Pain in Long-Term Cancer Survivors: Prevalence and Impact in a Cohort Composed Mostly of Breast Cancer Survivors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Subjects
2.3. Clinical Endpoints
2.4. Data Sources
2.5. Study Size
2.6. Statistical Methods
3. Results
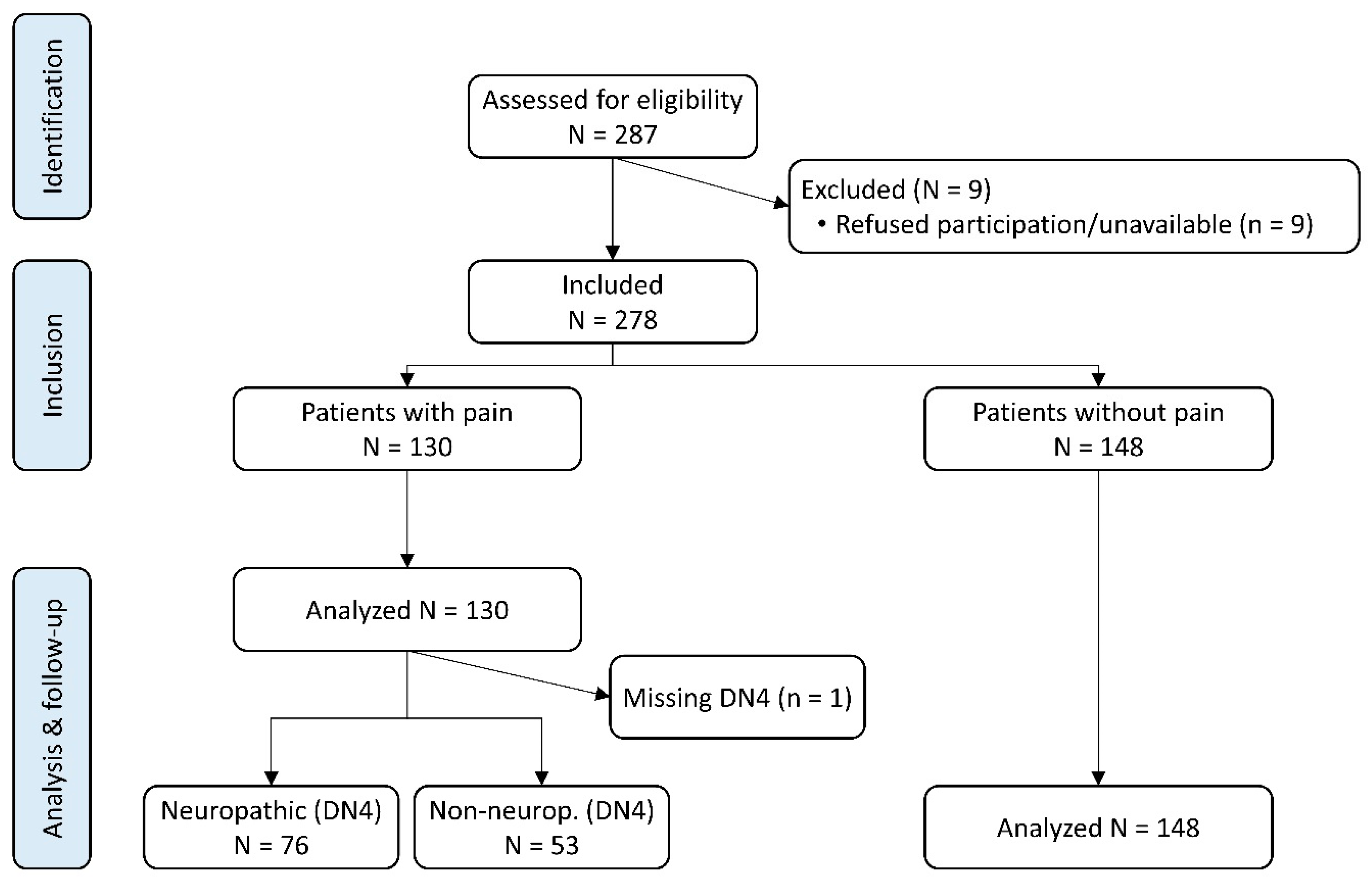
3.1. Patient Disposition, Pain Prevalence, Cancer, and Pain Characteristics
3.2. Clinical Endpoints, Pain Characteristics and Health Outcomes
3.3. Adjusted Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, M.I.; Kaasa, S.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.D. IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: Chronic cancer-related pain. Pain 2019, 160, 38–44. [Google Scholar] [CrossRef]
- De Angelis, R.; Sant, M.; Coleman, M.P.; Francisci, S.; Baili, P.; Pierannunzio, D.; Trama, A.; Visser, O.; Brenner, H.; Ardanaz, E.; et al. Cancer survival in Europe 1999–2007 by country and age: Results of EUROCARE--5-a population-based study. Lancet Oncol. 2014, 15, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Glare, P.A.; Davies, P.S.; Finlay, E.; Gulati, A.; Lemanne, D.; Moryl, N.; Oeffinger, K.C.; Paice, J.A.; Stubblefield, M.D.; Syrjala, K.L. Pain in cancer survivors. J. Clin. Oncol. 2014, 32, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Bayonas, A.; Jimenez-Fonseca, P.; Castanon, E.; Ramchandani-Vaswani, A.; Sanchez-Bayona, R.; Custodio, A.; Calvo-Temprano, D.; Virizuela, J.A. Chronic opioid therapy in long-term cancer survivors. Clin. Transl. Oncol. 2017, 19, 236–250. [Google Scholar] [CrossRef] [PubMed]
- van den Beuken-van Everdingen, M.H.; Hochstenbach, L.M.; Joosten, E.A.; Tjan-Heijnen, V.C.; Janssen, D.J. Update on Prevalence of Pain in Patients with Cancer: Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2016, 51, 1070–1090.e9. [Google Scholar] [CrossRef] [PubMed]
- Pachman, D.R.; Barton, D.L.; Swetz, K.M.; Loprinzi, C.L. Troublesome symptoms in cancer survivors: Fatigue, insomnia, neuropathy, and pain. J. Clin. Oncol. 2012, 30, 3687–3696. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.H.; Chwistek, M.; Mehta, R.S. Management of chronic pain in cancer survivors. Cancer J. 2008, 14, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.L.; Duffy, J.P. Surgical aspects of chronic post-thoracotomy pain. Eur. J. Cardiothorac. Surg. 2000, 18, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, G.; Marmiroli, P. Chemotherapy-induced peripheral neurotoxicity. Nat. Rev. Neurol. 2010, 6, 657–666. [Google Scholar] [CrossRef]
- Brown, M.R.; Ramirez, J.D.; Farquhar-Smith, P. Pain in cancer survivors. Br. J. Pain 2014, 8, 139–153. [Google Scholar] [CrossRef]
