Patient-Reported Outcomes after Laser Ablation for Bladder Tumours Compared to Transurethral Resection—A Prospective Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
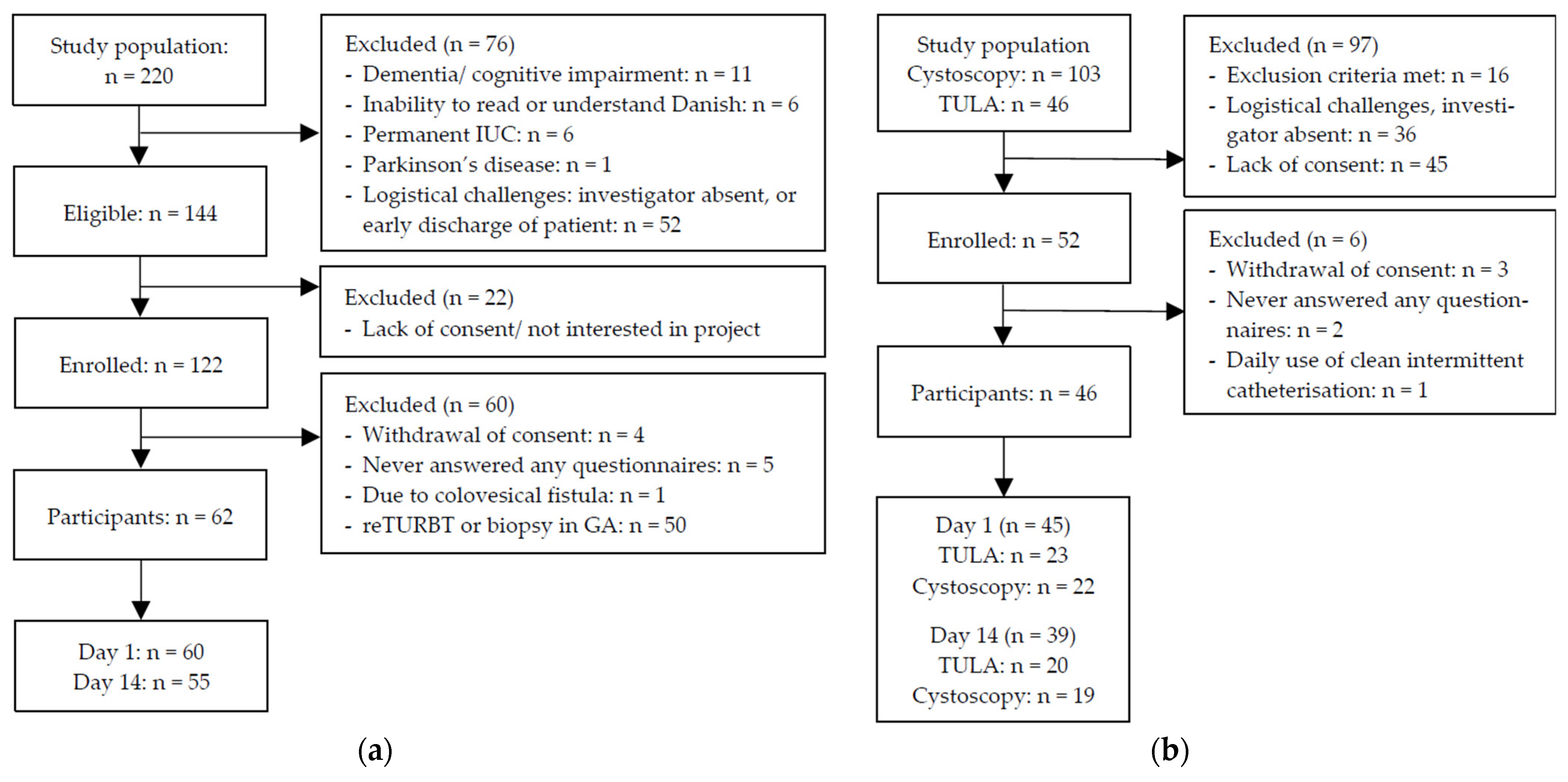
2.1. Patients
2.2. Procedures
2.3. Data Collection
2.4. Questionnaires
2.5. Demographic and Intraoperative Data
2.6. Statistical Analyses
2.7. Ethics
3. Results
3.1. Baseline Characteristics
3.2. Postoperative Outcome
3.2.1. PROTO
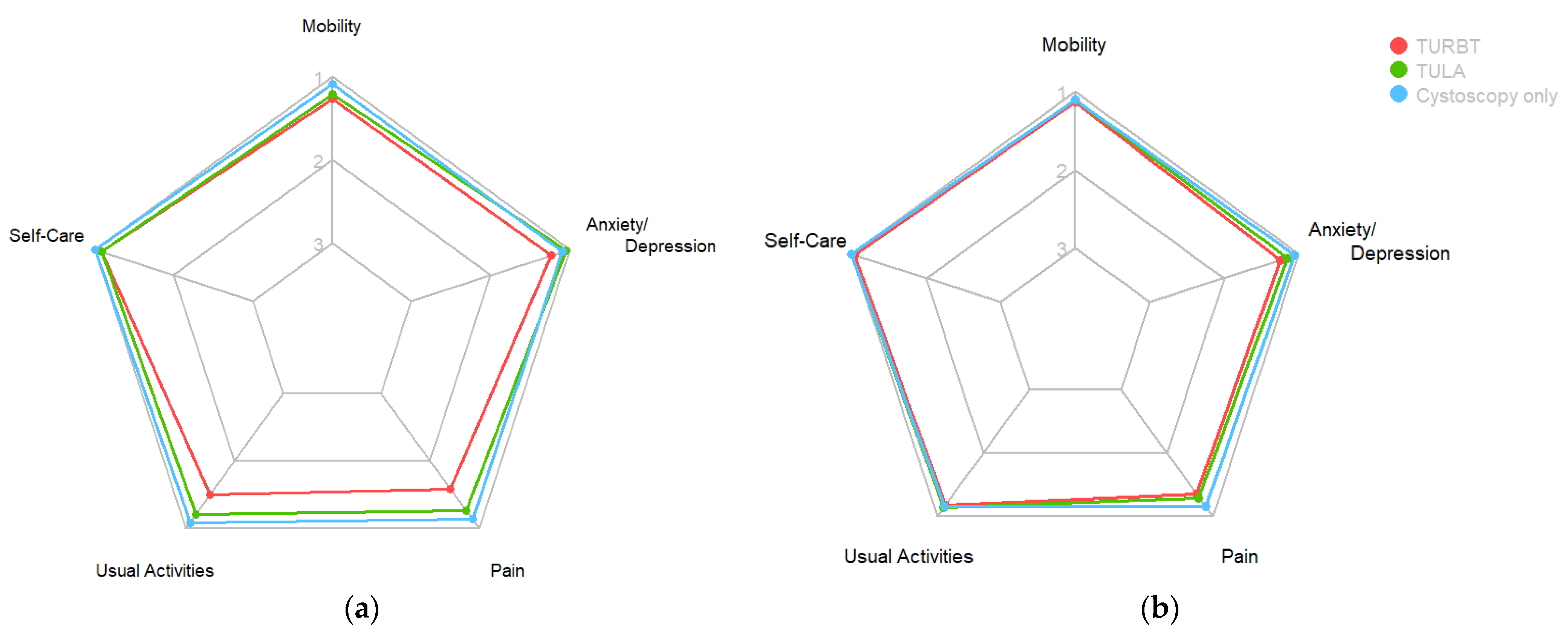
3.2.2. EQ-5D-3L and ICIQ-LUTS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.E.M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Denmark. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/208-denmark-fact-sheet.pdf (accessed on 1 March 2024).
- Ferlay, J.E.M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Bladder. Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/30-bladder-fact-sheet.pdf (accessed on 1 March 2024).
- Burger, M.; Catto, J.W.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Danish Bladder Cancer Group (DaBlaCa). National Clinical Guidelines: Diagnosing of Bladder Tumours—Pathology, Histology and Diagnostics. Available online: https://www.dmcg.dk/Kliniske-retningslinjer/kliniske-retningslinjer-opdelt-paa-dmcg/cancer-i-urinvejene/blaerecancer/udredning-af-blaretumorer---patologi-histologi-og-diagnostik/ (accessed on 23 April 2024).
