Effects of Short-Term Lenvatinib Administration Prior to Transarterial Chemoembolization for Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Data Collection and Eligibility Criteria
2.2. LEN Administration before TACE
2.3. Perfusion-CT
2.4. TACE Procedure
2.5. Evaluation
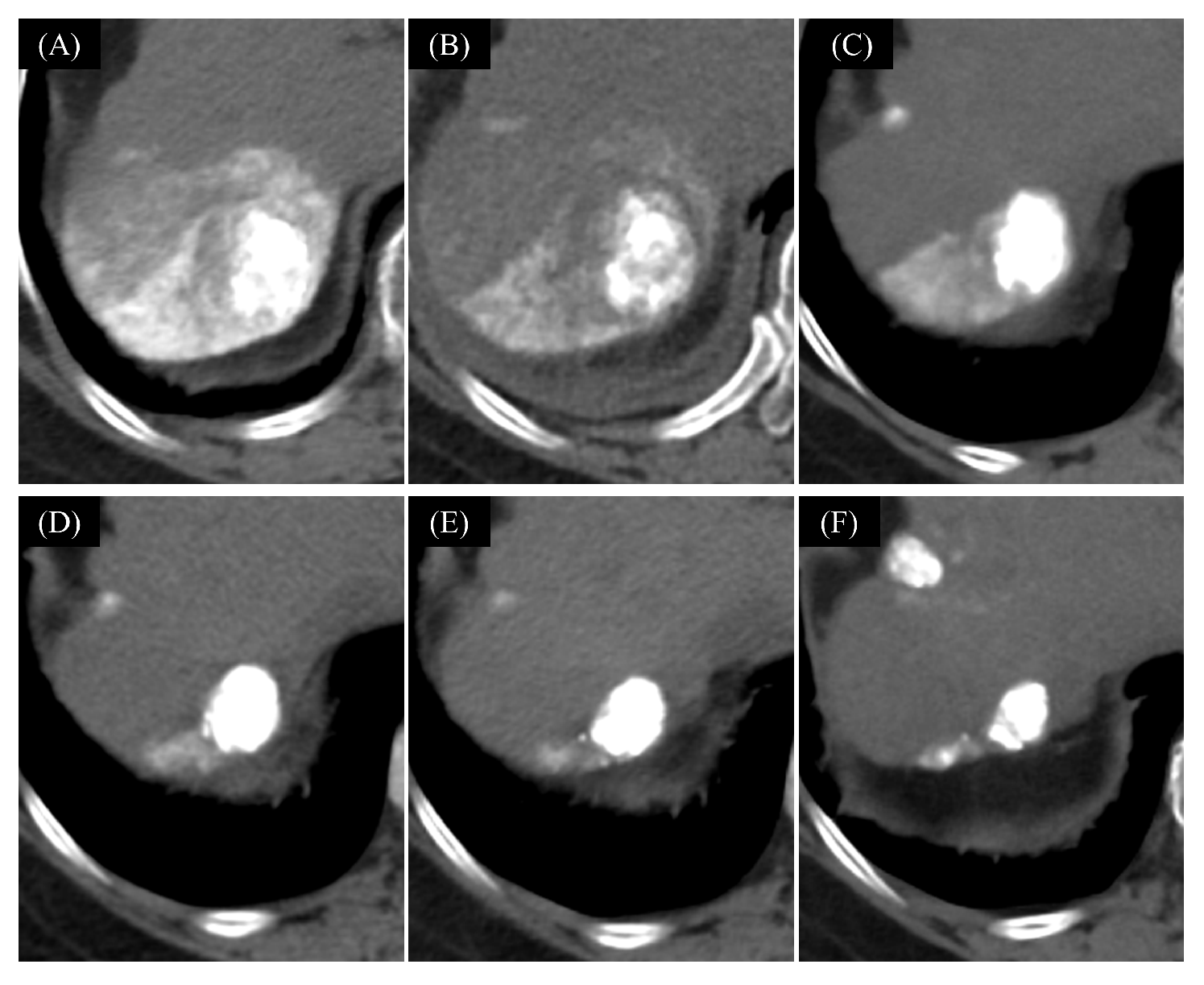
2.5.1. The Changes of Tumor Hemodynamics
2.5.2. Technical Feasibility of short-Term LEN-TACE
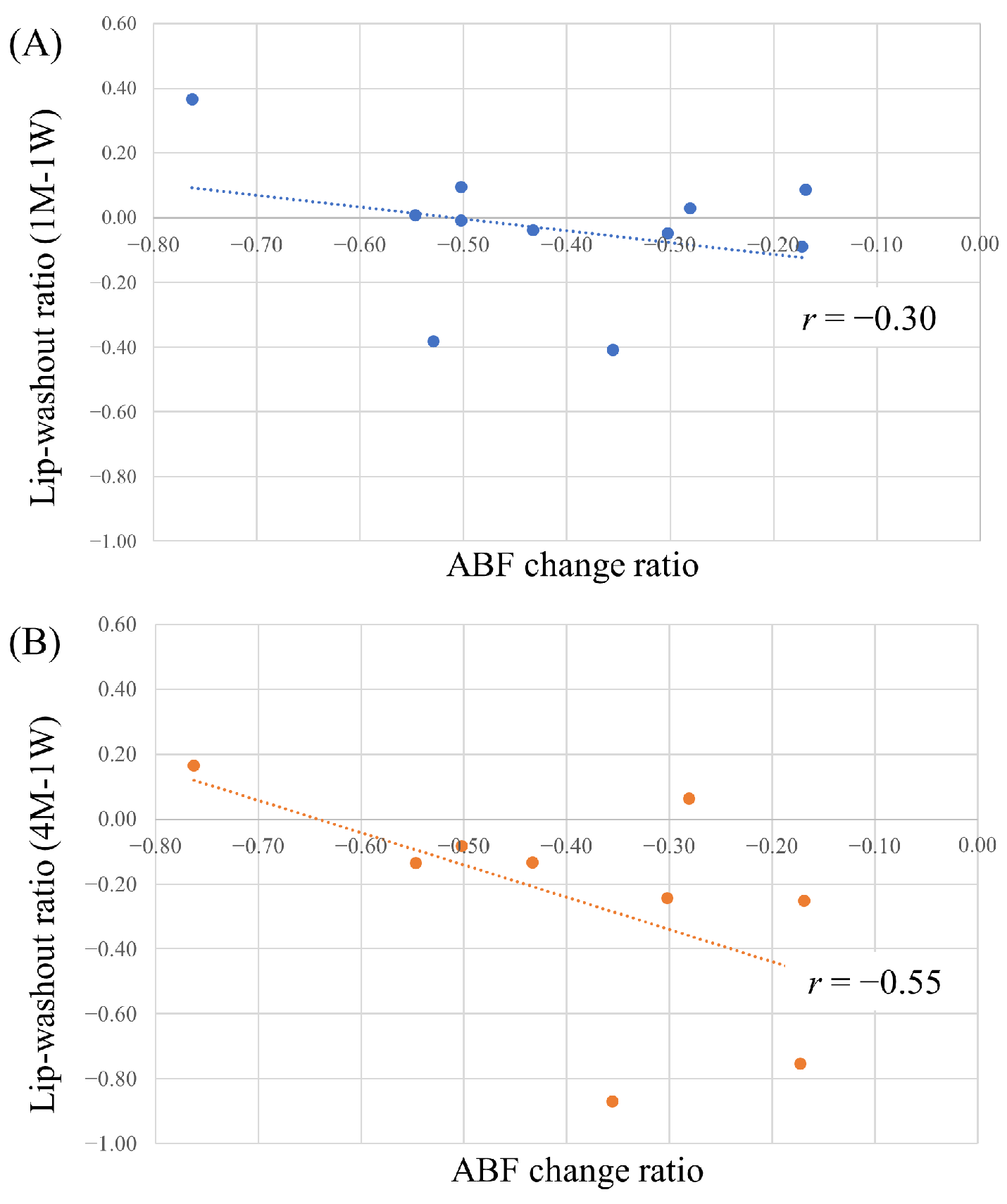
2.5.3. Correlation between Lipiodol Washout in Tumors and Effects of Lenvatinib
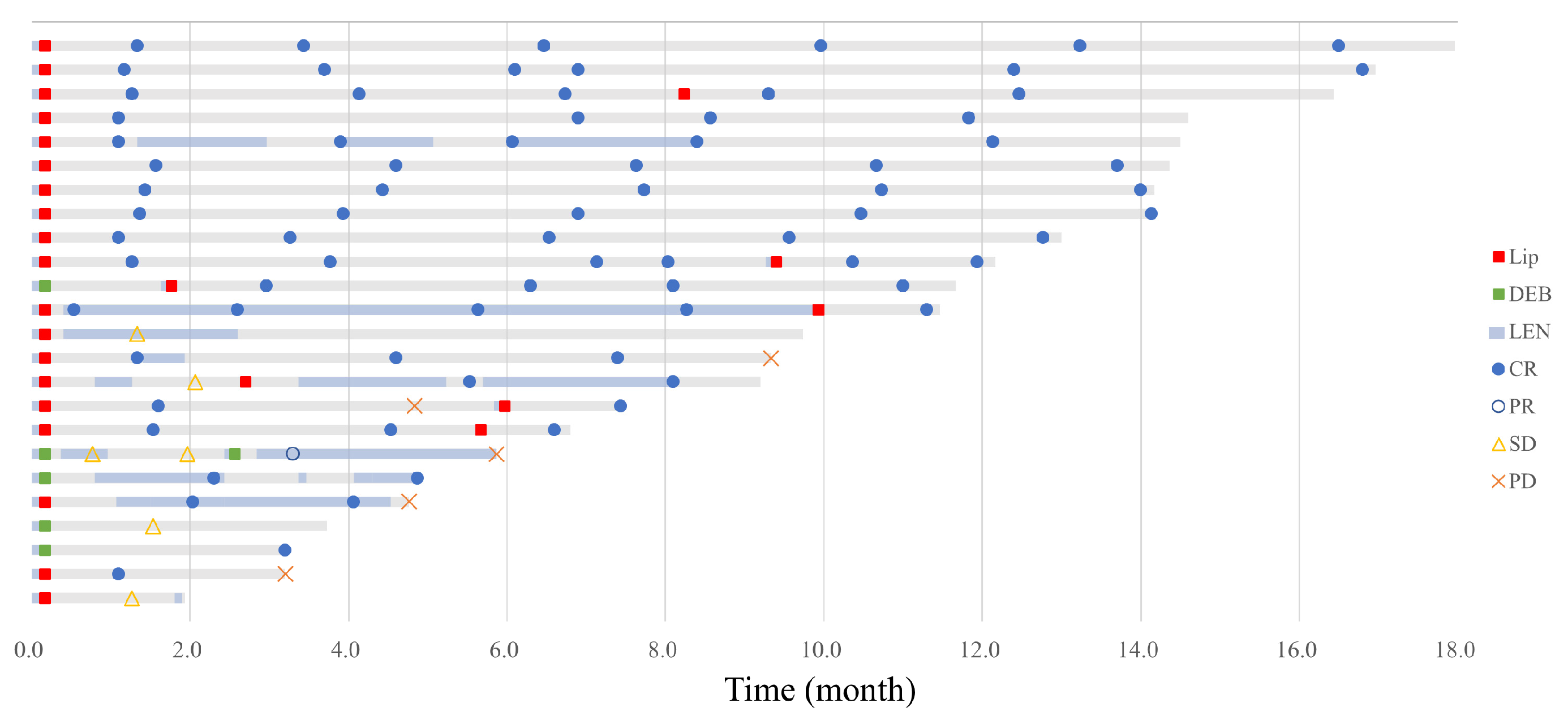
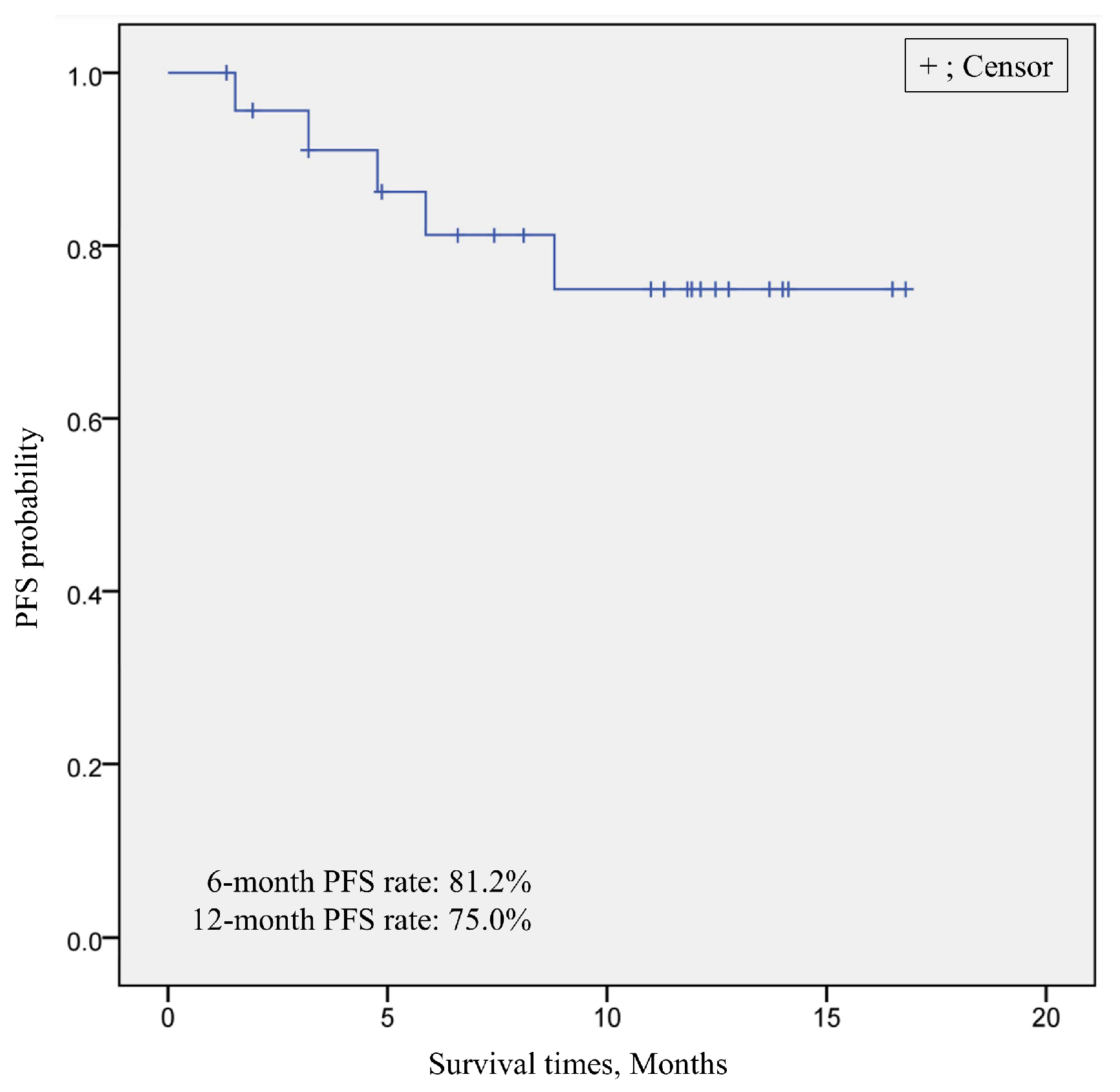
2.5.4. Tumor Response and Progression-Free Survival
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
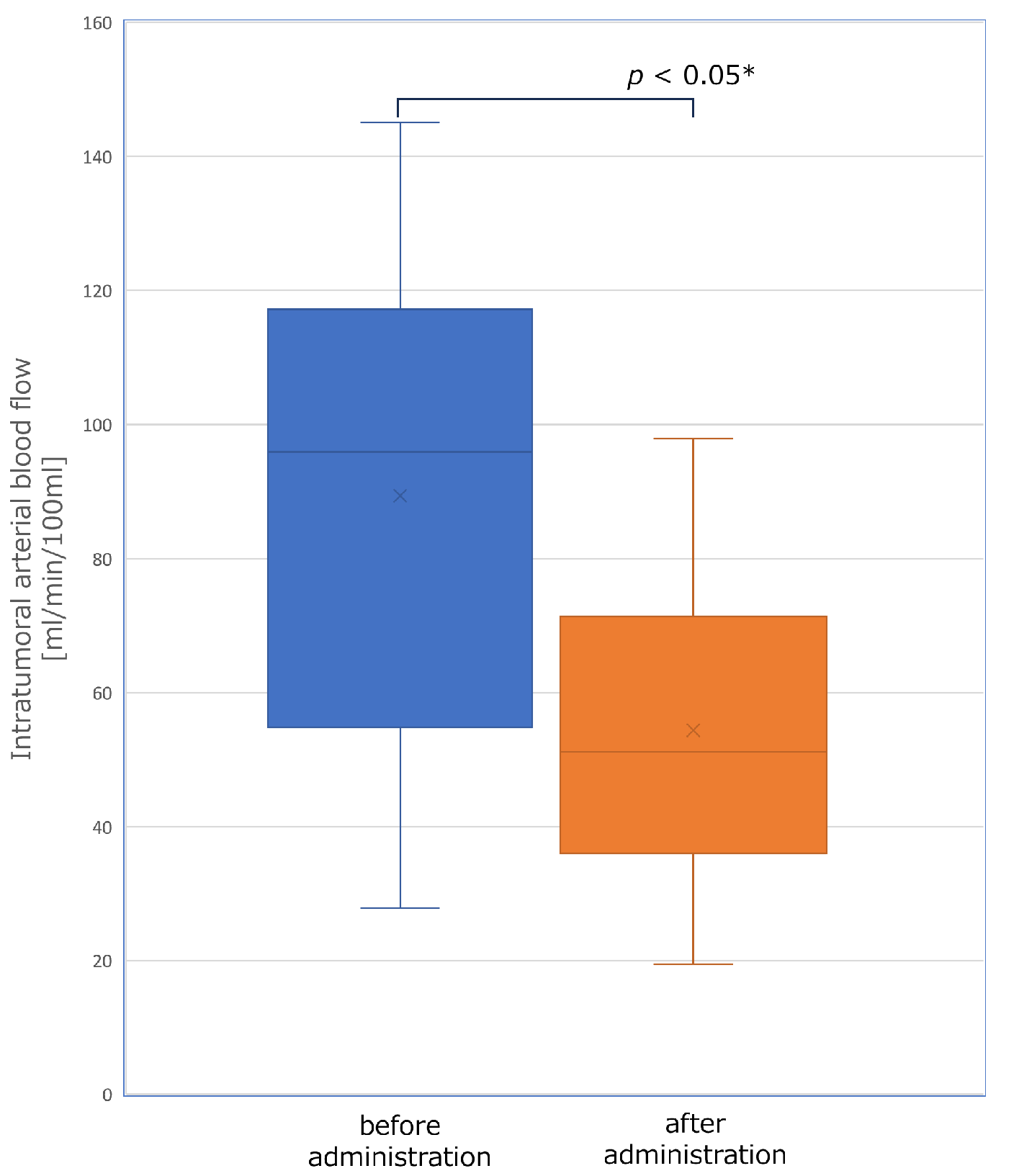
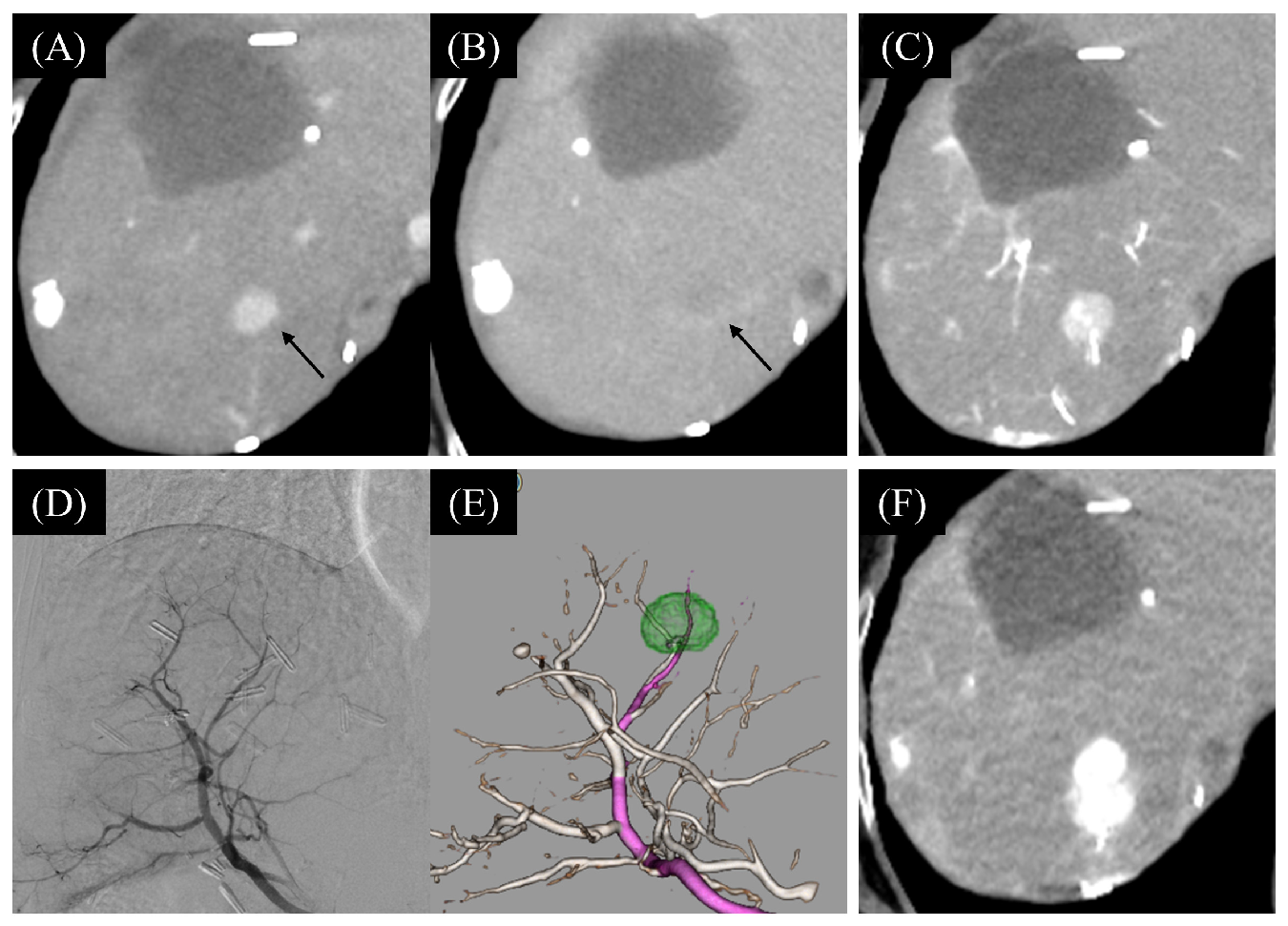
3.2. The Change of Tumor Hemodynamics
3.3. Technical Feasibility and Safety of Short-Term LEN-TACE
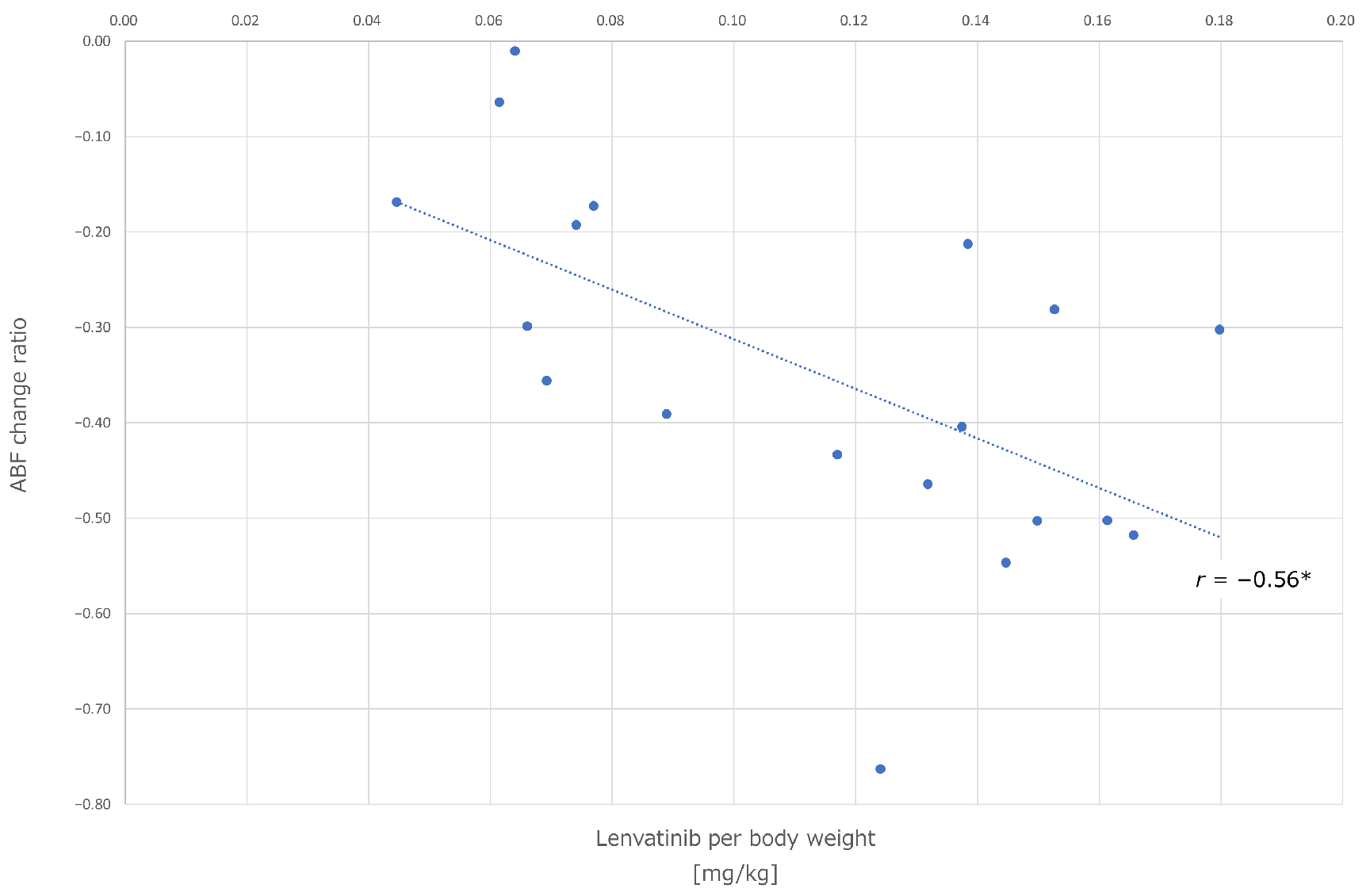
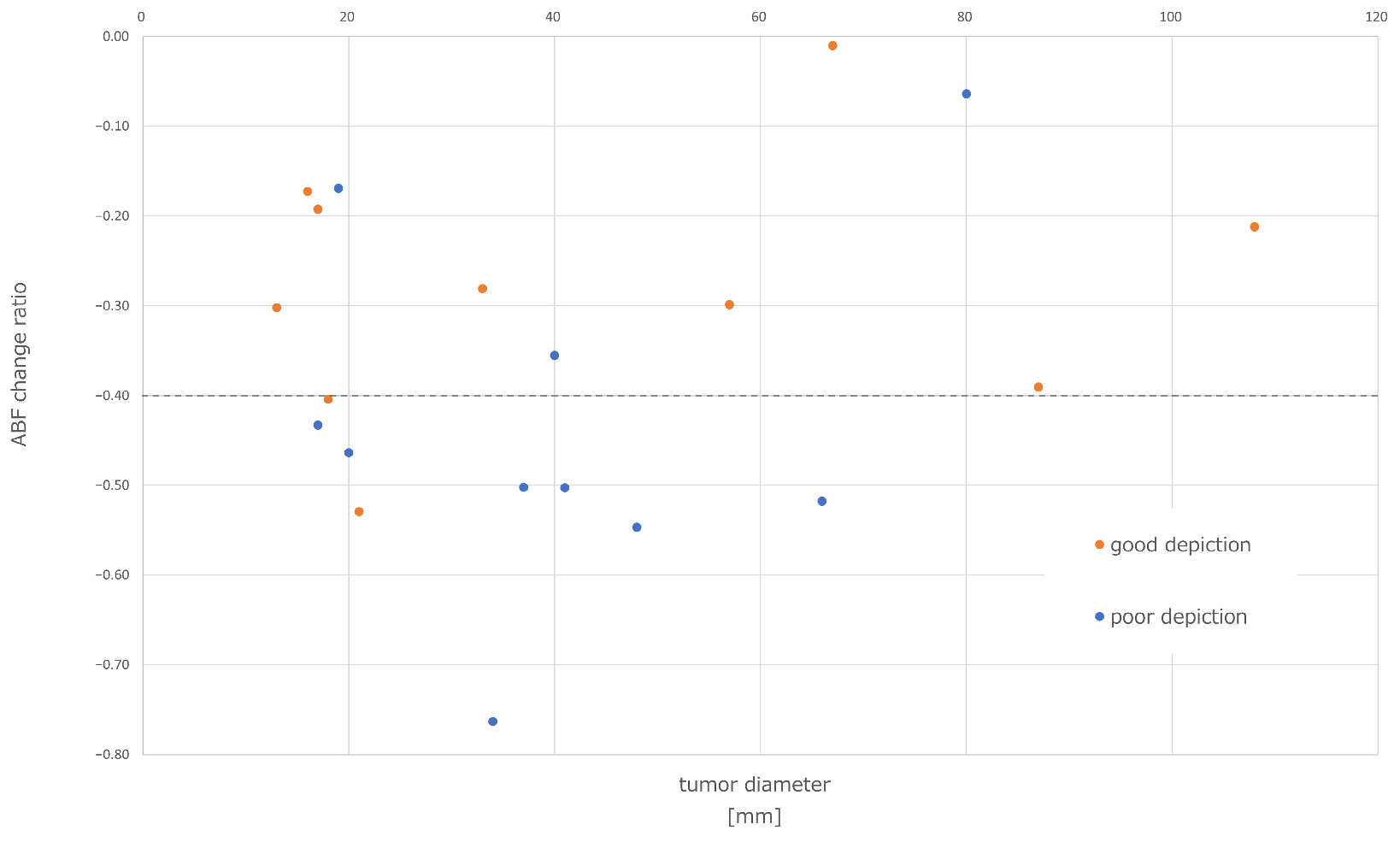
3.3.1. Correlation between Lipiodol Washout in Tumors and Effects of Lenvatinib
3.3.2. Treatment Outcome of Short-Term LEN-TACE
3.3.3. Comparison of the Treatment Outcome between cTACE and DEB-TACE
3.3.4. Correlation between Clinical Characteristics and Treatment Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ohishi, H.; Uchida, H.; Yoshimura, H.; Ohue, S.; Ueda, J.; Katsuragi, M.; Matsuo, N.; Hosogi, Y. Hepatocellular Carcinoma Detected by Iodized Oil. Use of Anticancer Agents. Radiology 1985, 154, 25–29. [Google Scholar] [CrossRef]
- Foerster, F.; Gairing, S.J.; Ilyas, S.I.; Galle, P.R. Emerging Immunotherapy for HCC: A Guide for Hepatologists. Hepatology 2022, 75, 1604–1626. [Google Scholar] [CrossRef]
