Simple Summary
The clinical significance of nivolumab plus ipilimumab in patients with recurrence after chemoradiotherapy followed by durvalumab remains unclear. We investigated the efficacy and safety of nivolumab plus ipilimumab in patients who relapsed after chemoradiotherapy followed by durvalumab. The 6- and 12-month PFS rates for nivolumab plus ipilimumab for relapse after durvalumab were 46.7% and 36.4%, indicating a long-tail plateau. Nivolumab plus ipilimumab was particularly effective in patients treated with durvalumab for 6 months or more. Therefore, nivolumab plus ipilimumab is a promising treatment option for patients who have relapsed after CCRT–durvalumab, with good tolerability.
Abstract
Nivolumab plus ipilimumab showed promising efficacy in patients with metastatic non-small-cell lung cancer (NSCLC). The efficacy of the nivolumab plus ipilimumab combination regimen in NSCLC patients who relapse after durvalumab consolidation following concurrent chemoradiotherapy (CCRT) has not been determined. Between January 2021 and June 2022, clinical data were retrospectively extracted from the medical records of patients with NSCLC who received nivolumab plus ipilimumab after CCRT and durvalumab consolidation. A total of 30 patients were included in this analysis. The median number of durvalumab treatment cycles was 11. Median PFS and OS with nivolumab plus ipilimumab were 4.2 months (95% confidence interval [CI]: 0.7–7.7) and 18.5 months (95% CI: 3.5–33.5), respectively. The 6-month and 12-month PFS rates were 46.7% (95% CI: 28.8–64.5) and 36.4% (95% CI: 19.0–53.7). In multivariate analysis, a significant correlation was observed between a durvalumab treatment duration of 6 months or more and PFS (p = 0.04) as well as OS (p = 0.001). Grade 3 adverse events, including pneumonitis, dermatitis, and colitis, occurred in 10% of the patients. This study suggests that nivolumab plus ipilimumab is effective, especially in patients who have received durvalumab for 6 months or more, and tolerable for patients who relapsed after durvalumab following CCRT.
1. Introduction
Immune checkpoint inhibitors (ICIs) are utilized as the standard systemic therapy in various types of cancer [1,2]. In patients with non-small-cell lung cancer (NSCLC), ICIs help achieve clinical efficacy as a first-line treatment, perioperative adjuvant or neoadjuvant therapy, and as a maintenance agent after concurrent chemoradiotherapy (CCRT) [3,4,5,6,7,8]. Durvalumab, a programmed cell death-ligand 1 (PD-L1) inhibitor, as consolidation therapy after CCRT, is standard care for patients with unresectable locally advanced NSCLC (LA-NSCLC), exhibiting a survival benefit of 5-year overall survival (OS) rate of 42.9% [9]. However, approximately two-thirds of patients who receive durvalumab are not curable, requiring a different treatment after relapse.
Anti-PD-(L)1 and anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) antibodies enhance anti-tumor immune responses through different phases and mechanisms [10]. PD-1 binds to the ligands PD-L1 and PD-L2 and inhibits T-cell proliferation, and production of interferon-γ, tumor necrosis factor-α, and IL-2, and reduces T-cell survival [11]. Some T cells with PD-1 expression called “exhausted” T cells experience high levels of stimulation or reduced CD4+ T-cell help [12]. CTLA-4 is a classical immune checkpoint molecule that acts as a CD28 homolog and binds with high affinity to its receptor B7-1 (CD80) or B7-2 (CD86). Unlike CD28, binding of CTLA-4 to B7 is not able to produce a stimulatory signal [13]. This competitive binding prevents the costimulatory signal provided by CD28 and B7-1/2 binding. Moreover, several immune checkpoint targets including T-cell immunoglobulin and ITIM domain, and lymphocyte activation gene-3, like T-cell immunoglobulin and mucin-domain containing-3, have been discovered and are being investigated in clinical research. However, only ICIs targeting PD-1/PD-L1 and CTLA-4 have been approved and widely used in various cancers. The combination therapy of nivolumab and ipilimumab demonstrated favorable 5-year survival rates even in patient populations with PD-L1 tumor proportion scores of less than 1%, where PD-1/PD-L1 inhibitors are considered less effective [14]. Moreover, previous studies have demonstrated the clinical benefits of PD-1 inhibitor plus ipilimumab in patients with metastatic melanoma who experienced resistance to PD-(L)1 inhibitor [15]. Among patients with recurrence after durvalumab consolidation therapy following CCRT (CCRT–durvalumab), some patients did not achieve sufficient enhancement of anti-tumor immunity with anti-PD-L1 monotherapy. For these patients, the combination of nivolumab plus ipilimumab might be potentially effective. However, the clinical significance of nivolumab plus ipilimumab in patients with recurrence after CCRT–durvalumab remains unclear.