- Collado, A.; Chéchile, G.E.; Salvador, J.; Vicente, J. Early complications of endoscopic treatment for superficial bladder tumors. J. Urol. 2000, 164, 1529–1532. [Google Scholar] [CrossRef] [PubMed]
- Avallone, M.A.; Sack, B.S.; El-Arabi, A.; Charles, D.K.; Herre, W.R.; Radtke, A.C.; Davis, C.M.; See, W.A. Ten-Year Review of Perioperative Complications after Transurethral Resection of Bladder Tumors: Analysis of Monopolar and Plasmakinetic Bipolar Cases. J. Endourol. 2017, 31, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Gregg, J.R.; McCormick, B.; Wang, L.; Cohen, P.; Sun, D.; Penson, D.F.; Smith, J.A.; Clark, P.E.; Cookson, M.S.; Barocas, D.A.; et al. Short term complications from transurethral resection of bladder tumor. Can. J. Urol. 2016, 23, 8198–8203. [Google Scholar] [PubMed]
- De Nunzio, C.; Franco, G.; Cindolo, L.; Autorino, R.; Cicione, A.; Perdonà, S.; Falsaperla, M.; Gacci, M.; Leonardo, C.; Damiano, R.; et al. Transuretral resection of the bladder (TURB): Analysis of complications using a modified Clavien system in an Italian real life cohort. Eur. J. Surg. Oncol. 2014, 40, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, K.; Christensen, K.B.; Vrang, M.L.; Hermann, G.G. Hospitalization for transurethral bladder resection reduces quality of life in Danish patients with non-muscle-invasive bladder tumour. Scand. J. Urol. 2016, 50, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, R.J.; van der Meijden, A.P.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.; Kurth, K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 2006, 49, 466–477. [Google Scholar] [CrossRef]
- European Association of Urology. EAU Guidelines. Edn. Presented at the EAU Annual Congress Milan. 2023. Available online: https://uroweb.org/guidelines/non-muscle-invasive-bladder-cancer (accessed on 10 August 2023).
- Wong, K.A.; Zisengwe, G.; Athanasiou, T.; O’Brien, T.; Thomas, K. Outpatient laser ablation of non-muscle-invasive bladder cancer: Is it safe, tolerable and cost-effective? BJU Int. 2013, 112, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Hermann, G.G.; Mogensen, K.; Rosthøj, S. Outpatient diode laser treatment of intermediate-risk non-invasive bladder tumors without sedation: Efficacy, safety and economic analysis. Scand. J. Urol. 2018, 52, 194–198. [Google Scholar] [CrossRef]
- Grover, S.; Raj, S.; Russell, B.; Mensah, E.; Nair, R.; Thurairaja, R.; Khan, M.S.; Thomas, K.; Malde, S. Long-term outcomes of outpatient laser ablation for recurrent non-muscle invasive bladder cancer: A retrospective cohort study. BJUI Compass 2022, 3, 124–129. [Google Scholar] [CrossRef] [PubMed]
- International Consultation on Incontinence Questionnaire Group—Bristol Urological Institute. International Consultation on Incontinence Questionnaire Male Lower Urinary Tract Symptoms Module (ICIQ-MLUTS). Available online: https://iciq.net/iciq-mluts (accessed on 8 August 2023).
- International Consultation on Incontinence Questionnaire Group—Bristol Urological Institute. International Consultation on Incontinence Questionnaire Female Lower Urinary Tract Symptoms Modules (ICIQ-FLUTS). Available online: https://iciq.net/iciq-fluts (accessed on 8 August 2023).
