Patterns of First Recurrence and Oncological Outcomes in Locally Advanced Cervical Cancer Patients: Does Surgical Staging Play a Role?
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Aortic Lymph Node Assessment and Primary Treatment
2.2. Recurrence Diagnosis
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Summary of Main Results
4.2. Results in the Context of the Published Literature
4.3. Strengths and Weaknesses
4.4. Implications for Practice and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Comission ECIS—European Cancer Information System. Available online: https://ecis.jrc.ec.europa.eu/ (accessed on 19 March 2024).
- Falcaro, M.; Castañon, A.; Ndlela, B.; Checchi, M.; Soldan, K.; Lopez-Bernal, J.; Elliss-Brookes, L.; Sasieni, P. The Effects of the National HPV Vaccination Programme in England, UK, on Cervical Cancer and Grade 3 Cervical Intraepithelial Neoplasia Incidence: A Register-Based Observational Study. Lancet 2021, 8, 2084–2092. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Zanetti, R.; Ferlay, J. (Eds.) Cancer Incidence in Five Continents; International Agency for Research on Cancer: Lyon, France, 2023; Volume XI. Available online: https://ci5.iarc.who.int (accessed on 5 December 2021).
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the Cervix Uteri: 2021 Update. Int. J. Gynecol. Obstet. 2021, 155, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie Meder, C.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients With Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Pötter, R.; Tanderup, K.; Kirisits, C.; de Leeuw, A.; Kirchheiner, K.; Nout, R.; Tan, L.T.; Haie-Meder, C.; Mahantshetty, U.; Segedin, B.; et al. The EMBRACE II Study: The Outcome and Prospect of Two Decades of Evolution within the GEC-ESTRO GYN Working Group and the EMBRACE Studies. Clin. Transl. Radiat. Oncol. 2018, 9, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Marnitz, S.; Tsunoda, A.T.; Martus, P.; Vieira, M.; Affonso Junior, R.J.; Nunes, J.; Budach, V.; Hertel, H.; Mustea, A.; Sehouli, J.; et al. Surgical versus Clinical Staging Prior to Primary Chemoradiation in Patients with Cervical Cancer FIGO Stages IIB-IVA: Oncologic Results of a Prospective Randomized International Multicenter (Uterus-11) Intergroup Study. Int. J. Gynecol. Cancer 2020, 30, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Feijoo, B.; Torné, A.; Tejerizo, Á.; Benito, V.; Hernández, A.; Ruiz, R.; Domingo, S.; Luna-Guibourg, R.; Llueca, A.; Coronado, P.; et al. Prognostic Value and Therapeutic Implication of Laparoscopic Extraperitoneal Paraaortic Staging in Locally Advanced Cervical Cancer: A Spanish Multicenter Study. Ann. Surg. Oncol. 2020, 27, 2829–2839. [Google Scholar] [CrossRef]