Based on these backgrounds, this retrospective study aimed to determine the clinical efficacy of nivolumab plus ipilimumab in patients with LA-NSCLC who relapsed after CCRT–durvalumab.
2. Materials and Methods
2.1. Patients
This multicenter, retrospective cohort study enrolled patients with LA-NSCLC who were treated with a nivolumab plus ipilimumab combination regimen due to cancer recurrence after CCRT–durvalumab treatment. The patients were evaluated at any of the four participating institutions in Japan and received nivolumab and ipilimumab regardless of whether chemotherapy was or was not concomitant, between January 2021 and June 2022. The eligibility criteria were as follows: (i) pathologically diagnosed NSCLC and (ii) progression after treatment with definitive CCRT (curative intent followed by consolidation durvalumab). Recurrence was defined as an apparent worsening on computed tomography scanning with or without histopathological proof of existing lung cancer. The local recurrence was defined as either the progression of an ipsilateral lung lesion contiguous with the primary tumor or a single mediastinal/hilar/supraclavicular lymph node metastasis. The clinical stages of all patients were classified according to the eight editions of the TNM classification system [16]. PD-L1 expression level was determined by the TPS, which was reported as a percentage on a scale of 0% to 100%. The TPS was evaluated using the Pharm Dx 22C3 PD-L1 assay (Agilent, Santa Clara, CA, USA).
This study was approved by the Clinical Research Review Board of Saitama Medical University (2022-018) and conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was not required because of the retrospective nature of the study.
2.2. Treatment and Follow-Up Evaluation
Durvalumab was administrated every 2 weeks at a dose of 10 mg/kg as consolidation therapy after CCRT until disease progression or unacceptable toxicity or discontinuation as determined by the treating physician for up to 12 months. Nivolumab at a dose of 240 mg/day every 2 weeks or 360 mg/day every 3 weeks was administered intravenously. Ipilimumab at a dose of 1 mg/kg was administered intravenously every 6 weeks until disease progression, unacceptable toxicity, or discontinuation as determined by the treating physician. When chemotherapy was used in combination with nivolumab and ipilimumab, the regimen consisted of carboplatin with an area under the curve (AUC) of 5, along with pemetrexed at 500 mg/m2 for non-squamous cell carcinoma, or carboplatin with an AUC of 5 along with paclitaxel at 200 mg/m2 for squamous cell carcinoma.
Any toxicity was assessed and graded using the Common Terminology Criteria for Adverse Events version 5.0. Treatment efficacy was evaluated by the attending physician and radiologist using the Response Evaluation Criteria in Solid Tumors version 1.1 [17]. Anonymized clinical data were recorded by local investigators using case report forms and stored in a password-protected secure system. The primary endpoint was to assess the progression-free survival (PFS) and PFS rate at 6 months as measured from the first administration of nivolumab and ipilimumab to progression or death from any cause. The secondary endpoints included OS, objective response rate (ORR), and safety. The analysis of PFS, OS, and ORR was conducted using the data cut-offs that were employed for the primary analysis of PFS.