- Pelizzaro, F.; Haxhi, S.; Penzo, B.; Vitale, A.; Giannini, E.G.; Sansone, V.; Rapaccini, G.L.; Di Marco, M.; Caturelli, E.; Magalotti, D.; et al. Transarterial Chemoembolization for Hepatocellular Carcinoma in Clinical Practice: Temporal Trends and Survival Outcomes of an Iterative Treatment. Front. Oncol. 2022, 12, 822507. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Park, C.; Chu, H.H.; Kim, J.H.; Kim, S.Y.; Alrashidi, I.; Gwon, D.I.; Yoon, H.-K.; Kim, N. Clinical Significance of the Initial and Best Responses after Chemoembolization in the Treatment of Intermediate-Stage Hepatocellular Carcinoma with Preserved Liver Function. J. Vasc. Interv. Radiol. 2020, 31, 1998–2006.e1. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Randomised, Multicentre Prospective Trial of Transarterial Chemoembolisation (TACE) plus Sorafenib as Compared with TACE Alone in Patients with Hepatocellular Carcinoma: TACTICS Trial. Gut 2020, 69, 1492–1501. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus Sorafenib in First-Line Treatment of Patients with Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 Non-Inferiority Trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Facciorusso, A.; Tartaglia, N.; Villani, R.; Serviddio, G.; Ramai, D.; Mohan, B.P.; Chandan, S.; Abd El Aziz, M.A.; Evangelista, J.; Cotsoglou, C.; et al. Lenvatinib versus Sorafenib as First-Line Therapy of Advanced Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Am. J. Transl. Res. 2021, 13, 2379–2387. [Google Scholar]
- Kudo, M.; Ueshima, K.; Saeki, I.; Ishikawa, T.; Inaba, Y.; Morimoto, N.; Aikata, H.; Tanabe, N.; Wada, Y.; Kondo, Y.; et al. A Phase 2, Prospective, Multicenter, Single-Arm Trial of Transarterial Chemoembolization Therapy in Combination Strategy with Lenvatinib in Patients with Unresectable Intermediate-Stage Hepatocellular Carcinoma: TACTICS-L Trial. Liver Cancer 2023, 13, 99–112. [Google Scholar] [CrossRef]