- Rubin, D.I. Brachial and lumbosacral plexopathies: A review. Clin. Neurophysiol. Pract. 2020, 5, 173–193. [Google Scholar] [CrossRef] [PubMed]
- Karri, J.; Lachman, L.; Hanania, A.; Marathe, A.; Singh, M.; Zacharias, N.; Orhurhu, V.; Gulati, A.; Abd-Elsayed, A. Radiotherapy-Specific Chronic Pain Syndromes in the Cancer Population: An Evidence-Based Narrative Review. Adv. Ther. 2021, 38, 1425–1446. [Google Scholar] [CrossRef] [PubMed]
- Badia, X.; Muriel, C.; Gracia, A.; Nunez-Olarte, J.M.; Perulero, N.; Galvez, R.; Carulla, J.; Cleeland, C.S.; Grupo, V. Validation of the Spanish version of the Brief Pain Inventory in patients with oncological pain. Med. Clin. 2003, 120, 52–59. [Google Scholar] [CrossRef]
- Bouhassira, D.; Attal, N.; Alchaar, H.; Boureau, F.; Brochet, B.; Bruxelle, J.; Cunin, G.; Fermanian, J.; Ginies, P.; Grun-Overdyking, A.; et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005, 114, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Herdman, M.; Badia, X.; Berra, S. EuroQol-5D: A simple alternative for measuring health-related quality of life in primary care. Aten. Primaria 2001, 28, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.J.; Blanch, J.; Peri, J.M.; De Pablo, J.; Pintor, L.; Bulbena, A. A validation study of the hospital anxiety and depression scale (HADS) in a Spanish population. Gen. Hosp. Psychiatry 2003, 25, 277–283. [Google Scholar] [CrossRef]
- Garcia Campayo, J.; Rodero, B.; Alda, M.; Sobradiel, N.; Montero, J.; Moreno, S. Validation of the Spanish version of the Pain Catastrophizing Scale in fibromyalgia. Med. Clin. 2008, 131, 487–492. [Google Scholar]
- Varela, N.; Guillen-Grima, F.; Perez-Cajaraville, J.J.; Perez-Hernandez, C.; Monedero, P. Assessment of the impact of pain on work productivity: Validation of the Spanish WPAI:Pain questionnaire. An. Sist. Sanit. Navar. 2016, 39, 77–85. [Google Scholar] [CrossRef]
- Wang, K.; Yee, C.; Tam, S.; Drost, L.; Chan, S.; Zaki, P.; Rico, V.; Ariello, K.; Dasios, M.; Lam, H.; et al. Prevalence of pain in patients with breast cancer post-treatment: A systematic review. Breast 2018, 42, 113–127. [Google Scholar] [CrossRef]
- Wang, L.; Cohen, J.C.; Devasenapathy, N.; Hong, B.Y.; Kheyson, S.; Lu, D.; Oparin, Y.; Kennedy, S.A.; Romerosa, B.; Arora, N.; et al. Prevalence and intensity of persistent post-surgical pain following breast cancer surgery: A systematic review and meta-analysis of observational studies. Br. J. Anaesth. 2020, 125, 346–357. [Google Scholar] [CrossRef]
- Moser, E.C.; Meunier, F. Cancer survivorship: A positive side-effect of more successful cancer treatment. EJC Suppl. 2014, 12, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Farquhar-Smith, P.; Magee, D. Pain in the Cancer Survivor. In Fundamentals of Cancer Pain Treatment, 1st ed.; Leitner, A., Chang, C., Eds.; Springer: Cham, Switzerland, 2021; pp. 57–84. [Google Scholar]
