Early Stage Breast Cancer: Does Histologic Subtype (Ductal vs. Lobular) Impact 5 Year Overall Survival?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient and Tumor Characteristics
3.2. Overall Survival
3.3. Cox Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 24 August 2023).
- American Cancer Society. Key Statistics for Breast Cancer. Available online: https://www.cancer.org/cancer/types/breast-cancer/about/how-common-is-breast-cancer.html (accessed on 24 August 2023).
- National Cancer Institute. Cancer Stat Facts: Female Breast Cancer. Available online: https://seer.cancer.gov/statfacts/html/breast.html (accessed on 24 August 2023).
- Makki, J. Diversity of breast carcinoma: Histological subtypes and clinical relevance. Clin. Med. Insights Pathol. 2015, 8, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Christgen, M.; Cserni, G.; Floris, G.; Marchio, C.; Djerroudi, L.; Kreipe, H.; Derksen, P.W.B.; Vincent-Salomon, A. Lobular Breast Cancer: Histomorphology and Different Concepts of a Special Spectrum of Tumors. Cancers 2021, 13, 3695. [Google Scholar] [CrossRef] [PubMed]
- Pramod, N.; Nigam, A.; Basree, M.; Mawalkar, R.; Mehra, S.; Shinde, N.; Tozbikian, G.; Williams, N.; Majumder, S.; Ramaswamy, B. Comprehensive review of molecular mechanisms and clinical features of invasive lobular cancer. Oncologist 2021, 26, e943–e953. [Google Scholar] [CrossRef] [PubMed]
- Ruibal, A.; Aguiar, P.; Río, M.D.C.D.; Menéndez, P.; Arias, J.I.; Herranz, M. Clinicopathological characteristics of infiltrating lobular breast carcinoma in elderly women: Preliminary results. Mol. Clin. Oncol. 2015, 3, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Arpino, G.; Bardou, V.J.; Clark, G.M.; Elledge, R.M. Infiltrating lobular carcinoma of the breast: Tumor characteristics and clinical outcome. Breast Cancer Res. 2004, 6, R149–R156. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, E.; Caraceni, G.; Gentile, D.; Gavazzi, F.; Zerbi, A.; Tinterri, C. A Rare Case of Duodenal Metastasis from Lobular Breast Cancer: From Diagnosis to Surgery. Case Rep. Oncol. 2023, 16, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Daniels, I.R.; Layer, G.T.; Chisholm, E.M. Bowel obstruction due to extrinsic compression by metastatic lobular carcinoma of the breast. J. R. Soc. Promot. Health 2002, 122, 61–62. [Google Scholar] [CrossRef] [PubMed]