- Jain, R.K. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Teleanu, D.M. Tumor Angiogenesis and Anti-Angiogenic Strategies for Cancer Treatment. J. Clin. Med. 2019, 9, 84. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis: An Organizing Principle for Drug Discovery? Nat. Rev. Drug Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef]
- Van der Veldt, A.A.M.; Lubberink, M.; Bahce, I.; Walraven, M.; de Boer, M.P.; Greuter, H.N.J.M.; Hendrikse, N.H.; Eriksson, J.; Windhorst, A.D.; Postmus, P.E.; et al. Rapid Decrease in Delivery of Chemotherapy to Tumors after Anti-VEGF Therapy: Implications for Scheduling of Anti-Angiogenic Drugs. Cancer Cell 2012, 21, 82–91. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular Mechanisms and Clinical Applications of Angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the Vasculature for Treatment of Cancer and Other Diseases. Physiol. Rev. 2011, 91, 1071–1121. [Google Scholar] [CrossRef]
- Hicklin, D.J.; Ellis, L.M. Role of the Vascular Endothelial Growth Factor Pathway in Tumor Growth and Angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef]
- Tachiiri, T.; Nishiofuku, H.; Maeda, S.; Sato, T.; Toyoda, S.; Matsumoto, T.; Chanoki, Y.; Minamiguchi, K.; Taiji, R.; Kunichika, H.; et al. Vascular Normalization Caused by Short-Term Lenvatinib Could Enhance Transarterial Chemoembolization in Hepatocellular Carcinoma. Curr. Oncol. 2023, 30, 4779–4786. [Google Scholar] [CrossRef]
- Wang, B.; Xu, H.; Gao, Z.Q.; Ning, H.F.; Sun, Y.Q.; Cao, G.W. Increased Expression of Vascular Endothelial Growth Factor in Hepatocellular Carcinoma after Transcatheter Arterial Chemoembolization. Acta Radiol. 2008, 49, 523–529. [Google Scholar] [CrossRef]