- Haenen, V.; Evenepoel, M.; De Baerdemaecker, T.; Meeus, M.; Devoogdt, N.; Morlion, B.; Dams, L.; Van Dijck, S.; Van der Gucht, E.; De Vrieze, T.; et al. Pain prevalence and characteristics in survivors of solid cancers: A systematic review and meta-analysis. Support. Care Cancer 2022, 31, 85. [Google Scholar] [CrossRef] [PubMed]
- Firkins, J.; Hansen, L.; Driessnack, M.; Dieckmann, N. Quality of life in “chronic” cancer survivors: A meta-analysis. J. Cancer Surviv. 2020, 14, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Cox-Martin, E.; Anderson-Mellies, A.; Borges, V.; Bradley, C. Chronic pain, health-related quality of life, and employment in working-age cancer survivors. J. Cancer Surviv. 2020, 14, 179–187. [Google Scholar] [CrossRef]
- Gallaway, M.S.; Townsend, J.S.; Shelby, D.; Puckett, M.C. Pain Among Cancer Survivors. Prev. Chronic Dis. 2020, 17, E54. [Google Scholar] [CrossRef] [PubMed]
- Heathcote, L.C.; Eccleston, C. Pain and cancer survival: A cognitive-affective model of symptom appraisal and the uncertain threat of disease recurrence. Pain 2017, 158, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Turk, D.C.; Okifuji, A. Psychological Aspects of Chronic Pain. In Principles and Practice of Pain Medicine, 3rd ed.; Warfield, C.A., Bajwa, Z.H., Wootton, R.J., Eds.; McGraw-Hill Education: New York, NY, USA, 2017; pp. 120–129. [Google Scholar]
- Paice, J.A. Pain in Cancer Survivors: How to Manage. Curr. Treat. Opt. Oncol. 2019, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Paice, J.A.; Portenoy, R.; Lacchetti, C.; Campbell, T.; Cheville, A.; Citron, M.; Constine, L.S.; Cooper, A.; Glare, P.; Keefe, F.; et al. Management of Chronic Pain in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 3325–3345. [Google Scholar] [CrossRef] [PubMed]
- Fabi, A.; Bhargava, R.; Fatigoni, S.; Guglielmo, M.; Horneber, M.; Roila, F.; Weis, J.; Jordan, K.; Ripamonti, C.I.; ESMO Guidelines Committee. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann. Oncol. 2020, 31, 713–723. [Google Scholar] [CrossRef]
- Meghani, S.H.; Vapiwala, N. Bridging the Critical Divide in Pain Management Guidelines From the CDC, NCCN, and ASCO for Cancer Survivors. JAMA Oncol. 2018, 4, 1323–1324. [Google Scholar] [CrossRef]