2.3. Statistical Analyses
PFS and OS were estimated using the Kaplan–Meier method. Univariate and multivariate analyses according to baseline characteristic subgroups were performed using Cox regression analysis. The statistical significance level was set at p-values of <0.05. All statistical analyses were performed using Microsoft Excel 2019 (Microsoft Corporation, Redmond, WA, USA) and JMP 14.0 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Characteristics of Patients and Treatment Information
A total of 30 patients were included in further analysis. At the data cut-off (31 December 2022), the median follow-up time was 10.3 months (range, 1.4–23.3). Patient characteristics are shown in Table 1. The median age of the patients was 72 years (range, 50–80) and there were 29 (96.7%) males. Fifteen patients (50%) had lung squamous cell carcinoma, 14 (46.7%) had adenocarcinoma, and 1 (3.3%) was diagnosed as not otherwise specified. Twenty-nine patients (96.7%) had an Eastern Cooperative Oncology Group performance status of 0 or 1. The TPS based on PD-L1 immunohistochemistry was <1% in 9 (30%) patients, 1–49% in 14 (46.7%) patients, and ≥50% in 5 (16.7%) patients. Of 30 patients, driver mutations and rearrangements, including EGFR, ALK, and ROS1, were not identified in 25 patients. Additionally, five patients did not undergo driver oncogene testing. The median number of administrations of durvalumab was 10.5 (range, 1–26) and the median treatment duration with durvalumab was 5.8 months (range, 0.03–11.9). The median time from completion of irradiation to recurrence was 6.8 months (range, 1.7–30.7). Of 30 patients, 26 patients received nivolumab plus ipilimumab without chemotherapy (CheckMate 227 regimen), while 4 patients received nivolumab plus ipilimumab in combination with platinum-doublet chemotherapy (CheckMate 9LA regimen). Detailed information on CCRT–durvalumab including chemotherapy regimen are shown in Supplementary Table S1.

Table 1.
Patient characteristics.
3.2. Efficacy and Survival
The median PFS and OS with nivolumab plus ipilimumab were 4.2 months (95% CI: 0.7–7.7) and 18.5 months (95% CI: 3.5–33.5), respectively. The median time to treatment failure was 5.4 months (95% CI: 0–11.1) (Figure 1). The 6-month PFS rate was 46.7% (95% CI: 28.8–64.5), and the 12-month PFS rate was 36.4% (95% CI: 19.0–53.7). After nivolumab plus ipilimumab treatment, partial response was observed in 7 patients, stable disease in 14 patients, and progression of disease in 9 patients. The ORR was 23.3% (95% CI: 12–41), and the disease control rate was 70% (95% CI: 52–83) (Table 2).
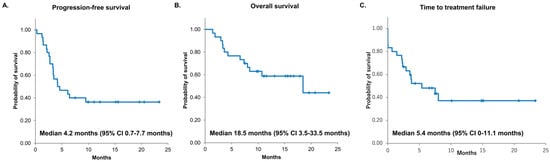
Figure 1.
Kaplan–Meier survival curves of PFS (A), OS (B), and TTF (C). PFS, progression-free survival; OS, overall survival: TTF, time to treatment failure: CI, confidential interval; and NR, not reached.

Table 2.
Tumor response.
The results of the univariate and multivariate analyses for PFS and OS are summarized in Table 3. Three factors had a prognostic significance in univariate analysis for PFS: treatment cycles of durvalumab (p = 0.005), treatment duration of durvalumab for 6 months or more (p = 0.005), and time to recurrence from final CCRT (p = 0.04). Likewise, three were identified as having prognostic significance in univariate analysis for OS: treatment cycles of durvalumab (p = 0.005), dose completion of durvalumab (p = 0.02), and treatment duration of durvalumab for 6 months or more (p = 0.005). As the factors related to durvalumab administration were similar, a multivariate analysis was conducted by selecting relevant factors with low p-values to mitigate multicollinearity. Duration of durvalumab treatment for 6 months or more was an independent prognostic factor for both PFS (hazard ratio 0.3 [95% CI: 0.1–1], p = 0.04) and OS (hazard ratio 0.1 [95% CI: 0.02–0.4], p = 0.0007). Supplementary Figure S1 shows swimmer plots illustrating the treatment duration with durvalumab and nivolumab plus ipilimumab. Of 30 patients, 17 patients were undergoing treatment with nivolumab and ipilimumab.

Table 3.
Univariate and multivariate analyses of predictive factors related to PFS and OS.
