Laparoscopic Treatment of Bulky Nodes in Primary and Recurrent Ovarian Cancer: Surgical Technique and Outcomes from Two Specialized Italian Centers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
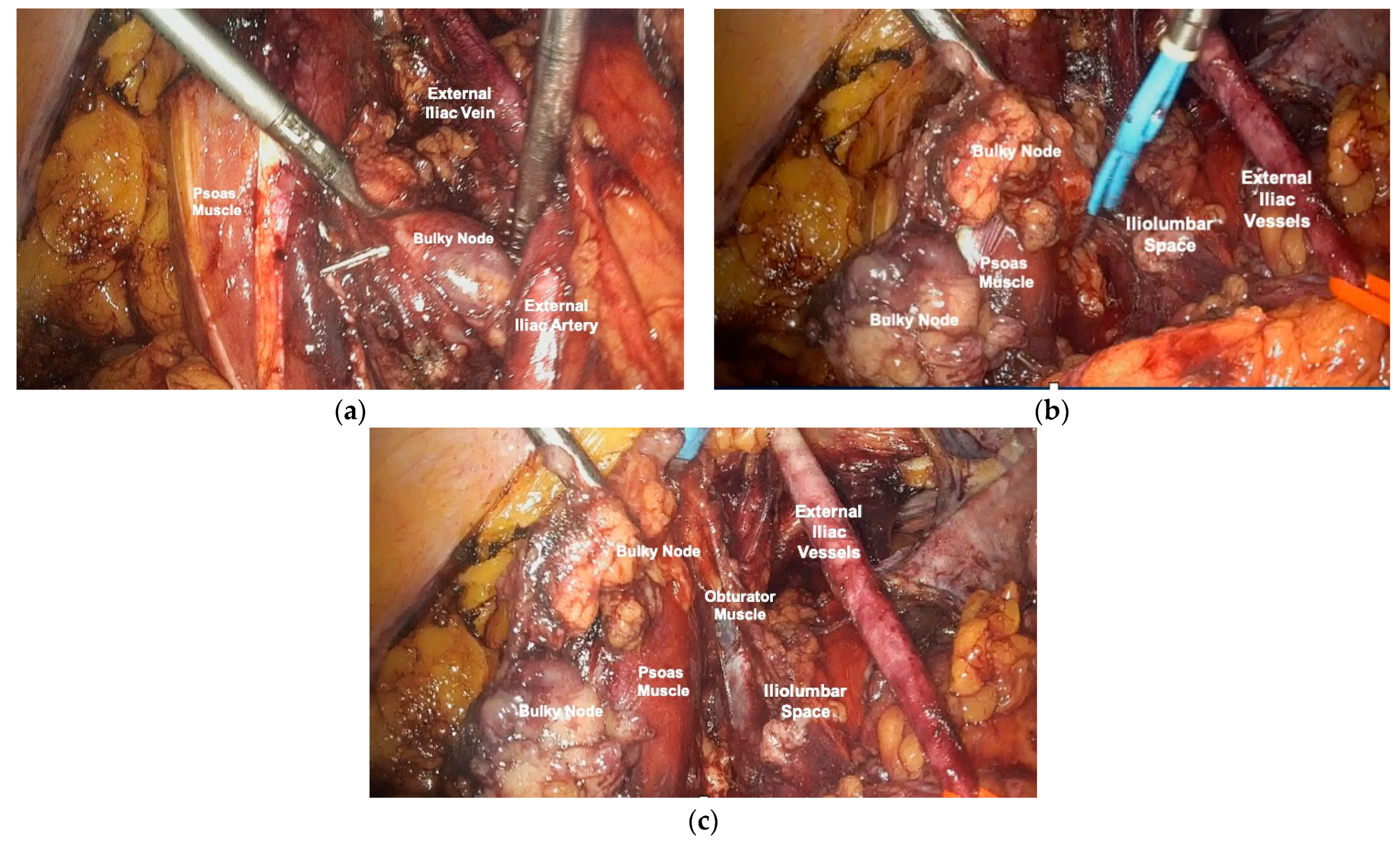
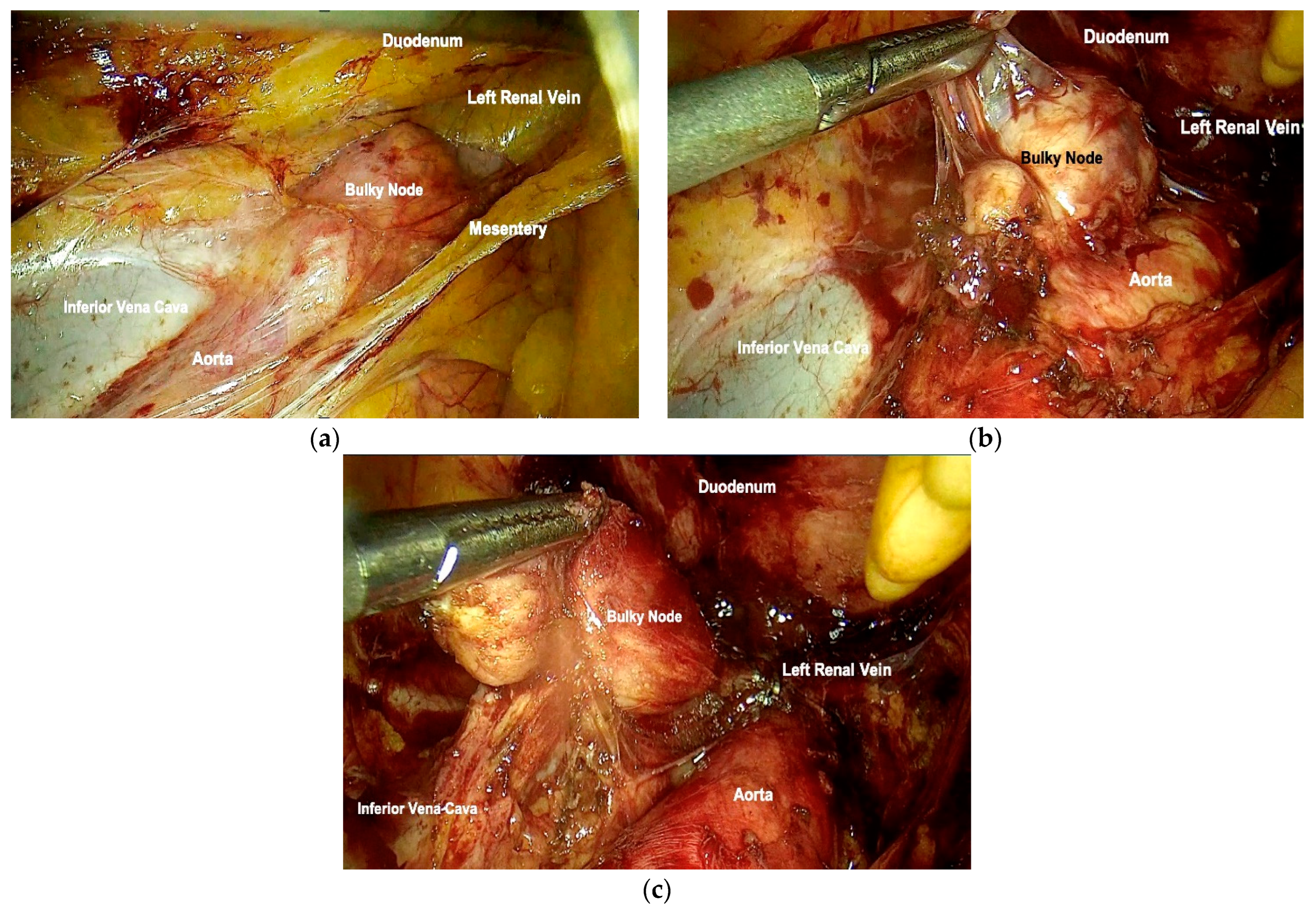
2.3. Surgical Technique
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Soerjomataram, I.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ceccaroni, M.; Roviglione, G.; Bruni, F.; Clarizia, R.; Ruffo, G.; Salgarello, M.; Peiretti, M.; Uccella, S. Laparoscopy for primary cytoreduction with multivisceral resections in advanced ovarian cancer: Prospective validation. “The times they are a-changin”? Surg. Endosc. 2018, 32, 2026–2037. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Jiang, Q.; Luo, X.; Chen, M.; Yuan, L.; Yao, L. Cytoreductive surgery is feasible in patients with limited regional platinum-resistant recurrent ovarian cancer. World J. Surg. Oncol. 2023, 21, 375. [Google Scholar] [CrossRef] [PubMed]
- du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 2009, 115, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, A.; Ditto, A.; Giorda, G.; Gadducci, A.; Greggi, S.; Daniele, A.; Fuso, L.; Panuccio, E.; Scaffa, C.; Raspagliesi, F.; et al. Secondary cytoreductive surgery for isolated lymph node recurrence of epithelial ovarian cancer: A multicenter study. Eur. J. Surg. Oncol. 2014, 40, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Cosio, S.; Zola, P.; Sostegni, B.; Fuso, L.; Sartori, E. Prognostic factors and clinical outcome of patients with recurrent early-stage epithelial ovarian cancer: An Italian multicenter retrospective study. Int. J. Gynecol. Cancer 2013, 23, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Gueli Alletti, S.; Capozzi, V.A.; Rosati, A.; De Blasis, I.; Cianci, S.; Vizzielli, G.; Uccella, S.; Gallotta, V.; Fanfani, F.; Fagotti, A.; et al. Laparoscopy vs. laparotomy for advanced ovarian cancer: A systematic review of the literature. Minerva Med. 2019, 110, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Goicoechea, J.; Wang, Y.; McGorray, S.; Saleem, M.D.; Carbajal Mamani, S.L.; Pomputius, A.F.; Markham, M.