- Bratthauer, G.L.; Miettinen, M.; Tavassoli, F.A. Cytokeratin immunoreactivity in lobular intraepithelial neoplasia. J. Histochem. Cytochem. 2003, 51, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lei, C.; Zhang, Y.; Zhang, J.; Ji, F.; Pan, W.; Zhang, L.; Gao, H.; Yang, M.; Li, J.; et al. Comparison of Overall Survival Between Invasive Lobular Breast Carcinoma and Invasive Ductal Breast Carcinoma: A Propensity Score Matching Study Based on SEER Database. Front. Oncol. 2020, 10, 590643. [Google Scholar] [CrossRef] [PubMed]
- Li, C.I.; Anderson, B.O.; Porter, P.; Holt, S.K.; Daling, J.R.; Moe, R.E. Changing incidence rate of invasive lobular breast carcinoma among older women. Cancer 2000, 88, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Oesterreich, S.; Nasrazadani, A.; Zou, J.; Carleton, N.; Onger, T.; Wright, M.D.; Li, Y.; Demanelis, K.; Ramaswamy, B.; Tseng, G.; et al. Clinicopathological Features and Outcomes Comparing Patients With Invasive Ductal and Lobular Breast Cancer. JNCI J. Natl. Cancer Inst. 2022, 114, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Dian, D.; Herold, H.; Mylonas, I.; Scholz, C.; Janni, W.; Sommer, H.; Friese, K. Survival analysis between patients with invasive ductal and invasive lobular breast cancer. Arch. Gynecol. Obstet. 2009, 279, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Wasif, N.; Maggard, M.A.; Ko, C.Y.; Giuliano, A.E. Invasive Lobular vs. Ductal Breast Cancer: A Stage-Matched Comparison of Outcomes. Ann. Surg. Oncol. 2010, 17, 1862–1869. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Ishiguro, J.; Kotani, H.; Hisada, T.; Ichikawa, M.; Gondo, N.; Yoshimura, A.; Kondo, N.; Hattori, M.; Sawaki, M.; et al. Comparison of clinical outcomes between luminal invasive ductal carcinoma and luminal invasive lobular carcinoma. BMC Cancer 2016, 16, 248. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, L.; Mascaro, A.; Poccia, I.; Andrich, R.; Amini, M.; Costarelli, L.; Cortese, G.; Farina, M.; Vitelli, C. Lobular Breast Cancer: Same Survival and Local Control Compared with Ductal Cancer, but Should Both Be Treated the Same Way? Analysis of an Institutional Database over a 10-Year Period. Ann. Surg. Oncol. 2012, 19, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, T.; Kuukasjärvi, T.; Huhtala, H.; Alarmo, E.-L.; Holli, K.; Kallioniemi, A.; Pylkkänen, L. The impact of lobular and ductal breast cancer histology on the metastatic behavior and long term survival of breast cancer patients. Breast 2013, 22, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Batra, H.; Mouabbi, J.A.; Ding, Q.; Sahin, A.A.; Raso, M.G. Lobular Carcinoma of the Breast: A Comprehensive Review with Translational Insights. Cancers 2023, 15, 5491. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; El-Sayed, M.E.; Powe, D.G.; Green, A.R.; Habashy, H.; Grainge, M.J.; Robertson, J.F.; Blamey, R.; Gee, J.; Nicholson, R.I.; et al. Invasive lobular carcinoma of the breast: Response to hormonal therapy and outcomes. Eur. J. Cancer 2008, 44, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.I.; Jeong, J.; Lee, S.; Ryu, J.M.; Lee, Y.J.; Lee, J.Y.; Hwang, K.-T.; Kim, H.; Kim, S.; Lee, S.B.; et al. Survival Outcomes in Premenopausal Patients With Invasive Lobular Carcinoma. JAMA Netw. Open 2023, 6, e2342270. [Google Scholar] [CrossRef] [PubMed]
- Aleshire, M.E.; Adegboyega, A.; Escontrías, O.A.; Edward, J.; Hatcher, J. Access to Care as a Barrier to Mammography for Black Women. Policy Polit. Nurs. Pract. 2021, 22, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Baquet, C.R.; Mishra, S.I.; Commiskey, P.; Ellison, G.L.; DeShields, M. Breast Cancer Epidemiology in Blacks and Whites: Disparities in Incidence, Mortality, Survival Rates and Histology. J. Natl. Med. Assoc. 2008, 100, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, L.A.; Shriver, C.D.; Haricharan, S.; Ellsworth, R.E. Survival Disparities in US Black Compared to White Women with Hormone Receptor Positive-HER2 Negative Breast Cancer. Int. J. Environ. Res. Public Health 2023, 20, 2903. [Google Scholar] [CrossRef] [PubMed]
- Sukniam, K.; Kasbi, A.A.; Ashary, M.A.; Popp, K.; Attwood, K.; George, A.; Gabriel, E. Disparities in Time to Treatment for Breast Cancer. Anticancer Res. 2022, 42, 5813–5818. [Google Scholar] [CrossRef] [PubMed]
- Smith-Gagen, J.; Carrillo, J.E.; Ang, A.; Pérez-Stable, E.J. Practices That Reduce the Latina Survival Disparity after Breast Cancer. J. Womens Health 2013, 22, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Yedjou, C.G.; Tchounwou, P.B.; Payton, M.; Miele, L.; Fonseca, D.D.; Lowe, L.; Alo, R.A. Assessing the Racial and Ethnic Disparities in Breast Cancer Mortality in the United States. Int. J. Environ. Res. Public Health 2017, 14, 486. [Google Scholar] [CrossRef]
- Ooi, S.L.; Martinez, M.E.; Li, C.I. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res. Treat. 2011, 127, 729–738. [Google Scholar] [CrossRef]
- Denu, R.A. Reported Biologic Differences in Breast Cancer by Race Due to Disparities in Screening. JAMA Oncol. 2018, 4, 883. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.M.; Cole, S.R.; Tse, C.-K.; Perou, C.M.; Carey, L.A.; Foulkes, W.D.; Dressler, L.G.; Geradts, J.; Millikan, R.C. Intrinsic Breast Tumor Subtypes, Race, and Long-Term Survival in the Carolina Breast Cancer Study. Clin. Cancer Res. 2010, 16, 6100–6110. [Google Scholar] [CrossRef] [PubMed]