- Shim, J.H.; Park, J.-W.; Kim, J.H.; An, M.; Kong, S.-Y.; Nam, B.-H.; Choi, J.-I.; Kim, H.B.; Lee, W.J.; Kim, C.-M. Association between Increment of Serum VEGF Level and Prognosis after Transcatheter Arterial Chemoembolization in Hepatocellular Carcinoma Patients. Cancer Sci. 2008, 99, 2037–2044. [Google Scholar] [CrossRef]
- Kim, J.H.; Shim, J.H.; Lee, H.C.; Sung, K.-B.; Ko, H.-K.; Ko, G.-Y.; Gwon, D.I.; Kim, J.W.; Lim, Y.-S.; Park, S.H. New Intermediate-Stage Subclassification for Patients with Hepatocellular Carcinoma Treated with Transarterial Chemoembolization. Liver Int. 2017, 37, 1861–1868. [Google Scholar] [CrossRef]
- Hung, Y.-W.; Lee, I.-C.; Chi, C.-T.; Lee, R.-C.; Liu, C.-A.; Chiu, N.-C.; Hwang, H.-E.; Chao, Y.; Hou, M.-C.; Huang, Y.-H. Redefining Tumor Burden in Patients with Intermediate-Stage Hepatocellular Carcinoma: The Seven-Eleven Criteria. Liver Cancer 2021, 10, 629–640. [Google Scholar] [CrossRef]
- Ikeda, K.; Kobayashi, H.; Shinohara, S.; Ohkubo, K. An experimental trial for quantitative estimation of lipiodol using CT value—Application to hepatic arterial infusion therapy. Nihon. Igaku Hoshasen. Gakkai Zasshi 1990, 50, 84–86. [Google Scholar]
- Chen, R.; Geschwind, J.F.; Wang, Z.; Tacher, V.; Lin, M. Quantitative Assessment of Lipiodol Deposition after Chemoembolization: Comparison between Cone-Beam CT and Multidetector CT. J. Vasc. Interv. Radiol. 2013, 24, 1837–1844. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Kubo, S.; Sakamoto, M.; Tanaka, M.; Ikai, I.; Furuse, J.; Murakami, T.; Kadoya, M.; Kokudo, N.; et al. Response Evaluation Criteria in Cancer of the Liver (RECICL) (2015 Revised Version). Hepatol. Res. 2016, 46, 3–9. [Google Scholar] [CrossRef]