-J.; Castagno, J.C. Minimally invasive interval cytoreductive surgery in ovarian cancer: Systematic review and meta-analysis. J. Robot. Surg. 2019, 13, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Chi, D.S.; Abu-Rustum, N.R.; Sonoda, Y.; Ivy, J.; Rhee, E.; Moore, K.; Levine, D.A.; Barakat, R.R. The safety and efficacy of laparoscopic surgical staging of apparent stage I ovarian and fallopian tube cancers. Am. J. Obstet. Gynecol. 2005, 192, 1614–1619. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, D.W.; Yim, G.W.; Nam, E.J.; Kim, S.; Kim, Y.T. Staging laparoscopy for the management of early-stage ovarian cancer: A metaanalysis. Am. J. Obstet. Gynecol. 2013, 209, 58.e1–58.e8. [Google Scholar] [CrossRef]
- Falcetta, F.S.; Lawrie, T.A.; Medeiros, L.R.; da Rosa, M.I.; Edelweiss, M.I.; Stein, A.T.; Zelmanowicz, A.; Moraes, A.B.; Zanini, R.R.; Rosa, D.D. Laparoscopy versus laparotomy for FIGO stage I ovarian cancer. Cochrane Database Syst. Rev. 2016, 10, CD005344. [Google Scholar] [CrossRef] [PubMed]
- Brockbank, E.C.; Harry, V.; Kolomainen, D.; Mukhopadhyay, D.; Sohaib, A.; Bridges, J.E.; Nobbenhuis, M.a.E.; Shepherd, J.H.; Ind, T.E.J.; Barton, D.P.J. Laparoscopic staging for apparent early stage ovarian or fallopian tube cancer. First case series from a UK cancer centre and systematic literature review. Eur. J. Surg. Oncol. 2013, 39, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Borghi, C.; Leone Roberti Maggiore, U.; Ditto, A.; Signorelli, M.; Martinelli, F.; Chiappa, V.; Lopez, C.; Sabatucci, I.; Scaffa, C.; et al. Minimally Invasive Surgical Staging in Early-stage Ovarian Carcinoma: A Systematic Review and Meta-analysis. J. Minim. Invasive Gynecol. 2017, 24, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Macerox, N.; Pfiffer, T.; Köhler, C.; da Costa Miranda, V.; Estevez Diz, M.D.P.; Fukushima, J.T.; Baracat, E.C.; Carvalho, J.P. Oncologic Concerns regarding Laparoscopic Cytoreductive Surgery in Patients with Advanced Ovarian Cancer Submitted to Neoadjuvant Chemotherapy. Oncology 2015, 89, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Gueli Alletti, S.; Petrillo, M.; Vizzielli, G.; Bottoni, C.; Nardelli, F.; Costantini, B.; Quagliozzi, L.; Gallotta, V.; Scambia, G.; Fagotti, A. Minimally invasive versus standard laparotomic interval debulking surgery in ovarian neoplasm: A single-institution retrospective case-control study. Gynecol. Oncol. 2016, 143, 516–520. [Google Scholar] [CrossRef]
