 2023, 15, 5673. [Google Scholar] [CrossRef]
- Zhang, D.; Jones, R.R.; James, P.; Kitahara, C.M.; Xiao, Q. Associations between artificial light at night and risk for thyroid cancer: A large US cohort study. Cancer 2021, 127, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A.; Erren, T.C.; Piekarski, C.; Tamura, H.; Manchester, L.C. Light at night, chronodisruption, melatonin suppression, and cancer risk: A review. Crit. Rev. Oncog. 2007, 13, 303–328. [Google Scholar] [CrossRef]
- IARC Monographs Vol 123 Group. Carcinogenicity of some nitrobenzenes and other industrial chemicals. Lancet Oncol. 2018, 19, e681–e682. [Google Scholar] [CrossRef]
- Hsu, C.H.; Stedeford, T.; Okochi-Takada, E.; Ushijima, T.; Noguchi, H.; Muro-Cacho, C.; Holder, J.W.; Banasik, M. Framework analysis for the carcinogenic mode of action of nitrobenzene. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2007, 25, 155–184. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Integrated Risk Information System (IRIS) on Nitrobenzene; National Center for Environmental Assessment, Office of Research and Development: Washington, DC, USA, 1999.
- Cattley, R.C.; Everitt, J.I.; Gross, E.A.; Moss, O.R.; Hamm, T.E., Jr.; Popp, J.A. Carcinogenicity and toxicity of inhaled nitrobenzene in B6C3F1 mice and F344 and CD rats. Fundam. Appl. Toxicol. 1994, 22, 328–340. [Google Scholar] [CrossRef]
- Zasada, K.; Karbownik-Lewinska, M. Comparison of potential protective effects of melatonin and propylthiouracil against lipid peroxidation caused by nitrobenzene in the thyroid gland. Toxicol. Ind. Health 2015, 31, 1195–1201. [Google Scholar] [CrossRef]
- IARC. Some Chemicals that Cause Tumours of the Kidney or Urinary Bladder in Rodents and Some Other Substances. In Volume 73: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1999. [Google Scholar]
- Watanabe, S.; Togashi, S.; Fukui, T. Contribution of nitric oxide to potassium bromate-induced elevation of methaemoglobin concentration in mouse blood. Biol. Pharm. Bull. 2002, 25, 1315–1319. [Google Scholar] [CrossRef]
- Kakehashi, A.; Wei, M.; Fukushima, S.; Wanibuchi, H. Oxidative stress in the carcinogenicity of chemical carcinogens. Cancers 2013, 5, 1332–1354. [Google Scholar] [CrossRef]
- Karbownik, M.; Stasiak, M.; Zasada, K.; Zygmunt, A.; Lewinski, A. Comparison of potential protective effects of melatonin, indole-3-propionic acid, and propylthiouracil against lipid peroxidation caused by potassium bromate in the thyroid gland. J. Cell. Biochem. 2005, 95, 131–138. [Google Scholar] [CrossRef]
- World Health Organization. Preventing Disease through Healthy Environments: Exposure to Mercury: A Major Public Health Concern, 2nd ed. Available online: https://www.who.int/publications/i/item/9789240023567 (accessed on 10 January 2024).
- Skalny, A.V.; Aschner, M.; Sekacheva, M.I.; Santamaria, A.; Barbosa, F.; Ferrer, B.; Aaseth, J.; Paoliello, M.M.B.; Rocha, J.B.T.; Tinkov, A.A. Mercury and cancer: Where are we now after two decades of research? Food Chem. Toxicol. 2022, 164, 113001. [Google Scholar] [CrossRef]
- IARC. Beryllium, Cadmium, Mercury, and Exposures in the Glass Manufacturing Industry. In Volume 58: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1993. [Google Scholar]
- U.S. Environmental Protection Agency. Integrated Risk Information System (IRIS) on Mercury; National Center for Environmental Assessment, Office of Research and Development: Washington, DC, USA, 2000.