- Hiraoka, A.; Michitaka, K.; Kumada, T.; Izumi, N.; Kadoya, M.; Kokudo, N.; Kubo, S.; Matsuyama, Y.; Nakashima, O.; Sakamoto, M.; et al. Validation and Potential of Albumin-Bilirubin Grade and Prognostication in a Nationwide Survey of 46,681 Hepatocellular Carcinoma Patients in Japan: The Need for a More Detailed Evaluation of Hepatic Function. Liver Cancer 2017, 6, 325–336. [Google Scholar] [CrossRef]
- Kudo, M.; Han, K.-H.; Ye, S.-L.; Zhou, J.; Huang, Y.-H.; Lin, S.-M.; Wang, C.-K.; Ikeda, M.; Chan, S.L.; Choo, S.P.; et al. A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer 2020, 9, 245–260. [Google Scholar] [CrossRef]
- Une, N.; Takano-Kasuya, M.; Kitamura, N.; Ohta, M.; Inose, T.; Kato, C.; Nishimura, R.; Tada, H.; Miyagi, S.; Ishida, T.; et al. The Anti-Angiogenic Agent Lenvatinib Induces Tumor Vessel Normalization and Enhances Radiosensitivity in Hepatocellular Tumors. Med. Oncol. 2021, 38, 60. [Google Scholar] [CrossRef]
- Rimassa, L.; Danesi, R.; Pressiani, T.; Merle, P. Management of Adverse Events Associated with Tyrosine Kinase Inhibitors: Improving Outcomes for Patients with Hepatocellular Carcinoma. Cancer Treat Rev. 2019, 77, 20–28. [Google Scholar] [CrossRef]
- Lenvatinib for thyroid and renal cell cancer. Aust. Prescr. 2017, 40, 242–243. [CrossRef]
- Lencioni, R.; Kudo, M.; Erinjeri, J.; Qin, S.; Ren, Z.; Chan, S.; Arai, Y.; Heo, J.; Mai, A.; Escobar, J.; et al. EMERALD-1: A Phase 3, Randomized, Placebo-Controlled Study of Transarterial Chemoembolization Combined with Durvalumab with or without Bevacizumab in Participants with Unresectable Hepatocellular Carcinoma Eligible for Embolization. JCO 2024, 42, LBA432. [Google Scholar] [CrossRef]