- Gallotta, V.; Ghezzi, F.; Vizza, E.; Fagotti, A.; Ceccaroni, M.; Fanfani, F.; Chiantera, V.; Ercoli, A.; Rossitto, C.; Conte, C.; et al. Laparoscopic Management of Ovarian Cancer Patients with Localized Carcinomatosis and Lymph Node Metastases: Results of a Retrospective Multi-institutional Series. J. Minim. Invasive Gynecol. 2016, 23, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Radosa, J.C.; Radosa, M.P.; Schweitzer, P.A.; Juhasz-Boess, I.; Rimbach, S.; Solomayer, E.-F. Report of the survey on current opinions and practice of German Society for Gynecologic Endoscopy (AGE) members regarding the laparoscopic treatment of ovarian malignancies. Arch. Gynecol. Obstet. 2018, 297, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Vito Andrea, C.; Uccella, S.; Sozzi, G.; Ceccaroni, M.; Mautone, D.; Armano, G.; Franchi, M.; Chiantera, V.; Berretta, R. Primary site disease and recurrence location in ovarian cancer patients undergoing primary debulking surgery vs. interval debulking surgery. Eur. J. Surg. Oncol. 2021, 47, 1075–1082. [Google Scholar] [CrossRef]
- Magrina, J.F.; Cetta, R.L.; Chang, Y.-H.; Guevara, G.; Magtibay, P.M. Analysis of secondary cytoreduction for recurrent ovarian cancer by robotics, laparoscopy and laparotomy. Gynecol. Oncol. 2013, 129, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Gallotta, V.; Conte, C.; Giudice, M.T.; Nero, C.; Vizzielli, G.; Gueli Alletti, S.; Cianci, S.; Lodoli, C.; Di Giorgio, A.; De Rose, A.M.; et al. Secondary Laparoscopic Cytoreduction in Recurrent Ovarian Cancer: A Large, Single-Institution Experience. J. Minim. Invasive Gynecol. 2018, 25, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; Alvarez, R.D.; Backes, F.J.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.M.; Chitiyo, V.C.; Cristea, M.; et al. NCCN Guidelines® Insights: Ovarian Cancer, Version 3.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Rao, T.; Raju, K.; Iyer, R.R.; Ahmed, S.M.; Shah, M.; Nagaraju, R. Outcomes of Laparoscopic Optimal Interval Cytoreduction Surgery (LOICS) in Patients with Advanced Ovarian Cancers Having Low Burden Disease. Indian J. Surg. Oncol. 