- Tinterri, C.; Canavese, G.; Gentile, D. To Dissect or Not to Dissect? The Surgeon’s Perspective on the Prediction of Greater Than or Equal to 4 Axillary Lymph Node Metastasis in Early-Stage Breast Cancer: A Comparative Analysis of the Per-Protocol Population of the SINODAR-ONE Clinical Trial. Ann. Surg. Open 2024, 5, e405. [Google Scholar] [CrossRef]
- Rossi, L.; Stevens, D.; Pierga, J.-Y.; Lerebours, F.; Reyal, F.; Robain, M.; Asselain, B.; Rouzier, R. Impact of Adjuvant Chemotherapy on Breast Cancer Survival: A Real-World Population. PLoS ONE 2015, 10, e0132853. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.A.; Cronin, K.A.; Plevritis, S.K.; Fryback, D.G.; Clarke, L.; Zelen, M.; Mandelblatt, J.S.; Yakovlev, A.Y.; Habbema, J.D.F.; Feuer, E.J. Effect of Screening and Adjuvant Therapy on Mortality from Breast Cancer. N. Engl. J. Med. 2005, 353, 1784–1792. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [Google Scholar] [CrossRef] [PubMed]
- Truin, W.; Voogd, A.C.; Vreugdenhil, G.; Heiden-van der Loo, M.V.D.; Siesling, S.; Roumen, R.M. Effect of adjuvant chemotherapy in postmenopausal patients with invasive ductal versus lobular breast cancer. Ann. Oncol. 2012, 23, 2859–2865. [Google Scholar] [CrossRef] [PubMed]
- Pestalozzi, B.C.; Zahrieh, D.; Mallon, E.; Gusterson, B.A.; Price, K.N.; Gelber, R.D.; Holmberg, S.B.; Lindtner, J.; Snyder, R.; Thürlimann, B.; et al. Distinct Clinical and Prognostic Features of Infiltrating Lobular Carcinoma of the Breast: Combined Results of 15 International Breast Cancer Study Group Clinical Trials. J. Clin. Oncol. 2008, 26, 3006–3014. [Google Scholar] [CrossRef] [PubMed]
- Chamalidou, C.; Fohlin, H.; Albertsson, P.; Arnesson, L.-G.; Einbeigi, Z.; Holmberg, E.; Nordenskjöld, A.; Nordenskjöld, B.; Karlsson, P.; Linderholm, B. Survival patterns of invasive lobular and invasive ductal breast cancer in a large population-based cohort with two decades of follow up. Breast 2021, 59, 294–300. [Google Scholar] [CrossRef]
| Ductal | Lobular | Overall | p-Value | ||
|---|---|---|---|---|---|
| 8678 | 2407 | 11,085 | |||
| Age | <55 | 2389 (27.53%) | 595 (24.72%) | 2984 (26.92%) | 0.002 |
| 55–65 | 2636 (30.38%) | 696 (28.92%) | 3332 (30.06%) | ||
| >65 | 3653 (42.09%) | 1116 (46.36%) | 4769 (43.02%) | ||
| Race | White | 7337 (85.93%) | 2102 (88.80%) | 9439 (86.56%) | 0.005 |
| Black | 926 (10.85%) | 211 (8.914%) | 1137 (10.43%) | ||
| Native American | 31 (0.363%) | 5 (0.211%) | 36 (0.330%) | ||