- Llovet, J.M.; Vogel, A.; Madoff, D.C.; Finn, R.S.; Ogasawara, S.; Ren, Z.; Mody, K.; Li, J.J.; Siegel, A.B.; Dubrovsky, L.; et al. Randomized Phase 3 LEAP-012 Study: Transarterial Chemoembolization with or without Lenvatinib Plus Pembrolizumab for Intermediate-Stage Hepatocellular Carcinoma Not Amenable to Curative Treatment. Cardiovasc. Interv. Radiol. 2022, 45, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Guo, Y.; Hua, Y.; Zhao, M.; Xing, W.; Zhang, Y.; Liu, R.; Ren, Z.; Gu, S.; Lin, Z.; et al. TALENTACE: A Phase III, Open-Label, Randomized Study of on-Demand Transarterial Chemoembolization Combined with Atezolizumab + Bevacizumab or on-Demand Transarterial Chemoembolization Alone in Patients with Untreated Hepatocellular Carcinoma. J. Clin. Oncol. 2022, 40, TPS487. [Google Scholar] [CrossRef]
- Idée, J.-M.; Guiu, B. Use of Lipiodol as a Drug-Delivery System for Transcatheter Arterial Chemoembolization of Hepatocellular Carcinoma: A Review. Crit. Rev. Oncol. Hematol. 2013, 88, 530–549. [Google Scholar] [CrossRef] [PubMed]
- Kan, Z.; Madoff, D.C. Liver Anatomy: Microcirculation of the Liver. Semin. Intervent. Radiol. 2008, 25, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Arai, Y.; Inaba, Y.; Tanaka, T.; Sugawara, S.; Kodama, Y.; Aramaki, T.; Anai, H.; Morita, S.; Tsukahara, Y.; et al. Conventional or Drug-Eluting Beads? Randomized Controlled Study of Chemoembolization for Hepatocellular Carcinoma: JIVROSG-1302. Liver Cancer 2022, 11, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Nishiofuku, H.; Sato, T.; Maeda, S.; Minamiguchi, K.; Taiji, R.; Matsumoto, T.; Chanoki, Y.; Tachiiri, T.; Kunichika, H.; et al. Predictive Factors of Complete Response to Transarterial Chemoembolization in Intermediate Stage Hepatocellular Carcinoma beyond Up-To-7 Criteria. Cancers 2023, 15, 2609. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Tanaka, T.; Nishiohuku, H.; Sato, T.; Masada, T.; Matsumoto, T.; Anai, H.; Sakaguchi, H.; Sueyoshi, S.; Marugami, N.; et al. Transarterial-Chemoembolization Remains an Effective Therapy for Intermediate-Stage Hepatocellular Carcinoma with Preserved Liver Function. Hepatol. Res. 2020, 50, 1176–1185. [Google Scholar] [CrossRef]
- Milberg, O.; Gong, C.; Jafarnejad, M.; Bartelink, I.H.; Wang, B.; Vicini, P.; Narwal, R.; Roskos, L.; Popel, A.S. A QSP Model for Predicting Clinical Responses to Monotherapy, Combination and Sequential Therapy Following CTLA-4, PD-1, and PD-L1 Checkpoint Blockade. Sci. Rep. 2019, 9, 11286. [Google Scholar] [CrossRef]
| Characteristic | n = 25 |
|---|---|
| Age, median (range), years | 80 (63–90) |
| Weight, median (range), kg | 58.2 (44.5–89.8) |
| Sex Male, n (%) | 18 (72.0) |
| Etiology, % | |
| Hepatitis B | 2 (8.0) |
| Hepatitis C | 7 (28.0) |
| Non-B non-C | 16 (64.0) |
| Child-Pugh stage, n (%) | |
| A | 22 (88.0) |
| B | 3 (12.0) |
| mALBI grade, n (%) | |
| 1, 2a | 16 (64.0) |
| 2b | 9 (36.0) |
| AFP, n (%) | |
| <200 ng/mL | 20 (80.0) |
| ≥200 ng/mL | 5 (20.0) |
| Liver cancer staging, n (%) | |
| early | 15 (60.0) |
| intermediate | 10 (40.0) |
| TACE, n (%) | |
| cTACE | 19 (76.0) |
| DEB-TACE | 6 (24.0) |
| AEs, n (%) | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| pyrexia | 6 (24) | 0 | 0 | 0 |
| malaise | 8 (32) | 1 (4) | 0 | 0 |
| appetite loss | 8 (32) | 0 | 0 | 0 |
| nausea | 4 (16) | 0 | 0 | 0 |
| abdominal pain | 10 (40) | 0 | 0 | 0 |
| Dyspnea | 2 (8) | 0 | 0 | 0 |