- EuroQol Group. EQ-5D-3L|About. Available online: https://euroqol.org/eq-5d-instruments/eq-5d-3l-about/ (accessed on 8 August 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Pereira, J.F.; Pareek, G.; Mueller-Leonhard, C.; Zhang, Z.; Amin, A.; Mega, A.; Tucci, C.; Golijanin, D.; Gershman, B. The Perioperative Morbidity of Transurethral Resection of Bladder Tumor: Implications for Quality Improvement. Urology 2019, 125, 131–137. [Google Scholar] [CrossRef]
- Ghali, F.; Moses, R.A.; Raffin, E.; Hyams, E.S. What factors are associated with unplanned return following transurethral resection of bladder tumor? An analysis of a large single institution’s experience. Scand. J. Urol. 2016, 50, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Malde, S.; Grover, S.; Raj, S.; Yuan, C.; Nair, R.; Thurairaja, R.; Khan, M.S. A Systematic Review of the Efficacy and Safety of Outpatient Bladder Tumour Ablation. Eur. Urol. Focus. 2022, 8, 141–151. [Google Scholar] [CrossRef]
- Darrad, M.; Jah, S.; Ahmed, Z.; Syed, H. Long-Term Prospective Outcomes of Patients with Non-Muscle Invasive Bladder Cancer After Holmium Laser Ablation. J. Endourol. 2019, 33, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Planelles Gómez, J.; Olmos Sánchez, L.; Cardosa Benet, J.J.; Martínez López, E.; Vidal Moreno, J.F. Holmium YAG Photocoagulation: Safe and Economical Alternative to Transurethral Resection in Small Nonmuscle-Invasive Bladder Tumors. J. Endourol. 2017, 31, 674–678. [Google Scholar] [CrossRef]
- Lonati, C.; Esperto, F.; Scarpa, R.M.; Papalia, R.; Gómez Rivas, J.; Alvarez-Maestro, M.; Afferi, L.; Fankhauser, C.D.; Mattei, A.; Colombo, R.; et al. Bladder perforation during transurethral resection of the bladder: A comprehensive algorithm for diagnosis, management and follow-up. Minerva Urol. Nephrol. 2022, 74, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Beer, M.; Jocham, D.; Beer, A.; Staehler, G. Adjuvant laser treatment of bladder cancer: 8 years’ experience with the Nd-YAG laser 1064 nm. Br. J. Urol. 1989, 63, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, G.L.; Erikson, M.S.; Mogensen, K.; Rosthøj, S.; Hermann, G.G. Outpatient Photodynamic Diagnosis-guided Laser Destruction of Bladder Tumors Is as Good as Conventional Inpatient Photodynamic Diagnosis-guided Transurethral Tumor Resection in Patients with Recurrent Intermediate-risk Low-grade Ta Bladder Tumors. A Prospective Randomized Noninferiority Clinical Trial. Eur. Urol. 2022, 83, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Jønler, M.; Lund, L.; Bisballe, S. Holmium: YAG laser vaporization of recurrent papillary tumours of the bladder under local anaesthesia. BJU Int. 2004, 94, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Sievert, K.D.; Amend, B.; Nagele, U.; Schilling, D.; Bedke, J.; Horstmann, M.; Hennenlotter, J.; Kruck, S.; Stenzl, A. Economic aspects of bladder cancer: What are the benefits and costs? World J. Urol. 2009, 27, 295–300. [Google Scholar] [CrossRef] [PubMed]


| Overall N = 108 | TURBT N = 62 | TULA N = 23 | Cystoscopy Only N = 23 | |