- Pecorelli, S. Revised FIGO Staging for Carcinoma of the Vulva, Cervix, and Endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Feijoo, B.; Acosta, Ú.; Torné, A.; Gil-Ibáñez, B.; Hernández, A.; Domingo, S.; Gil-Moreno, A. Laparoscopic Debulking of Enlarged Pelvic Nodes during Surgical Para-Aortic Staging in Locally Advanced Cervical Cancer: A Retrospective Comparative Cohort Study. J. Minim. Invasive Gynecol. 2022, 29, 103–113. [Google Scholar] [CrossRef] [PubMed]
- de Foucher, T.; Bendifallah, S.; Ouldamer, L.; Bricou, A.; Lavoue, V.; Varinot, J.; Canlorbe, G.; Carcopino, X.; Raimond, E.; Monnier, L.; et al. Patterns of Recurrence and Prognosis in Locally Advanced FIGO Stage IB2 to IIB Cervical Cancer: Retrospective Multicentre Study from the FRANCOGYN Group. Eur. J. Surg. Oncol. 2019, 45, 659–665. [Google Scholar] [CrossRef]
- Yang, J.; Yang, J.; Cao, D.; Shen, K.; Ma, J.; Zhang, F. Completion Hysterectomy after Chemoradiotherapy for Locally Advanced Adeno-Type Cervical Carcinoma: Updated Survival Outcomes and Experience in Post Radiation Surgery. J. Gynecol. Oncol. 2020, 31, e16. [Google Scholar] [CrossRef]
- Gennigens, C.; De Cuypere, M.; Seidel, L.; Hermesse, J.; Barbeaux, A.; Forget, F.; Albert, A.; Jerusalem, G.; Kridelka, F. Correlation between Hematological Parameters and Outcome in Patients with Locally Advanced Cervical Cancer Treated by Concomitant Chemoradiotherapy. Cancer Med. 2020, 9, 8432–8443. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Huang, K.G.; Hong, J.H.; Lee, C.L.; Chou, H.H.; Chang, T.C.; Hsueh, S.; Huang, H.J.; Ng, K.K.; Tsai, C.S. Randomized Trial of Surgical Staging (Extraperitoneal or Laparoscopic) versus Clinical Staging in Locally Advanced Cervical Cancer. Gynecol. Oncol. 2003, 89, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Pomel, C.; Martinez, A.; Bourgin, C.; Beguinot, M.; Benoit, C.; Naik, R.; Dauplat, J.; Lebouedec, G.; Ferron, G. Survival Effect of Laparoscopic Para-Aortic Staging in Locally Advanced Cervical Cancer: A Retrospective Cohort Analysis. BJOG 2017, 124, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Dabi, Y.; Bendifallah, S.; Kolanska, K.; Boudy, A.S.; Querleu, D.; Akladios, C.; Zilberman, S.; Darai, E.; Touboul, C. Anatomical Basis of Lymph Node Detection in Gynecologic Cancers: A Review from a Surgical Perspective. Chin. Clin. Oncol. 