2023, 14, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Marchetti, C.; Loverro, M.; Giudice, M.T.; Rosati, A.; Gallotta, V.; Scambia, G.; Fagotti, A. Role of minimally invasive secondary cytoreduction in patients with recurrent ovarian cancer. Int. J. Gynecol. Cancer 2023, 33, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Gao, W.; Shi, P.; Xi, M.; Tang, W.; Zhang, J. Primary Laparoscopic Surgery Does Not Affect the Prognosis of Early-Stage Ovarian Clear Cell Cancer. Cancer Manag. Res. 2021, 13, 6403–6409. [Google Scholar] [CrossRef]
- Melamed, A.; Nitecki, R.; Boruta, D.M.; Del Carmen, M.G.; Clark, R.M.; Growdon, W.B.; Goodman, A.; Schorge, J.O.; Rauh-Hain, J.A. Laparoscopy Compared with Laparotomy for Debulking Ovarian Cancer after Neoadjuvant Chemotherapy. Obstet. Gynecol. 2017, 129, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Nitecki, R.; Rauh-Hain, J.A.; Melamed, A.; Pareja, R.; Sinno, A.; Mcnally, L.; Horowitz, N.; Iaco, P.D.; Michener, C.; May, T.; et al. Laparoscopic cytoreduction after neoadjuvant chemotherapy (LANCE): Feasibility phase of a randomized trial. Int. J. Gynecol. Cancer 2023, 30, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, F.R.; DeNoble, S.M.; Liu, C.S.; Cho, J.E.; Brown, D.N.; Chuang, L.; Gretz, H.; Saharia, P. The safety and efficacy of laparoscopic surgical staging and debulking of apparent advanced stage ovarian, fallopian tube, and primary peritoneal cancers. JSLS J. Soc. Laparoendosc. Surg. 2010, 14, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Fanning, J.; Yacoub, E.; Hojat, R. Laparoscopic-assisted cytoreduction for primary advanced ovarian cancer: Success, morbidity and survival. Gynecol. Oncol. 2011, 123, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Knisely, A.; Gamble, C.R.; St Clair, C.M.; Hou, J.Y.; Khoury-Collado, F.; Gockley, A.A.; Wright, J.D.; Melamed, A. The Role of Minimally Invasive Surgery in the Care of Women with Ovarian Cancer: A Systematic Review and Meta-analysis. J. Minim. Invasive Gynecol. 2021, 28, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.R.; Beyer, J.; Griffin, S.; Kanjanavaikoon, P. Extraperitoneal metastases from recurrent ovarian cancer. Int. J. Gynecol. Cancer 2012, 22, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, P.; Pagès, C.; Afchain, P.; Louvet, C.; Tournigand, C.; de Gramont, A. Isolated lymph node relapse of epithelial ovarian carcinoma: Outcomes and prognostic factors. Gynecol. Oncol. 