| Asian | 244 (2.858%) | 49 (2.070%) | 293 (2.687%) | ||
| Hispanic | No | 8035 (94.93%) | 2233 (94.98%) | 10,268 (94.94%) | 0.96 |
| Yes | 429 (5.069%) | 118 (5.019%) | 547 (5.058%) | ||
| Facility | Community Center | 946 (10.90%) | 240 (9.971%) | 1186 (10.70%) | 0.28 |
| Comprehensive Community Center | 4145 (47.76%) | 1136 (47.20%) | 5281 (47.64%) | ||
| Academic Center | 2160 (24.89%) | 640 (26.59%) | 2800 (25.26%) | ||
| Other | 1427 (16.44%) | 391 (16.24%) | 1818 (16.40%) | ||
| Grade | 1 | 1681 (20.15%) | 543 (23.66%) | 2224 (20.91%) | <0.001 |
| 2 | 4104 (49.19%) | 1459 (63.57%) | 5563 (52.29%) | ||
| 3 | 2558 (30.66%) | 293 (12.77%) | 2851 (26.80%) | ||
| T Clinical | T1 | 5750 (66.26%) | 1556 (64.64%) | 7306 (65.91%) | 0.15 |
| T2 | 2928 (33.74%) | 851 (35.36%) | 3779 (34.09%) | ||
| Lymphovascular Invasion | No | 4609 (61.14%) | 1507 (72.04%) | 6116 (63.50%) | <0.001 |
| Yes | 2930 (38.86%) | 585 (27.96%) | 3515 (36.50%) | ||
| ER Status | Negative | 1074 (12.43%) | 86 (3.588%) | 1160 (10.51%) | <0.001 |
| Positive | 7564 (87.57%) | 2311 (96.41%) | 9875 (89.49%) | ||
| PR Status | Negative | 1728 (20.03%) | 315 (13.17%) | 2043 (18.54%) | <0.001 |
| Positive | 6901 (79.97%) | 2077 (86.83%) | 8978 (81.46%) | ||
| Her2 Status | Negative | 5700 (85.88%) | 1563 (83.99%) | 7263 (85.47%) | 0.044 |
| Positive | 937 (14.12%) | 298 (16.01%) | 1235 (14.53%) | ||
| Nodes Positive | Mean Median/IQR | 1.195 1.0 (1.0, 1.0) | 1.25 1.0 (1.0, 1.0) | 1.207 1.0 (1.0, 1.0) | <0.001 |
| Surgery | Lumpectomy | 5853 (67.47%) | 1375 (57.13%) | 7228 (65.22%) | <0.001 |
| Mastectomy | 2803 (32.31%) | 1029 (42.75%) | 3832 (34.58%) | ||
| None | 19 (0.219%) | 3 (0.125%) | 22 (0.199%) | ||
| Radiation | Adjuvant | 5807 (66.92%) | 1582 (65.72%) | 7389 (66.66%) | 0.25 |
| Intraoperative | 58 (0.668%) | 10 (0.416%) | 68 (0.613%) | ||
| Neoadjuvant | 9 (0.104%) | 5 (0.208%) | 14 (0.126%) | ||
| Perioperative | 10 (0.115%) | 1 (0.042%) | 11 (0.099%) | ||
| None | 2692 (31.02%) | 783 (32.53%) | 3475 (31.35%) | ||
| Unknown | 102 (1.175%) | 26 (1.080%) | 128 (1.155%) | ||
| Chemotherapy | Adjuvant | 6966 (80.27%) | 2004 (83.26%) | 8970 (80.92%) | <0.001 |
| Neoadjuvant | 235 (2.708%) | 52 (2.160%) | 287 (2.589%) | ||
| Perioperative | 194 (2.236%) | 54 (2.243%) | 248 (2.237%) | ||
| None | 829 (9.553%) | 218 (9.057%) | 1047 (9.445%) | ||
| Unknown | 454 (5.232%) | 79 (3.282%) | 533 (4.808%) | ||
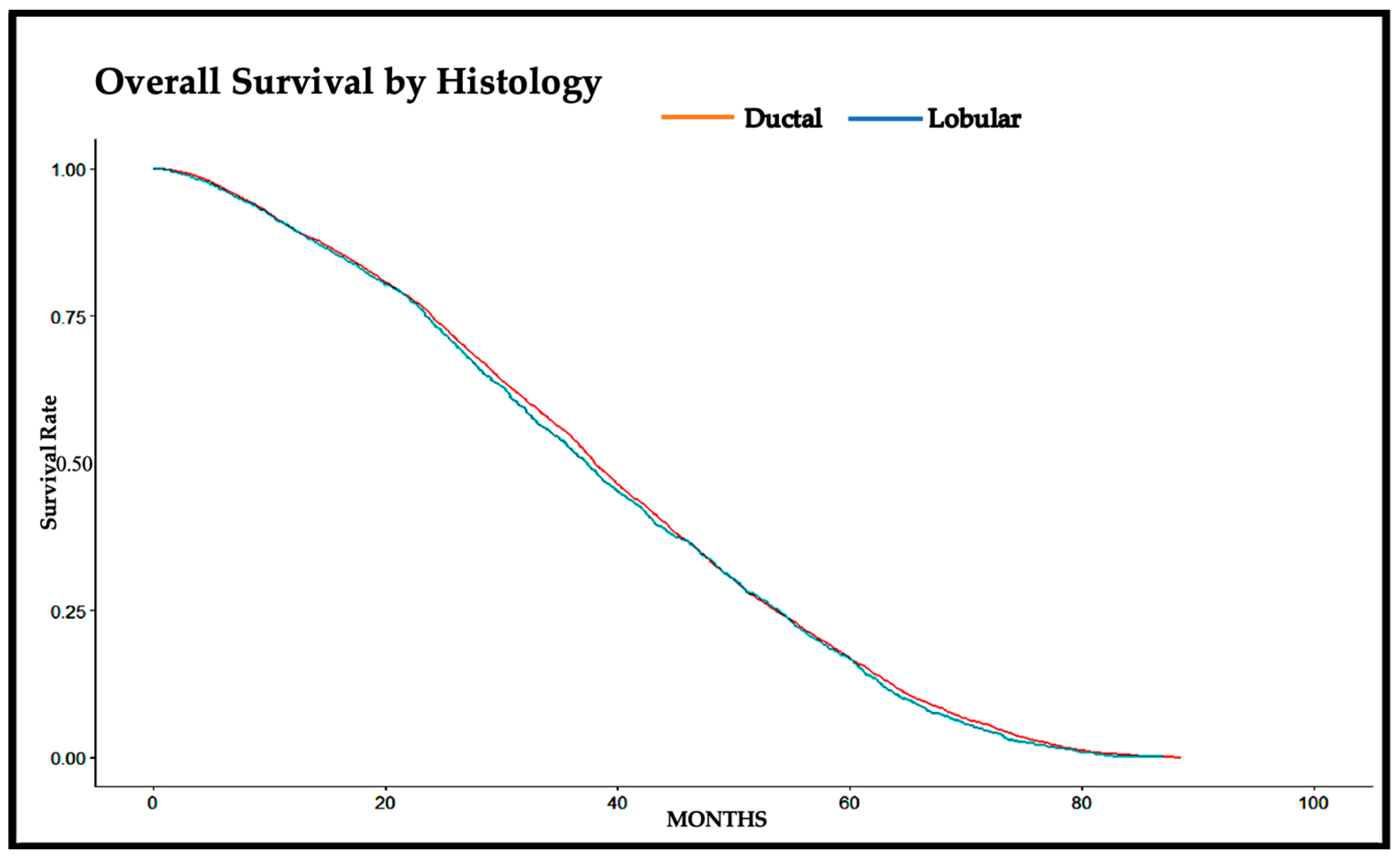
| Median (Months) | 0.95 LCL | 0.95 UCL | 5 Yr Survival Rate | p-Value | |