- Lemay, K.; Wilson, K.G.; Buenger, U.; Jarvis, V.; Fitzgibbon, E.; Bhimji, K.; Dobkin, P.L. Fear of pain in patients with advanced cancer or in patients with chronic noncancer pain. Clin. J. Pain 2011, 27, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Whitney, C.A.; Dorfman, C.S.; Shelby, R.A.; Keefe, F.J.; Gandhi, V.; Somers, T.J. Reminders of cancer risk and pain catastrophizing: Relationships with cancer worry and perceived risk in women with a first-degree relative with breast cancer. Fam. Cancer 2019, 18, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Attal, N.; Cruccu, G.; Baron, R.; Haanpaa, M.; Hansson, P.; Jensen, T.S.; Nurmikko, T. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur. J. Neurol. 2010, 17, 1113-e1188. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpaa, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.L.; Lacchetti, C.; Dworkin, R.H.; Lavoie Smith, E.M.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Gavin, P.; Lavino, A.; Lustberg, M.B.; et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2014, 32, 1941–1967. [Google Scholar] [CrossRef] [PubMed]
- Hamood, R.; Hamood, H.; Merhasin, I.; Keinan-Boker, L. Chronic pain and other symptoms among breast cancer survivors: Prevalence, predictors, and effects on quality of life. Breast Cancer Res. Treat. 2018, 167, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Beckwee, D.; Leysen, L.; Meuwis, K.; Adriaenssens, N. Prevalence of aromatase inhibitor-induced arthralgia in breast cancer: A systematic review and meta-analysis. Support. Care Cancer 2017, 25, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Anekar, A.A.; Cascella, M. WHO Analgesic Ladder; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- De Groef, A.; Meeus, M.; Heathcote, L.C.; Wiles, L.; Catley, M.; Vogelzang, A.; Olver, I.; Runciman, W.B.; Hibbert, P.; Dams, L.; et al. Treating persistent pain after breast cancer: Practice gaps and future directions. J. Cancer Surviv. 2023, 17, 1698–1707. [Google Scholar] [CrossRef]
- Freynhagen, R.; Parada, H.A.; Calderon-Ospina, C.A.; Chen, J.; Rakhmawati Emril, D.; Fernandez-Villacorta, F.J.; Franco, H.; Ho, K.Y.; Lara-Solares, A.; Li, C.C.; et al. Current understanding of the mixed pain concept: A brief narrative review. Curr. Med. Res. Opin. 2019, 35, 1011–1018. [Google Scholar] [CrossRef]
- Mulvey, M.R.; Boland, E.G.; Bouhassira, D.; Freynhagen, R.; Hardy, J.; Hjermstad, M.J.; Mercadante, S.; Perez, C.; Bennett, M.I. Neuropathic pain in cancer: Systematic review, performance of screening tools and analysis of symptom profiles. Br. J. Anaesth. 2017, 119, 765–774. [Google Scholar] [CrossRef]
- Singhal, S.; Dickerson, J.; Glover, M.J.; Roy, M.; Chiu, M.; Ellis-Caleo, T.; Hui, G.; Tamayo, C.; Loecher, N.; Wong, H.N.; et al. Patient-reported outcome measurement implementation in cancer survivors: A systematic review. J. Cancer Surviv. 2024, 18, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Gatchel, R.J.; McGeary, D.D.; McGeary, C.A.; Lippe, B. Interdisciplinary chronic pain management: Past, present, and future. Am. Psychol. 