2007, 104, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Debnath, S.; Sharma, A.; Kamboj, M.; Mohanty, A.; Rawal, S. Isolated lymph node recurrence in epithelial ovarian cancer—Management and outcome. J. Visc. Surg. 2023, 160, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Sehouli, J.; Vergote, I.; Ferron, G.; Reuss, A.; Meier, W.; Greggi, S.; Mosgaard, B.J.; Selle, F.; Guyon, F.; et al. Randomized Trial of Cytoreductive Surgery for Relapsed Ovarian Cancer. N. Engl. J. Med. 2021, 385, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Tinelli, R.; Dellino, M.; Nappi, L.; Sorrentino, F.; D’Alterio, M.N.; Angioni, S.; Bogani, G.; Pisconti, S.; Silvestris, E. Eradication of Isolated Para-Aortic Nodal Recurrence in a Patient with an Advanced High Grade Serous Ovarian Carcinoma: Our Experience and Review of Literature. Medicina 2022, 58, 244. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Costantini, B.; Gallotta, V.; Cianci, S.; Ronsini, C.; Petrillo, M.; Pacciani, M.; Scambia, G.; Fanfani, F. Minimally invasive secondary cytoreduction plus HIPEC versus open surgery plus HIPEC in isolated relapse from ovarian cancer: A retrospective cohort study on perioperative outcomes. J. Minim. Invasive Gynecol. 2015, 22, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, A.G.Z.; Graul, A.; Yu, M.C.; Halko, A.; Chi, D.S.; Zivanovic, O.; Gardner, G.J.; Sonoda, Y.; Barakat, R.R.; Abu-Rustum, N.R.; et al. Minimal access surgery compared to laparotomy for secondary surgical cytoreduction in patients with recurrent ovarian carcinoma: Perioperative and oncologic outcomes. Gynecol. Oncol. 2017, 146, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Gallotta, V.; Giudice, M.T.; Conte, C.; Sarandeses, A.V.; D’Indinosante, M.; Federico, A.; Tortorella, L.; Carbone, M.V.; Gueli Alletti, S.; Vizzielli, G.; et al. Minimally invasive salvage lymphadenectomy in gynecological cancer patients: A single institution series. Eur. J. Surg. Oncol. 2018, 44, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Choi, J.S.; Lee, J.H.; Bae, J.W.; Eom, J.M.; Kim, J.T.; Oh, S. Laparoscopic Lymphadenectomy for Isolated Lymph Node Recurrence in Gynecologic Malignancies. J. Minim. Invasive Gynecol. 