For further analysis, patients were divided into three groups: those who progressed after less than 6 months of treatment with durvalumab, those who progressed after 6–12 months, and those who relapsed after 12 months with completion of durvalumab. The Kaplan–Meier curves are shown in Supplementary Figure S2. Compared to a median PFS of 3.5 months (95% CI: 1.5–6.4) for the 14 patients who relapsed less than 6 months after completion of radiation, the PFS for those who relapsed at 6–12 months was not reached (95% CI: 0.3 not reached, p = 0.10), and for the group of patients who completed 12 months of durvalumab, the PFS was also not reached (95% CI: 2.1 not reached, p = 0.09). Compared to a median OS of 8.2 months (95% CI: 3.3 not reached) for the patients who relapsed less than 6 months after completion of radiation, the OS for those who relapsed at 6–12 months was 10.7 months (95% CI: 3 not reached, p = 0.61), and for the group of patients who completed 12 months of durvalumab, the OS was 18.5 months (95% CI: 3.6 not reached, p = 0.08).
3.3. Safety
Treatment safety profiles assessed by common terminology criteria for immune-related adverse events (irAEs) are presented in Table 4. The most common irAEs were liver dysfunction (20%), skin disorders (20%), hyperthyroidism or hypothyroidism (16.7%), pneumonitis (10%), arthralgia (10%), and diarrhea (6.7%). Grade 3 irAEs included pneumonitis (3.3%), skin disorders (3.3%), and diarrhea (3.3%). None of the patients experienced treatment-related deaths. There were no reports of unexpected irAEs.

Table 4.
IrAEs of nivolumab plus ipilimumab.
The AEs during durvalumab administration are listed in Supplementary Table S2. To compare AEs during durvalumab and during nivolumab plus ipilimumab treatment, the list of AEs for each patient is shown in Supplementary Table S3. The same AEs occurred in 26.7% (8/30) of patients, of which 2 patients had a worse grade with nivolumab plus ipilimumab than with durvalumab (Figure 2).
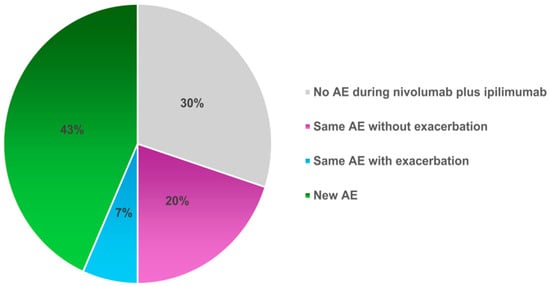
Figure 2.
Comparison of AEs during durvalumab with those during nivolumab plus ipilimumab treatment. The percentages of patients treated with nivolumab plus ipilimumab were divided into those who did not experience the same AEs observed during treatment with durvalumab, those who experienced the same AEs, and those who experienced new AE. AE, adverse events.
4. Discussion
The present study revealed the moderate efficacy of nivolumab plus ipilimumab in patients with LA-NSCLC relapse after CCRT–durvalumab treatment. Our study referenced a previous report that indicated that 24-week progression is the most accurate predictor of survival in NSCLC patients treated with ICIs [18]. The CheckMate 227 trial investigating nivolumab and ipilimumab in metastatic NSCLC patients showed a 6-month PFS rate of 40–50% [14]. In our study, the primary endpoint of the 6-month PFS rate was 46.7%, indicating a survival benefit of nivolumab plus ipilimumab even after CCRT–durvalumab. Of note, nivolumab plus ipilimumab resulted in a 12-month PFS rate of 36.4%. In addition, NSCLC patients who had received durvalumab for 6 months or more had significantly better PFS and OS. To the best of our knowledge, this is the first study to show that the combination of nivolumab and ipilimumab demonstrated survival benefits in patients with relapse after CCRT–durvalumab.