2014, 69, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Zhao, F.; Brell, J.; Lewis, M.A.; Loprinzi, C.L.; Weiss, M.; Fisch, M.J. Neuropathic symptoms, quality of life, and clinician perception of patient care in medical oncology outpatients with colorectal, breast, lung, and prostate cancer. J. Cancer Surviv. 2015, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.A.; Zhao, F.; Jones, D.; Loprinzi, C.L.; Brell, J.; Weiss, M.; Fisch, M.J. Neuropathic Symptoms and Their Risk Factors in Medical Oncology Outpatients with Colorectal vs. Breast, Lung, or Prostate Cancer: Results from a Prospective Multicenter Study. J. Pain Symptom Manag. 2015, 49, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
| With Pain (n = 130) | Without Pain (n = 148) | p-Value | |
|---|---|---|---|
| Age (years), mean (SD) | 63.9 (11.3) | 63.6 (11.7) | 0.975 2 |
| Gender: male, n (%) | 3 (2.3) | 25 (16.9) | <0.001 3 |
| Gender: female, n (%) | 127 (97.7) | 123 (83.1) | |
| BMI (Kg/m2), mean (SD) | 27.2 (5.3) | 25.7 (3.9) | 0.015 2 |
| Race: Caucasian, n (%) | 118 (90.8) | 133 (92.4) | 0.663 3 |
| Race: Asian, n (%) | 1 (0.8) | 1 (0.7) | |
| Race: Latin-American, n (%) | 9 (6.9) | 10 (6.9) | |
| Race: Middle-Eastern, n (%) | 2 (1.5) | - | |
| Marital status: single, n (%) | 24 (18.5) | 30 (20.3) | 0.932 4 |
| Marital status: married, n (%) | 69 (53.1) | 76 (51.4) | |
| Marital status: divorced, n (%) | 18 (13.9) | 17 (11.5) | |
| Marital status: widow(er), n (%) | 19 (14.6) | 20 (13.5) | |
| Educational attainment: no studies, n (%) | 6 (4.7) | 7 (5.0) | 0.727 4 |
| Educational attainment: secondary, n (%) | 82 (63.6) | 83 (58.9) | |
| Educational attainment: superior, n (%) | 41 (31.8) | 51 (36.2) | |
| Employment status: active, n (%) 1 | 45 (34.62) | 65 (44.22) | 0.103 4 |
| Employment status: inactive, n (%) 1 | 85 (65.38) | 82 (55.78) | |
| Paid employment, n (%) | 32 (24.6) | 55 (37.2) | 0.022 4 |
| Toxic habits: active smoker, n (%) | 20 (15.38) | 22 (14.86) | 0.939 4 |
| Toxic habits: active drinker, n (%) | 13 (10.00) | 22 (14.86) | 0.207 4 |
| Toxic habits: other, n (%) | 1 (0.77) | 1 (0.68) | >0.999 3 |
| Pain-related associated chronic conditions: any, n (%) | 59 (57.84) | 26 (36.11) | <0.001 4 |
| Pain-related assoc. chronic cond.: osteoporosis, n (%) | 26 (44.07) | 15 (57.69) | 0.241 4 |
| Pain-related assoc. chronic cond.: diab. neuropathy, n (%) | 1 (1.69) | - | >0.999 3 |
| No. of patients per pain type: nociceptive somatic, n (%) | 129 (99.2) | NA | NA |
| No. of patients per pain type: nociceptive visceral, n (%) | 2 (1.5) | NA | |
| No. of patients per pain type: neuropathic, n (%) | 57 (43.9) | NA | |
| No. of patients per pain type: mixed, n (%) | 57 (44.2) | NA |