2021, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Delara, R.; Magrina, J.; Magtibay, P.; Yi, J.; Langstraat, C.; Robertson, M.; Dinh, T.; Butler, K. Comparing Survival Outcomes between Surgical and Radiographic Lymph Node Assessment in Locally Advanced Cervical Cancer: A Propensity Score-Matched Analysis. Gynecol. Oncol. 2020, 156, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, Y.; Leblanc, E.; Querleu, D.; Castelain, B.; Papageorgiou, T.H.; Lambaudie, E.; Narducci, F. Prospective Evaluation of Surgical Staging of Advanced Cervical Cancer via a Laparoscopic Extraperitoneal Approach. Gynecol. Oncol. 2003, 91, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Bebia, V.; Monreal-Clua, S.; Pérez-Benavente, A.; Franco-Camps, S.; Díaz-Feijoo, B.; Gil-Moreno, A. Potential Strategies for Prevention of Tumor Spillage in Minimally Invasive Radical Hysterectomy. J. Gynecol. Oncol. 2020, 31, e73. [Google Scholar] [CrossRef] [PubMed]
- Gouy, S.; Seebacher, V.; Chargari, C.; Terroir, M.; Grimaldi, S.; Ilenko, A.; Maulard, A.; Genestie, C.; Leary, A.; Pautier, P.; et al. False Negative Rate at 18F-FDG PET/CT in Para-Aortic Lymphnode Involvement in Patients with Locally Advanced Cervical Cancer: Impact of PET Technology. BMC Cancer 2021, 21, 135. [Google Scholar] [CrossRef]
- Martinez, A.; Voglimacci, M.; Lusque, A.; Ducassou, A.; Gladieff, L.; Dupuis, N.; Angeles, M.A.; Martinez, C.; Tanguy Le Gac, Y.; Chantalat, E.; et al. Tumour and Pelvic Lymph Node Metabolic Activity on FDG-PET/CT to Stratify Patients for Para-Aortic Surgical Staging in Locally Advanced Cervical Cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1252–1260. [Google Scholar] [CrossRef]
- Martinez, A.; Lecuru, F.; Bizzarri, N.; Chargari, C.; Ducassou, A.; Fagotti, A.; Fanfani, F.; Scambia, G.; Cibula, D.; Feijoo, B.D.; et al. AOrtic LymphAdenectomy in Locally Advanced Cervical Cancer (PAROLA Trial): A GINECO, ENGOT, and GCIG Study. Int. J. Gynecol. Cancer 2023, 33, 293–298. [Google Scholar] [CrossRef] [PubMed]

| Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | No PALND (n = 288) | PALND (n = 634) | Total (n = 922) | p Value | No PALND (n = 546) | PALND (n = 546) | Total (n = 1092) | p Value |
| Median (IQR) or n(%) | Mean (SD), Median (IQR) or n(%) | |||||||