- Zhu, X.; Kusaka, Y.; Sato, K.; Zhang, Q. The endocrine disruptive effects of mercury. Environ. Health Prev. Med. 2000, 4, 174–183. [Google Scholar] [CrossRef]
- Pamphlett, R.; Doble, P.A.; Bishop, D.P. Mercury in the human thyroid gland: Potential implications for thyroid cancer, autoimmune thyroiditis, and hypothyroidism. PLoS ONE 2021, 16, e0246748. [Google Scholar] [CrossRef]
- Buonaurio, F.; Astolfi, M.L.; Pigini, D.; Tranfo, G.; Canepari, S.; Pietroiusti, A.; D’Alessandro, I.; Sisto, R. Oxidative Stress Biomarkers in Urine of Metal Carpentry Workers Can Be Diagnostic for Occupational Exposure to Low Level of Welding Fumes from Associated Metals. Cancers 2021, 13, 3167. [Google Scholar] [CrossRef]
- Webster, A.M.; Pinion, D.; Pineda, E.; Aboueisha, H.; Hussein, M.H.; Fawzy, M.S.; Toraih, E.A.; Kandil, E. Elucidating the link between thyroid cancer and mercury exposure: A review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2024, 31, 12841–12855. [Google Scholar] [CrossRef]
- Rao, M.V.; Chhunchha, B. Protective role of melatonin against the mercury induced oxidative stress in the rat thyroid. Food Chem. Toxicol. 2010, 48, 7–10. [Google Scholar] [CrossRef]
- Lisco, G.; De Tullio, A.; Triggiani, D.; Zupo, R.; Giagulli, V.A.; De Pergola, G.; Piazzolla, G.; Guastamacchia, E.; Sabbà, C.; Triggiani, V. Iodine Deficiency and Iodine Prophylaxis: An Overview and Update. Nutrients 2023, 15, 1004. [Google Scholar] [CrossRef]
- Poul, J.M.; Huet, S.; Godard, T.; Sanders, P. Lack of genotoxicity of potassium iodate in the alkaline comet assay and in the cytokinesis-block micronucleus test. Comparison to potassium bromate. Food Chem. Toxicol. 2004, 42, 203–209. [Google Scholar] [CrossRef]
- Milczarek, M.; Stępniak, J.; Lewiński, A.; Karbownik-Lewińska, M. Potassium iodide, but not potassium iodate, as a potential protective agent against oxidative damage to membrane lipids in porcine thyroid. Thyroid. Res. 2013, 6, 10. [Google Scholar] [CrossRef]
- Toyokuni, S.; Kong, Y.; Cheng, Z.; Sato, K.; Hayashi, S.; Ito, F.; Jiang, L.; Yanatori, I.; Okazaki, Y.; Akatsuka, S. Carcinogenesis as Side Effects of Iron and Oxygen Utilization: From the Unveiled Truth toward Ultimate Bioengineering. Cancers 2020, 12, 3320. [Google Scholar] [CrossRef]
- Toyokuni, S. Role of iron in carcinogenesis: Cancer as a ferrotoxic disease. Cancer Sci. 2009, 100, 9–16. [Google Scholar] [CrossRef]
- Fonseca-Nunes, A.; Jakszyn, P.; Agudo, A. Iron and cancer risk--a systematic review and meta-analysis of the epidemiological evidence. Cancer Epidemiol. Biomark. Prev. 2014, 23, 12–31. [Google Scholar] [CrossRef]
- Zoeller, R.T.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Vom Saal, F.S. Endocrine-disrupting chemicals and public health protection: A statement of principles from The Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef]
- Calsolaro, V.; Pasqualetti, G.; Niccolai, F.; Caraccio, N.; Monzani, F. Thyroid Disrupting Chemicals. Int. J. Mol. Sci. 2017, 18, 2583. [Google Scholar] [CrossRef]
- Gładysz, A.K.; Stępniak, J.; Karbownik-Lewińska, M. Exogenous Melatonin Protects against Oxidative Damage to Membrane Lipids Caused by Some Sodium/Iodide Symporter Inhibitors in the Thyroid. Antioxidants 2023, 12, 1688. [Google Scholar] [CrossRef]
- Sarkar, D.; Chandra, A.; Chattopadyay, S.; Biswas, M.; Das, S.; Singh, L.; Ray, I. Possible mechanism of bamboo shoots (Bambusa balcooa) induced thyroid disruption—An in vitro study. Hum. Exp. Toxicol. 2021, 40, 483–496. [Google Scholar] [CrossRef]
- Adedara, I.A.; Ojuade, T.J.D.; Olabiyi, B.F.; Idris, U.F.; Onibiyo, E.M.; Ajeigbe, O.F.; Farombi, E.O. Taurine Ameliorates Renal Oxidative Damage and Thyroid Dysfunction in Rats Chronically Exposed to Fluoride. Biol. Trace Elem. Res. 2017, 175, 388–395. [Google Scholar] [CrossRef]