| Hypertension | 0 | 1 (4) | 0 | 0 |
| Hypoalbuminemia | 13 (52) | 3 (12) | 0 | 0 |
| Bil increased | 3 (12) | 3 (12) | 0 | 0 |
| AST increased | 3 (12) | 3 (12) | 12 (48) | 7 (28) |
| ALT increased | 6 (24) | 6 (24) | 11 (44) | 0 |
| ALP increased | 1 (4) | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tachiiri, T.; Minamiguchi, K.; Taiji, R.; Sato, T.; Toyoda, S.; Matsumoto, T.; Chanoki, Y.; Kunichika, H.; Yamauchi, S.; Shimizu, S.; et al. Effects of Short-Term Lenvatinib Administration Prior to Transarterial Chemoembolization for Hepatocellular Carcinoma. Cancers 2024, 16, 1624. https://doi.org/10.3390/cancers16091624
Tachiiri T, Minamiguchi K, Taiji R, Sato T, Toyoda S, Matsumoto T, Chanoki Y, Kunichika H, Yamauchi S, Shimizu S, et al. Effects of Short-Term Lenvatinib Administration Prior to Transarterial Chemoembolization for Hepatocellular Carcinoma. Cancers. 2024; 16(9):1624. https://doi.org/10.3390/cancers16091624
Chicago/Turabian StyleTachiiri, Tetsuya, Kiyoyuki Minamiguchi, Ryosuke Taiji, Takeshi Sato, Shohei Toyoda, Takeshi Matsumoto, Yuto Chanoki, Hideki Kunichika, Satoshi Yamauchi, Sho Shimizu, and et al. 2024. "Effects of Short-Term Lenvatinib Administration Prior to Transarterial Chemoembolization for Hepatocellular Carcinoma" Cancers 16, no. 9: 1624. https://doi.org/10.3390/cancers16091624
APA StyleTachiiri, T., Minamiguchi, K., Taiji, R., Sato, T., Toyoda, S., Matsumoto, T., Chanoki, Y., Kunichika, H., Yamauchi, S., Shimizu, S., Nishiofuku, H., Marugami, N., Tsuji, Y., Namisaki, T., Yoshiji, H., & Tanaka, T. (2024). Effects of Short-Term Lenvatinib Administration Prior to Transarterial Chemoembolization for Hepatocellular Carcinoma. Cancers, 16(9), 1624. https://doi.org/10.3390/cancers16091624