2012, 19, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Sanna, E.; Madeddu, C.; Lavra, F.; Oppi, S.; Scartozzi, M.; Giorgio Calò, P.; Macciò, A. Laparoscopic management of isolated nodal recurrence in gynecological malignancies is safe and feasible even for large metastatic nodes up to 8 cm: A prospective case series. Int. J. Surg. 2022, 104, 106744. [Google Scholar] [CrossRef] [PubMed]
- Certelli, C.; Russo, S.A.; Palmieri, L.; Foresta, A.; Pedone Anchora, L.; Vargiu, V.; Santullo, F.; Fagotti, A.; Scambia, G.; Gallotta, V. Minimally-Invasive Secondary Cytoreduction in Recurrent Ovarian Cancer. Cancers 2023, 15, 4769. [Google Scholar] [CrossRef] [PubMed]
- Loverro, M.; Ergasti, R.; Conte, C.; Gallitelli, V.; Nachira, D.; Scaglione, G.; Fagotti, A.; Scambia, G.; Gallotta, V. Minimally Invasive Secondary Cytoreductive Surgery for Superficial Celiac and Cardio-Phrenic Isolated Nodal Recurrence of Ovarian Cancer. Ann. Surg. Oncol. 2022, 29, 2603–2604. [Google Scholar] [CrossRef] [PubMed]
- Tsibulak, I.; Fotopoulou, C. Tumor biology and impact on timing of surgery in advanced epithelial ovarian cancer. Int. J. Gynecol. Cancer 2023, 33, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Deffieux, X.; Castaigne, D.; Pomel, C. Role of laparoscopy to evaluate candidates for complete cytoreduction in advanced stages of epithelial ovarian cancer. Int. J. Gynecol. Cancer 2006, 16 (Suppl. S1), 35–40. [Google Scholar] [CrossRef] [PubMed]
| Patient | Age at Surgery | Histotype | FIGO Stage at Diagnosis (FIGO 2021) | BRCA Status | PDS, IDS or SCS | Site of Adenopathy | Maximum Nodal Diameter (mm) | Patient Status |
|---|---|---|---|---|---|---|---|---|
| 1 | 43 | High-grade serous | IIIC | wt | PDS | Pelvic | 60 | AWD |
| 2 | 60 | High-grade endometrioid | IIIA1 | wt | PDS | Pelvic and para-aortic | 30 | AWD |
| 3 | 50 | High-grade serous | IIIC | mBRCA1 | SCS | Pelvic | 15 | NED |
| 4 | 64 | High-grade serous | IIIA1 | wt | PDS | Pelvic and para-aortic | 35 | NED |
| 5 | 55 | Clear Cell | IIIC | mBRCA1 | PDS | Pelvic | 35 | NED |
| 6 | 52 | High-grade serous | IIIA2 | wt | SCS | Pelvic | 50 | AWD |
| 7 | 65 | High-grade serous | IIIA2 | wt | PDS | Pelvic | 35 | AWD |
| 8 | 67 | High-grade serous | IIIA2 | wt | SCS | Pelvic | 30 | AWD |
| 9 | 57 | High-grade serous | IIIA | wt | PDS | Pelvic | 30 | AWD |