|---|---|---|---|---|
| Male sex, n (%) | 94 (87%) | 51 (82%) | 22 (96%) | 21 (91%) |
| Median age (IQR) | 74.0 (67.0, 78.0) | 70.5 (64.2, 75.8) | 77.0 (70.5, 80.5) | 76.0 (72.0, 81.5) |
| Median BMI (IQR) | 27.0 (25.0, 29.0) | 27.0 (25.0, 29.0) | 27.5 (24.2, 28.8) | 26.0 (23.0, 29.5) |
| ASA Classification | ||||
| 1 | 22 (20%) | 10 (16%) | 5 (22%) | 7 (30%) |
| 2 | 55 (51%) | 35 (56%) | 9 (39%) | 11 (48%) |
| 3 | 28 (26%) | 17 (27%) | 7 (30%) | 4 (17%) |
| 4 | 3 (2.8%) | 0 (0%) | 2 (8.7%) | 1 (4.3%) |
| Diabetic | 21 (19%) | 13 (21%) | 4 (17%) | 4 (17%) |
| In anticoagulant treatment | 51 (47%) | 27 (44%) | 12 (52%) | 12 (52%) |
| Previous bladder cancer diagnosis | 55 (51%) | 14 (23%) | 22 (96%) | 19 (83%) |
| Tumour appearance | ||||
| No tumour tissue | 17 (16%) | 1 (1.6%) | 0 (0%) | 16 (70%) |
| Papillary | 65 (60%) | 41 (66%) | 19 (83%) | 5 (22%) |
| Sessile | 5 (4.6%) | 1 (1.6%) | 3 (13%) | 1 (4.3%) |
| Solid | 16 (15%) | 16 (26%) | 0 (0%) | 0 (0%) |
| Unspecified | 5 (4.6%) | 3 (4.8%) | 1 (4.3%) | 1 (4.3%) |
| Tumour number | ||||
| Single | 61 (67%) | 44 (72%) | 14 (61%) | 3 (43%) |
| Multiple | 30 (33%) | 17 (28%) | 9 (39%) | 4 (57%) |
| Size of largest tumour | ||||
| ≤1 cm | 41 (45%) | 22 (36%) | 19 (83%) | 0 (0%) |
| 2–3 cm | 26 (29%) | 22 (36%) | 2 (8.7%) | 2 (29%) |
| ≥4 cm | 12 (13%) | 12 (20%) | 0 (0%) | 0 (0%) |
| Unspecified | 12 (13%) | 5 (8.2%) | 2 (8.7%) | 5 (71%) |
| Tumour stage | ||||
| T2-4 | 9 (8.3%) | 9 (15%) | 0 (0%) | |
| T1 (a + b) | 11 (10%) | 11 (18%) | 0 (0%) | |
| Ta | 49 (45%) | 31 (50%) | 18 (78%) | |
| CIS | 6 (5.6%) | 5 (8.1%) | 1 (4.3%) | |
| T0 | 23 (21%) | 0 (0%) | 0 (0%) | |
| Tumour grade | ||||
| High | 29 (27%) | 26 (42%) | 3 (13%) | |
| Low | 42 (39%) | 28 (45%) | 14 (61%) | |
| Papilloma | 1 (0.9%) | 1 (1.6%) | 0 (0%) |
| DAY 1 (during the First 24 h) | DAY 14 (during the First Two Weeks) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Overall N = 105 | TURBT N = 60 | TULA N = 23 | Cystoscopy Only, N = 22 | p 1 | Overall N = 94 | TURBT N = 55 | TULA N = 20 | Cystoscopy Only, N = 19 | p 1 |
| Postoperative haematuria | 55 (52%) | 48 (80%) | 4 (17%) | 3 (14%) | <0.001 | 51 (54%) | 42 (76%) | 7 (35%) | 2 (11%) | <0.001 |
| >2 days | 27 (29%) | 21 (38%) | 6 (30%) | 0 (0%) | ||||||
| ≥8 days | 13 (14%) | 12 (22%) | 1 (5%) | 0 (0%) | ||||||
| AUR | 7 (6.7%) | 7 (11.7%) | 0 (0%) | 0 (0%) | 2 (2%) | 2 (3.6%) | 0 (0%) | 0 (0%) | ||
| Catheter when leaving hospital | 38 (36%) | 36 (60%) | 1 (4.3%) | 1 (4.5%) | <0.001 | |||||
| Subsequent catheter | 8 (8.5%) | 7 (13%) | 1 (5%) | 0 (0%) | 0.3 | |||||
| Pain | 58 (55%) | 43 (72%) | 11 (48%) | 4 (18%) | <0.001 | 44 (47%) | 34 (63%) | 7 (35%) | 3 (16%) | <0.001 |
| Mean NRS score (SD) | 3.12 (2.99) | 4.42 (2.99) | 1.91 (1.86) | 0.71 (1.74) | <0.001 | 1.57 (2.18) | 2.20 (2.41) | 0.75 (1.02) | 0.56 (1.69) | <0.001 |
| Use of painkillers | 55 (52%) | 48 (80%) | 6 (26%) | 1 (4.5%) | <0.001 | 26 (28%) | 22 (41%) | 2 (10%) | 2 (11%) | 0.005 |
| UTI | 8 (9%) | 5 (9%) | 1 (5%) | 2 (11%) | 0.9 | |||||