| With Pain (n = 130) | Without Pain (n = 148) | p-Value | |
|---|---|---|---|
| Elapsed time (years) since tumour diagnosis, mean (SD) | 11.8 (5.9) | 11.4 (5.2) | 0.632 5 |
| Age at cancer diagnosis, mean (SD) | 51.9 (12.1) | 52.2 (12.2) | 0.697 5 |
| Number of reported tumours: one, n (%) | 109 (83.9) | 138 (93.2) | 0.013 6 |
| Number of reported tumours: two, n (%) | 21 (16.2) | 10 (6.8) | |
| Types of primary tumour: inv. duct. breast ca., n (%) 1 | 84 (64.6) | 78 (52.7) | 0.211 7 |
| Types of primary tumour: breast carcinoma, n (%) 1 | 7 (5.4) | 8 (5.4) | |
| Types of primary tumour: colon adenocarcinoma, n (%) 1,2 | 4 (3.1) | 7 (4.7) | |
| Types of primary tumour: ductal breast carcinoma, n (%) 1,3 | 4 (3.1) | 6 (4.1) | |
| Types of primary tumour: inv. lob. breast cancer, n (%) 1,3 | 6 (4.6) | 4 (2.7) | |
| Types of primary tumour: colorectal adenocarcinoma, n (%) 1,2 | 2 (1.5) | 7 (4.7) | |
| Types of primary tumour: sigmoid adenocarcinoma, n (%) 1,2 | 1 (0.8) | 4 (2.7) | |
| Types of primary tumour: nodular melanoma, n (%) 1 | 1 (0.8) | 3 (2.0) | |
| Types of primary tumour: invasive breast carcinoma, n (%) 1,3 | - | 3 (2.0) | |
| Types of primary tumour: epidermoid lung carcinoma, n (%) 1 | - | 3 (2.0) | |
| Patients who underwent surgical treatment, n (%) | 128 (98.5) | 145 (98.0) | >0.999 7 |
| No. of surgical interventions required: one, n (%) | 34 (26.6) | 67 (46.2) | 0.001 6 |
| No. of surgical interventions required: two, n (%) | 71 (55.5) | 68 (46.9) | |
| No. of surgical interventions required: three or more, n (%) | 23 (18.0) | 10 (6.9) | |
| Patients who received CT, n (%) | 104 (80.0) | 127 (85.8) | 0.197 6 |
| Number of CT cycles required: one, n (%) | 10 (9.6) | 25 (19.7) | 0.118 6 |
| Number of CT cycles required: two, n (%) | 21 (20.2) | 21 (16.5) | |
| Number of CT cycles required: three, n (%) | 54 (51.9) | 66 (52.0) | |
| Number of CT cycles required: four or more, n (%) | 19 (18.3) | 15 (11.8) | |
| Most common CT delivered: alkylating agents, n (%) 4 | 80 (61.5) | 69 (46.6) | 0.013 6 |
| Most common CT delivered: anthracyclines, n (%) 4 | 80 (61.5) | 64 (43.2) | 0.002 6 |
| Most common CT delivered: antimetabolites, n (%) 4 | 35 (26.9) | 59 (39.9) | 0.023 6 |
| Most common CT delivered: taxanes, n (%) 4 | 56 (43.1) | 50 (33.8) | 0.111 6 |
| Most common CT delivered: aromatase inhibitors, n (%) 4 | 63 (49.6) | 39 (27.3) | <0.001 6 |
| Most common CT delivered: tamoxifen, n (%) 4 | 56 (43.4) | 47 (32.4) | 0.061 6 |
| Most common CT delivered: platinum analogues, n (%) 4 | 12 (9.2) | 36 (24.3) | 0.001 6 |
| Patients who received RT, n (%) | 99 (76.2) | 91 (61.5) | 0.009 6 |
| Number of RT sessions required: one, n (%) | 93 (93.9) | 85 (93.4) | 0.880 6 |