| Age (years) | 54 (45–61) | 49 (41–58) | 51 (42–60) | <0.001 | 47 (43–61) | 49 (41–59) | 48 (41–60) | 0.493 |
| Size (mm) | 50 (44–65) | 50 (40–55) | 50 (40–60) | <0.001 | 50 (35–60) | 50 (40–57) | 50 (40–60) | 0.754 |
| Histological subtype | 0.690 | 0.094 | ||||||
| Squamous | 223 (77.4) | 514 (81.1) | 737 (79.9) | 447 (81.9) | 442 (81.0) | 889 (81.4) | ||
| Adenocarcinoma | 48 (16.7) | 90 (14.2) | 138 (15.0) | 86 (15.8) | 77 (14.1) | 163 (14.9) | ||
| Adenosquamous | 8 (2.8) | 15 (2.4) | 23 (2.5) | 3 (0.5) | 13 (2.4) | 16 (1.5) | ||
| Undifferentiated | 7 (2.4) | 10 (1.6) | 17 (1.8) | 7 (1.3) | 10 (1.8) | 17 (1.6) | ||
| Other | 2 (0.7) | 5 (0.8) | 7 (0.8) | 3 (0.5) | 4 (0.7) | 7 (0.6) | ||
| FIGO 2009 Staging | <0.001 | 0.845 | ||||||
| IB2—IIA2 | 38 (13.2) | 187 (29.5) | 225 (24.4) | 173 (31.7) | 169 (31.0) | 342 (31.3) | ||
| IIB—IVA | 250 (86.8) | 447 (70.5) | 697 (75.6) | 373 (68.3) | 377 (69.0) | 750 (68.7) | ||
| Nodal status | 0.171 | 0.199 | ||||||
| N0 | 183 (63.8) | 422 (67.1) | 605 (66.0) | 351 (64.3) | 378 (69.2) | 729 (66.8) | ||
| N1 | 100 (34.8) | 189 (30.0) | 289 (31.6) | 177 (32.4) | 150 (27.5) | 327 (29.9) | ||
| Nx | 4 (1.4) | 18 (2.9) | 22 (2.4) | 18 (3.3) | 18 (3.3) | 36 (3.3) | ||
| Radiotherapy initial volume | <0.001 | 0.936 | ||||||
| Pelvic | 118 (41.0) | 489 (77.1) | 607 (65.8) | 450 (82.4) | 452 (82.8) | 902 (82.6) | ||
| Pelvic and Aortic | 167 (58.0) | 107 (16.9) | 274 (29.7) | 96 (17.6) | 94 (17.2) | 190 (17.4) | ||
| Unknown | 3 (1.0) | 38 (6.0) | 41 (4.5) | NA | NA | NA | ||
| Total dose (Gy) | 45 (45–46) | 45 (45–50) | 45 (45–50) | 0.201 | 45 (45–46) | 45 (45–50) | 45 (45–47) | 0.007 |
| Length of treatment (days) | 37 (35–42) | 38 (35–44) | 38 (35–43) | 0.376 | 38 (34–43) | 38 (35–44) | 38 (35–43) | 0.154 |
| Brachytherapy | 0.196 | 0.507 | ||||||
| Yes | 255 (88.5) | 580 (91.5) | 835 (90.6) | 498 (91.2) | 505 (92.5) | 1003 (91.8) | ||
| No | 33 (11.5) | 54 (8.5) | 87 (9.4) | 48 (8.8) | 41 (7.5) | 89 (8.2) | ||
| No PALND (n = 546) | PALND (n = 546) | Total (n = 1092) | p Value | |
|---|---|---|---|---|
| Any recurrence | 82 (15.0) | 153 (28.0) | 235 (21.5) | <0.001 |
| Local recurrence | 47 (8.6) | 48 (8.8) | 95 (8.7) | 1 |
| Regional recurrence | 13 (2.4) | 61 (11.2) | 74 (6.8) | <0.001 |
| Pelvic recurrence | 7 (1.3) | 37 (6.8) | 44 (4.0) | <0.001 |
| Aortic recurrence | 5 (0.9) | 25 (4.6) | 30 (2.7) | <0.001 |
| Patterns of regional recurrence | ||||
| Exclusively pelvic recurrence | 7 (58.3) | 33 (56.9) | 40 (57.1) | 1 |