| Contact with healthcare system | 22 (21%) | 22 (37%) | 0 (0%) | 0 (0%) | <0.001 | 28 (30%) | 27 (50%) | 1 (5%) | 0 (0%) | <0.001 |
| DAY 1 (during the First 24 h) | ||||||
|---|---|---|---|---|---|---|
| Outcome | Overall N Mdn (IQR) | TURBT N Mdn (IQR) | TULA N Mdn (IQR) | Cystoscopy Only N Mdn (IQR) | p 1 | Scoring Range ** |
| EQ-5D-3L HRQoL | 105 6 (5, 7) | 60 6 (6, 7.2) | 23 5 (5, 6.5) | 22 5 (5, 5) | <0.001 | [5–15] |
| ICIQ M-LUTS * | ||||||
| Voiding | 55 7 (5, 8.5) | 16 8 (6, 9) | 20 7 (4.75, 10) | 19 5 (4, 6.5) | 0.011 | [0–20] |
| Incontinence | 56 3 (1, 5) | 16 4 (3, 6) | 21 2 (1, 5) | 19 2 (0.5, 3.5) | 0.019 | [0–24] |
| Frequency | 57 1 (0, 2) | 17 1 (0, 2) | 21 1 (0, 2) | 19 0 (0, 1) | 0.13 | [0–4] |
| Nocturia | 56 1.5 (1, 3) | 17 2 (1, 3) | 20 2 (1, 3) | 19 1 (1, 2) | 0.7 | [0–4] |
| ICIQ F-LUTS * | ||||||
| Filling | 10 7 (3.75, 9) | 7 7 (6.50, 9) | 1 1 (1, 1) | 2 6.5 (4.75, 8.25) | 0.4 | [0–16] |
| Voiding | 11 0 (0, 1) | 8 0.5 (0, 1.25) | 1 0 (0, 0) | 2 0.50 (0.25, 0.75) | 0.7 | [0–12] |
| Incontinence | 10 4 (1, 7) | 7 3 (1, 7) | 1 5 (5, 5) | 2 6 (3.50, 8.50) | 0.9 | [0–20] |
| DAY 14 (during the first two weeks) | ||||||
| Outcome | Overall N Mdn (IQR) | TURBT N Mdn (IQR) | TULA N Mdn (IQR) | Cystoscopy only N Mdn (IQR) | p 1 | Scoring range ** |
| EQ-5D-3L HRQoL | 93 5 (5, 6) | 55 5 (5, 6) | 20 5 (5, 6) | 18 5 (5, 6) | 0.5 | [0–15] |
| ICIQ M-LUTS * | ||||||
| Voiding | 74 7 (5, 10) | 41 8 (6, 11) | 17 7 (5, 9) | 16 7 (4.8, 8) | 0.2 | [0–20] |
| Incontinence | 74 3 (2, 5) | 41 4 (2, 5) | 18 3 (2, 3.75) | 15 2 (1.5, 4.5) | 0.3 | [0–24] |
| Frequency | 78 1 (0, 2) | 43 2 (1, 3) | 18 1 (0, 2) | 17 0 (0, 1) | <0.001 | [0–4] |
| Nocturia | 78 2 (1, 3) | 43 2 (1.5, 3) | 18 1.5 (1, 2) | 17 1 (1, 2) | 0.042 | [0–4] |
| ICIQ F-LUTS * | ||||||
| Filling | 13 5 (3, 7) | 10 5 (3.2, 7) | 1 1 (1, 1) | 2 7.5 (5.2, 9.8) | 0.3 | [0–16] |
| Voiding | 13 1 (0, 1) | 10 0.5 (0, 1) | 1 0 (0, 0) | 2 2 (1.5, 2.5) | 0.2 | [0–12] |
| Incontinence | 13 1 (0, 4) | 10 0 (0, 3.25) | 1 1 (1, 1) | 27.5 (5.25, 9.75) | 0.2 | [0–20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deacon, N.N.; Nielsen, N.K.; Jensen, J.B. Patient-Reported Outcomes after Laser Ablation for Bladder Tumours Compared to Transurethral Resection—A Prospective Study. Cancers 2024, 16, 1630. https://doi.org/10.3390/cancers16091630
Deacon NN, Nielsen NK, Jensen JB. Patient-Reported Outcomes after Laser Ablation for Bladder Tumours Compared to Transurethral Resection—A Prospective Study. Cancers. 2024; 16(9):1630. https://doi.org/10.3390/cancers16091630
Chicago/Turabian StyleDeacon, Nina Nordtorp, Ninna Kjær Nielsen, and Jørgen Bjerggaard Jensen. 2024. "Patient-Reported Outcomes after Laser Ablation for Bladder Tumours Compared to Transurethral Resection—A Prospective Study" Cancers 16, no. 9: 1630. https://doi.org/10.3390/cancers16091630
APA StyleDeacon, N. N., Nielsen, N. K., & Jensen, J. B. (2024). Patient-Reported Outcomes after Laser Ablation for Bladder Tumours Compared to Transurethral Resection—A Prospective Study. Cancers, 16(9), 1630. https://doi.org/10.3390/cancers16091630