| Number of RT sessions required: two or more, n (%) | 6 (6.1) | 6 (6.6) |
| With Pain (n = 130) | Without Pain (n = 148) | p-Value | |
|---|---|---|---|
| Patients with positive (≥4) DN4 scores (n = 130, 100%), n (%) | 76 (58.9) | NA | - |
| EQ5D: Quality of Life Index (n = 276, 99.3%), median (IQR) 1 | 0.6 (0.4) | 1 (0.2) | <0.001 7 |
| EQ5D: health status score (n = 268, 96.4%), median (IQR) 2 | 70 (30) | 80 (20) | <0.001 7 |
| HADS: anxiety score (n = 278, 100%), median (IQR) 3 | 4.5 (8) | 3 (4) | <0.001 7 |
| HADS: depression score (n = 277, 99.6%), median (IQR) 3 | 3 (7) | 1 (2) | <0.001 7 |
| HADS: total score (n = 277, 99.6%), median (IQR) 4 | 9 (13) | 4 (6) | <0.001 7 |
| PCS: total score (n = 269, 96.8%), median (IQR) 5 | 7 (18) | 1 (2) | <0.001 7 |
| WPAI-GH: cur. paid employment (2022) (n = 278, 100%), n (%) | 29 (22.3) | 51 (34.5) | 0.026 8 |
| WPAI-GH: presenteeism score (n = 80, 100%), median (IQR) 6 | 0 (0) | 0 (0) | 0.561 7 |
| WPAI-GH: disability score (n = 278, 100%), median (IQR) 6 | 0 (40) | 0 (30) | 0.029 7 |
| WPAI-Pain: cur. paid employment (2022) (n = 278, 100%), n (%) | 32 (24.6) | 55 (37.2) | 0.024 8 |
| WPAI-Pain: disability score (n = 277, 99.6%), median (IQR) | 50 (70) | 0 (0) | <0.001 7 |
| Symptoms associated to pain: insomnia (n = 277, 99.6%), n (%) | 84 (65.1) | 56 (37.8) | <0.001 8 |
| Symptoms associated to pain: fatigue (n = 277, 99.6%), n (%) | 75 (58.1) | 56 (38.1) | 0.001 8 |
| Estimate 1 | Lower 95% CI Limit 1 | Upper 95% CI Limit 1 | p-Value | |
|---|---|---|---|---|
| Intercept | −1.26 | −4.41 | 1.89 | 0.432 |
| Age, change per additional year | −0.02 | −0.05 | 0.01 | 0.243 |
| Male gender | −2.60 | −4.29 | −0.92 | 0.002 |
| BMI, change per 1-unit (kg/m2) increase | 0.07 | 0.01 | 0.14 | 0.029 |
| Being married | 0.12 | −0.48 | 0.73 | 0.688 |
| Having superior studies | 0.22 | −0.44 | 0.89 | 0.513 |
| Smoking or routine alcohol intake | 0.08 | −0.63 | 0.80 | 0.820 |
| Having any chronic disease | 1.43 | 0.78 | 2.08 | <0.001 |
| Time since cancer onset, chg. per year | 0.02 | −0.03 | 0.08 | 0.430 |
| Having had breast cancer | −1.49 | −2.89 | −0.08 | 0.038 |
| Having had advanced cancer | −0.51 | −1.37 | 0.36 | 0.251 |
| Two surgeries (vs. one or none) | 0.49 | −0.19 | 1.17 | 0.157 |
| Three or more surgeries (vs. one or none) | 1.91 | 0.81 | 3.01 | 0.001 |
| One or two chemotherapies (vs. none) | −1.08 | −2.30 | 0.15 | 0.085 |
| Three or more chemotherapies (vs. none) | −1.45 | −3.00 | 0.09 | 0.065 |
| Radiotherapy | 0.52 | −0.21 | 1.24 | 0.161 |
| Vinca alkaloids or platinum compounds | −0.09 | −1.44 | 1.26 | 0.896 |
| Taxanes | 0.12 | −0.63 | 0.86 | 0.755 |
| Anthracyclines | 0.72 | −0.85 | 2.29 | 0.370 |