| Exclusively aortic recurrence | 5 (41.7) | 21 (36.2) | 26 (37.1) | |
| Pelvic and aortic concomitant recurrence | 0 (0.0) | 4 (6.9) | 4 (5.7) | |
| Distant recurrences | 38 (7.0) | 85 (15.6) | 123 (11.3) | <0.001 |
| Lung/Pleura | 14 | 33 | 47 | |
| Liver | 7 | 20 | 27 | |
| Supradiaphragmatic lymph nodes | 11 | 14 | 25 | |
| Peritoneum | 0 | 23 | 23 | |
| Bone | 1 | 12 | 13 | |
| Psoas muscle (LND surgical bed) | 0 | 4 | 4 | |
| Abdominal Wall | 0 | 1 | 1 | |
| Inguinal lymph nodes | 0 | 2 | 2 | |
| Adrenal gland | 6 | 1 | 7 | |
| Brain | 0 | 2 | 2 | |
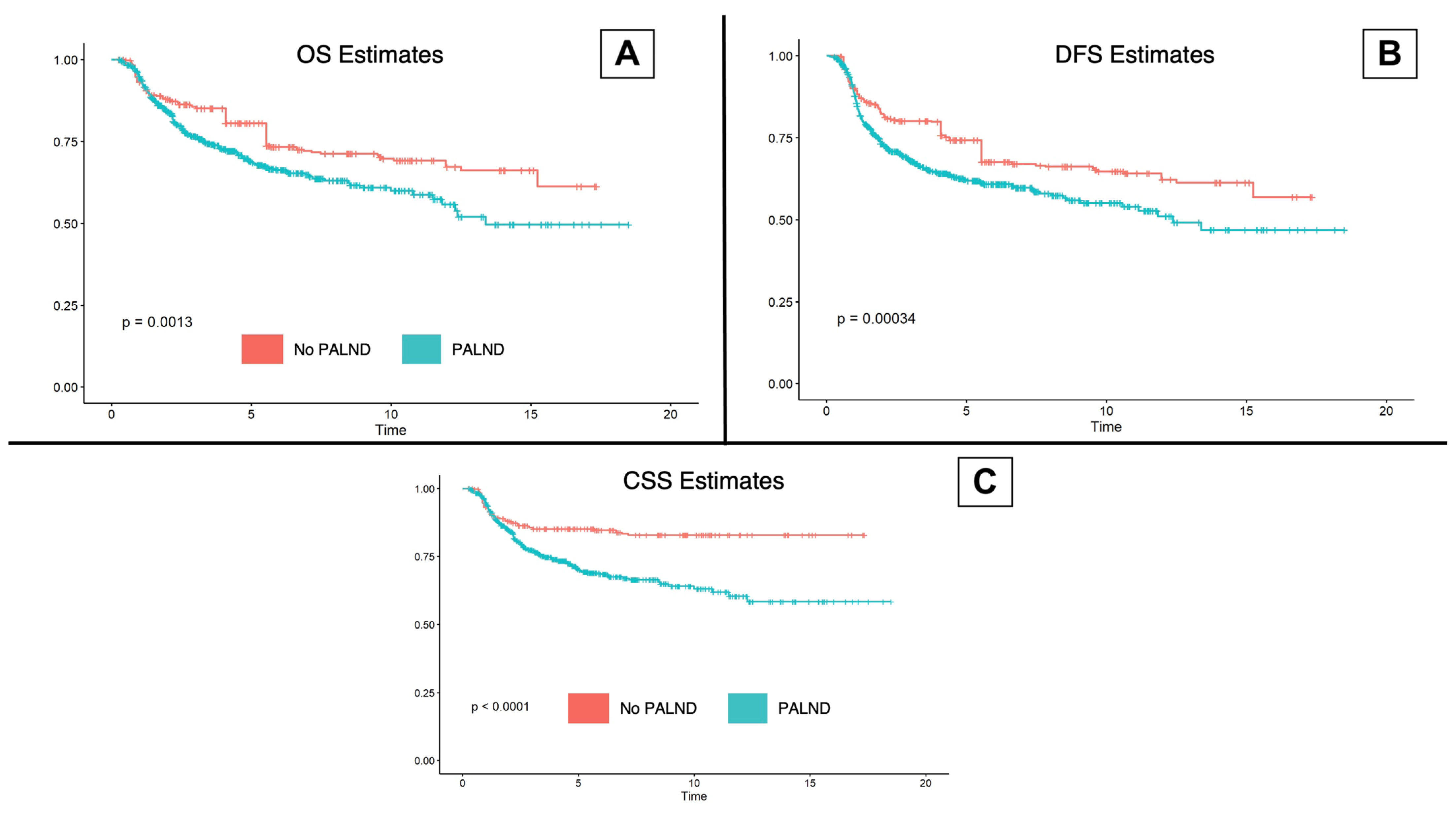
| 5-year overall survival (%, CI 95%) | 80.7 (77.1–84.4) | 68.9 (64.6–73.4) | 74.6 (71.7–77.5) | <0.001 |
| 5-year cancer-specific survival (%, CI 95%) | 85.1 (81.9–88.4) | 70.3 (66.1–74.8) | 77.6 (74.9–80.4) | <0.001 |
| 5-year disease-free survival (%, CI 95%) | 74.3 (70.4–78.4) | 61.9 (57.6–66.5) | 67.9 (65.0–71.0) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bebia, V.; Díaz-Feijoo, B.; Tejerizo, Á.; Torne, A.; Benito, V.; Hernández, A.; Gorostidi, M.; Domingo, S.; Bradbury, M.; Luna-Guibourg, R.; et al. Patterns of First Recurrence and Oncological Outcomes in Locally Advanced Cervical Cancer Patients: Does Surgical Staging Play a Role? Cancers 2024, 16, 1423. https://doi.org/10.3390/cancers16071423
Bebia V, Díaz-Feijoo B, Tejerizo Á, Torne A, Benito V, Hernández A, Gorostidi M, Domingo S, Bradbury M, Luna-Guibourg R, et al. Patterns of First Recurrence and Oncological Outcomes in Locally Advanced Cervical Cancer Patients: Does Surgical Staging Play a Role? Cancers. 2024; 16(7):1423. https://doi.org/10.3390/cancers16071423
Chicago/Turabian StyleBebia, Vicente, Berta Díaz-Feijoo, Álvaro Tejerizo, Aureli Torne, Virginia Benito, Alicia Hernández, Mikel Gorostidi, Santiago Domingo, Melissa Bradbury, Rocío Luna-Guibourg, and et al. 2024. "Patterns of First Recurrence and Oncological Outcomes in Locally Advanced Cervical Cancer Patients: Does Surgical Staging Play a Role?" Cancers 16, no. 7: 1423. https://doi.org/10.3390/cancers16071423
APA StyleBebia, V., Díaz-Feijoo, B., Tejerizo, Á., Torne, A., Benito, V., Hernández, A., Gorostidi, M., Domingo, S., Bradbury, M., Luna-Guibourg, R., & Gil-Moreno, A., on behalf of the SPAIN-GOG Group. (2024). Patterns of First Recurrence and Oncological Outcomes in Locally Advanced Cervical Cancer Patients: Does Surgical Staging Play a Role? Cancers, 16(7), 1423. https://doi.org/10.3390/cancers16071423