|---|---|---|---|---|---|
| Ductal | 38.05 | 37.59 | 38.74 | 0.168 | 0.20 |
| Lobular | 37.39 | 36.11 | 38.47 | 0.167 |
| Feature | Hazard Ratio | 95% CI | p-Value | |
|---|---|---|---|---|
| Histology | Ductal | 1.0 | ||
| Lobular | 1.026 | 0.96–1.08 | 0.40 | |
| Age | <55 | 1.0 | ||
| 55–65 | 1.047 | 0.98–1.11 | 0.15 | |
| >65 | 0.9805 | 0.91–1.05 | 0.61 | |
| Race | White | 1.0 | ||
| Black | 1.106 | 1.01–1.20 | 0.019 | |
| Native American | 1.169 | 0.75–1.79 | 0.48 | |
| Asian | 1.413 | 1.23–1.62 | <0.001 | |
| Hispanic | No | 1.0 | ||
| Yes | 1.175 | 1.05–1.31 | 0.004 | |
| Facility | Community Center | 1.0 | ||
| Comprehensive Community Center | 0.8357 | 0.77–0.90 | <0.001 | |
| Academic Center | 0.7694 | 0.70–0.84 | <0.001 | |
| Other | 0.7742 | 0.70–0.85 | <0.001 | |
| Grade | 1 | 1.0 | ||
| 2 | 1.006 | 0.94–1.06 | 0.86 | |
| 3 | 0.9014 | 0.83–0.97 | 0.006 | |
| T Clinical | T1 | 1.0 | ||
| T2 | 1.03 | 0.97–1.08 | 0.28 | |
| Surgery | Lumpectomy | 1.0 | ||
| Mastectomy | 0.9613 | 0.89–1.02 | 0.24 | |
| None | 1.013 | 0.52–1.96 | 0.97 | |
| Radiation | No | 1.0 | ||
| Yes | 0.9801 | 0.91–1.05 | 0.57 | |
| Chemotherapy | No | 1.0 | ||
| Yes | 0.8528 | 0.77–0.93 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mubarak, F.; Kowkabany, G.; Popp, R.; Bansal, S.; Ahmed, S.H.; Sharan, S.; Sukniam, K.B.; Raikot, S.R.; Jimenez, P.B.; Popp, K.; et al. Early Stage Breast Cancer: Does Histologic Subtype (Ductal vs. Lobular) Impact 5 Year Overall Survival? Cancers 2024, 16, 1509. https://doi.org/10.3390/cancers16081509
Mubarak F, Kowkabany G, Popp R, Bansal S, Ahmed SH, Sharan S, Sukniam KB, Raikot SR, Jimenez PB, Popp K, et al. Early Stage Breast Cancer: Does Histologic Subtype (Ductal vs. Lobular) Impact 5 Year Overall Survival? Cancers. 2024; 16(8):1509. https://doi.org/10.3390/cancers16081509
Chicago/Turabian StyleMubarak, Fatima, Gabrielle Kowkabany, Reed Popp, Shivam Bansal, Syeda Hoorulain Ahmed, Seema Sharan, Kulkaew B. Sukniam, Swathi R. Raikot, Paola Berrios Jimenez, Kyle Popp, and et al. 2024. "Early Stage Breast Cancer: Does Histologic Subtype (Ductal vs. Lobular) Impact 5 Year Overall Survival?" Cancers 16, no. 8: 1509. https://doi.org/10.3390/cancers16081509
APA StyleMubarak, F., Kowkabany, G., Popp, R., Bansal, S., Ahmed, S. H., Sharan, S., Sukniam, K. B., Raikot, S. R., Jimenez, P. B., Popp, K., Manaise, H. K., & Gabriel, E. (2024). Early Stage Breast Cancer: Does Histologic Subtype (Ductal vs. Lobular) Impact 5 Year Overall Survival? Cancers, 16(8), 1509. https://doi.org/10.3390/cancers16081509