| Nitrogen mustards | −0.01 | −0.85 | 2.29 | 0.370 |
| Aromatase inhibitors | 0.75 | 0.04 | 1.46 | 0.038 |
| Estimate 1 | Lower 95% CI Limit 1 | Upper 95% CI Limit 1 | p-Value | |
|---|---|---|---|---|
| Intercept | 0.26 | −4.33 | 4.87 | 0.909 |
| Age, change per additional year | −0.05 | −0.10 | 0.01 | 0.029 |
| Male gender | −1.11 | −4.21 | 2.00 | 0.489 |
| BMI, change per 1-unit (kg/m2) increase | 0.12 | 0.03 | 0.21 | 0.010 |
| Being married | 0.63 | −0.30 | 1.56 | 0.185 |
| Having any chronic disease | 0.01 | −1.14 | 1.17 | 0.987 |
| Time since cancer onset, chg. per year | −0.03 | −0.12 | 0.07 | 0.631 |
| Having had breast cancer | −1.84 | −4.04 | 0.36 | 0.101 |
| Having had advanced cancer | 0.47 | −0.90 | 1.84 | 0.501 |
| Two surgeries (vs. one or none) | 0.95 | −0.20 | 2.09 | 0.105 |
| Three or more surgeries (vs. one or none) | 1.51 | <0.01 | 3.01 | 0.049 |
| One or two chemotherapies (vs. none) | 1.22 | −0.81 | 3.24 | 0.239 |
| Three or more chemotherapies (vs. none) | 1.69 | −0.82 | 4.20 | 0.187 |
| Radiotherapy | 0.85 | −0.44 | 2.13 | 0.198 |
| Vinca alkaloids or platinum compounds | −0.97 | −3.14 | 1.20 | 0.381 |
| Taxanes | −0.33 | −1.49 | 0.83 | 0.578 |
| Anthracyclines | 0.59 | −2.31 | 3.49 | 0.692 |
| Nitrogen mustards | −1.70 | −4.77 | 1.36 | 0.277 |
| Aromatase inhibitors | 0.16 | −0.90 | 1.23 | 0.757 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, C.; Ochoa, D.; Sánchez, N.; Ballesteros, A.I.; Santidrián, S.; López, I.; Mondéjar, R.; Carnaval, T.; Villoria, J.; Colomer, R. Pain in Long-Term Cancer Survivors: Prevalence and Impact in a Cohort Composed Mostly of Breast Cancer Survivors. Cancers 2024, 16, 1581. https://doi.org/10.3390/cancers16081581
Pérez C, Ochoa D, Sánchez N, Ballesteros AI, Santidrián S, López I, Mondéjar R, Carnaval T, Villoria J, Colomer R. Pain in Long-Term Cancer Survivors: Prevalence and Impact in a Cohort Composed Mostly of Breast Cancer Survivors. Cancers. 2024; 16(8):1581. https://doi.org/10.3390/cancers16081581
Chicago/Turabian StylePérez, Concepción, Dolores Ochoa, Noelia Sánchez, Ana Isabel Ballesteros, Sheila Santidrián, Isabel López, Rebeca Mondéjar, Thiago Carnaval, Jesús Villoria, and Ramón Colomer. 2024. "Pain in Long-Term Cancer Survivors: Prevalence and Impact in a Cohort Composed Mostly of Breast Cancer Survivors" Cancers 16, no. 8: 1581. https://doi.org/10.3390/cancers16081581
APA StylePérez, C., Ochoa, D., Sánchez, N., Ballesteros, A. I., Santidrián, S., López, I., Mondéjar, R., Carnaval, T., Villoria, J., & Colomer, R. (2024). Pain in Long-Term Cancer Survivors: Prevalence and Impact in a Cohort Composed Mostly of Breast Cancer Survivors. Cancers, 16(8), 1581. https://doi.org/10.3390/cancers16081581