- Akinrinde, A.S.; Tijani, M.; Awodele, O.A.; Oyagbemi, A.A. Fluoride-induced hepatotoxicity is prevented by L-Arginine supplementation via suppression of oxidative stress and stimulation of nitric oxide production in rats. Toxicol. Environ. Health Sci. 2021, 13, 57–64. [Google Scholar] [CrossRef]
- Kitahara, J.; Seko, Y.; Imura, N. Possible involvement of active oxygen species in selenite toxicity in isolated rat hepatocytes. Arch. Toxicol. 1993, 67, 497–501. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Ali, S.N.; Arif, A.; Ansari, F.A.; Mahmood, R. Cytoprotective effect of taurine against sodium chlorate-induced oxidative damage in human red blood cells: An ex vivo study. Amino Acids 2022, 54, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Sirasanagandla, S.R.; Al-Huseini, I.; Sakr, H.; Moqadass, M.; Das, S.; Juliana, N.; Abu, I.F. Natural Products in Mitigation of Bisphenol A Toxicity: Future Therapeutic Use. Molecules 2022, 27, 5384. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.G.; Correia, J.; Adiga, D.; Rai, P.S.; Dsouza, H.S.; Chakrabarty, S.; Kabekkodu, S.P. A comprehensive review on the carcinogenic potential of bisphenol A: Clues and evidence. Environ. Sci. Pollut. Res. Int. 2021, 28, 19643–19663. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.D.; Xavier, L.L.F.; Gonçalves, C.F.L.; Santos-Silva, A.P.; Paiva-Melo, F.D.; Freitas, M.L.; Fortunato, R.S.; Alves, L.M.; Ferreira, A.C.F. Bisphenol A increases hydrogen peroxide generation by thyrocytes both in vivo and in vitro. Endocr. Connect. 2018, 7, 1196–1207. [Google Scholar] [CrossRef] [PubMed]

| IARC Group | Agent/Dose | Species/Organ/ Tissue/Cellular Compartment | Effect of Carcinogen | Dose of Melatonin, Which Reduced or Prevented the Effect of Carcinogen | Refs. |
|---|---|---|---|---|---|
| 1 | Hexavalent chromium/potassium dichromate 25 mg/kg/day for 2 months | Adult male albino rats of Wistar strain | Follicular hyperplasia, follicular enlargement | 10 mg/kg/day for 2 months | [92] |
| 1 | Radiotherapy/ionizing radiation total dose of 16–18 Gy | Adult female rats | Increased inflammation, vacuolization, degradation, swelling, and necrosis in the thyroid gland | 10–50 mg/kg 10–15 min before exposure | [79,80] |
| 1 | Radioiodinetherapy /iodine-131 111 MBq | Adult rats | Oxidative stress parameters in the liver | 12 mg/kg/day | [101] |
| Radioiodinetherapy /iodine-131 370 to 740 MBq | Hyperthyroid patients | Chromosomal damage in lymphocyte | 300 mg | [102] | |
| 2A | Night-shift work | Humans | Indirect evidence on carcinogenesis | Not documented in the literature | - |
| 2B | Nitrobenzene 7.5–10.0 mM | Porcine thyroid homogenates | ↑MDA + 4-HDA in the thyroid | 0.0001 mM | [112] |
| 2B | Bromium/potassium bromate 110 mg/kg | Adult rats | ↑MDA + 4-HDA in the thyroid | 15 mg/kg twice daily for 10 days | [116] |
| 3 | Mercury/mercury chloride 2 and 4 mg/kg, orally | Adult male albino rats of Wistar strain | ↑MDA ↓SOD, ↓CAT, ↓GPx, ↓GR, ↓GSH | 5 mg/kg, ip | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stępniak, J.; Karbownik-Lewińska, M. Protective Effects of Melatonin against Carcinogen-Induced Oxidative Damage in the Thyroid. Cancers 2024, 16, 1646. https://doi.org/10.3390/cancers16091646
Stępniak J, Karbownik-Lewińska M. Protective Effects of Melatonin against Carcinogen-Induced Oxidative Damage in the Thyroid. Cancers. 2024; 16(9):1646. https://doi.org/10.3390/cancers16091646
Chicago/Turabian StyleStępniak, Jan, and Małgorzata Karbownik-Lewińska. 2024. "Protective Effects of Melatonin against Carcinogen-Induced Oxidative Damage in the Thyroid" Cancers 16, no. 9: 1646. https://doi.org/10.3390/cancers16091646
APA StyleStępniak, J., & Karbownik-Lewińska, M. (2024). Protective Effects of Melatonin against Carcinogen-Induced Oxidative Damage in the Thyroid. Cancers, 16(9), 1646. https://doi.org/10.3390/cancers16091646