| 10 | 59 | High-grade serous | IIIA | wt | SCS | Pelvic | 30 | AWD |
| 11 | 56 | High-grade serous | IIIC | mBRCA1 | IDS | Pelvic | 10 | NED |
| 12 | 60 | High-grade serous | IVB | wt | IDS | Pelvic | 30 | AWD |
| 13 | 46 | High-grade serous | IIIC | wt | PDS | Pelvic | 10 | AWD |
| 14 | 80 | High-grade serous | IIIB | wt | IDS | Pelvic | 10 | AWD |
| 15 | 46 | High-grade endometrioid | IIIC | wt | PDS | Pelvic | 30 | AWD |
| 16 | 79 | High-grade serous | IIIC | wt | IDS | Pelvic | 30 | AWD |
| 17 | 74 | High-grade serous | IVB | mBRCA1 | IDS | Pelvic | 35 | AWD |
| 18 | 57 | High-grade serous | IIIC | wt | PDS | Pelvic | 20 | AWD |
| 19 | 59 | High-grade serous | IIIC | wt | SCS | Pelvic | 15 | NED |
| 20 | 59 | Clear Cell | IIIC | wt | IDS | Pelvic | 10 | AWD |
| 21 | 51 | High-grade serous | IVB | wt | IDS | Pelvic | 30 | AWD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniele, A.; Rosso, R.; Ceccaroni, M.; Roviglione, G.; D’Ancona, G.; Peano, E.; Clignon, V.; Calandra, V.; Puppo, A. Laparoscopic Treatment of Bulky Nodes in Primary and Recurrent Ovarian Cancer: Surgical Technique and Outcomes from Two Specialized Italian Centers. Cancers 2024, 16, 1631. https://doi.org/10.3390/cancers16091631
Daniele A, Rosso R, Ceccaroni M, Roviglione G, D’Ancona G, Peano E, Clignon V, Calandra V, Puppo A. Laparoscopic Treatment of Bulky Nodes in Primary and Recurrent Ovarian Cancer: Surgical Technique and Outcomes from Two Specialized Italian Centers. Cancers. 2024; 16(9):1631. https://doi.org/10.3390/cancers16091631
Chicago/Turabian StyleDaniele, Alberto, Roberta Rosso, Marcello Ceccaroni, Giovanni Roviglione, Gianmarco D’Ancona, Elisa Peano, Valentino Clignon, Valerio Calandra, and Andrea Puppo. 2024. "Laparoscopic Treatment of Bulky Nodes in Primary and Recurrent Ovarian Cancer: Surgical Technique and Outcomes from Two Specialized Italian Centers" Cancers 16, no. 9: 1631. https://doi.org/10.3390/cancers16091631
APA StyleDaniele, A., Rosso, R., Ceccaroni, M., Roviglione, G., D’Ancona, G., Peano, E., Clignon, V., Calandra, V., & Puppo, A. (2024). Laparoscopic Treatment of Bulky Nodes in Primary and Recurrent Ovarian Cancer: Surgical Technique and Outcomes from Two Specialized Italian Centers. Cancers, 16(9), 1631. https://doi.org/10.3390/cancers16091631