Tremendous attention has been paid to the optimal treatment for patients experiencing relapse after CCRT–durvalumab. Other treatments for this population include cytotoxic agents, platinum-based regimens, or a combination of those plus PD-(L)1 inhibitor [19]. Imai et al. showed that the platinum-based regimen resulted in a response rate of 16.7% and PFS of 4.2 months for patients who relapsed after chemoradiotherapy without durvalumab [20]. Miyawaki et al. discussed the benefit of platinum-based chemotherapy in similar patients without durvalumab [21]. The current study showed that the efficacy of nivolumab and ipilimumab is comparable to or better than those studies, despite cases of relapse after durvalumab. Amino et al. reported a favorable response rate of 45% for the anti-PD-1 antibody regimen in 20 patients with recurrence after CRT without durvalumab in stage III NSCLC [22]. Nonetheless, based on the available evidence, it appears that the re-challenge of PD-(L)1 inhibitors offers limited benefits [23]. Simultaneous inhibition of both PD-(L)1 and CTLA-4 could restore the anti-tumor immune response in patients previously treated with either PD-1 or CTLA-4 inhibitors alone. In malignant melanoma, the addition of CTLA-4 inhibitor after PD-1 inhibitor may improve PFS rates [15,24]. In both malignant melanoma and lung cancer, Kaplan–Meier curves from long-term follow-up analyses showed that the nivolumab plus ipilimumab therapy was superior to nivolumab alone with or without chemotherapy [14,25,26]. Considering the factors that contributed to the favorable outcome of the combination of CTLA-4 in this study, it is possible that the anti-tumor immune responses were activated because all patients were post-irradiation [22]. In addition, the selection bias, in which the attending physician included patients who were expected to benefit from nivolumab plus ipilimumab, should also be considered.
In the present study, patients who relapsed after the completion of durvalumab treatment tended to benefit from nivolumab plus ipilimumab, suggesting that these patients may have had cancer reactivation due to the ICI treatment discontinuation. This study could not clarify whether PD-1 inhibitor plus CTLA-4 inhibitor or PD-(L)1 inhibitor alone was sufficient to re-initiate or maintain the anti-tumor effect in such patients. Meanwhile, no significant differences in survival were associated with PD-L1 expression level, metastatic disease, or recurrence after the end of durvalumab treatment. Consistent with previous reports, distant metastases were the most common form of recurrence in this study [27]. It was assumed that the local recurrence cases included a population in which the anti-tumor immune system was relatively active in suppressing extensive progression, such as distant metastases. However, the results of the current study did not indicate that patients with local recurrence had a significantly better prognosis.
Nivolumab plus ipilimumab combination regimens for patients who had experienced radiation therapy did not appear to increase toxicity compared with the CheckMate 227 trial [7,8]. Of note, eight patients in the current study had the same AEs as during durvalumab treatment, and two patients had noticeable grade worsening compared to during durvalumab treatment, including skin disorder and pneumonitis of grade 2. Although grade 3 or 4 pneumonitis, dermatitis, and colitis were identified in the current study, the short follow-up period may have led to an underestimation of AEs. Treatment-related grade 3–4 AEs were invariably higher compared to anti-PD-1 antibodies alone in any reports [28,29]. Therefore, more attention should be paid when prescribing treatment with nivolumab plus ipilimumab.
Several limitations of our study warrant mention. First, it was a retrospective study with a small size. Therefore, the reported values may be under- or overestimated. Large trials are required to examine whether parameters such as PD-L1 < 1% and the pattern of local recurrence can predict treatment benefits. Four patients treated with the CheckMate 9LA regimen were included in this study. Compared to CheckMate 227 regimen cases, they had similar PFS and OS without significant differences (data not shown). We believe that the CheckMate 9LA regimen may be preferred in patients with large tumor volume or rapid disease progression, and this regimen could be a treatment option for patients with recurrence after CCRT–durvalumab. This study suggested the potential efficacy of nivolumab plus ipilimumab combination therapy in patients with recurrent NSCLC following durvalumab consolidation therapy. However, it should be noted that adherence to clinical guidelines and regulatory agency policies is imperative when considering its clinical use. Second, the advantages of ICIs for long-term outcomes were not evaluated. Although the Pacific study demonstrated a significant improvement in survival with durvalumab consolidation therapy, approximately 45% of patients experienced recurrence after CCRT–durvalumab within one year [9]. Therefore, we believe it is advisable to generate data demonstrating the efficacy of nivolumab plus ipilimumab in these patients as soon as possible. Although the observation period was short, a tail plateau was observed with this treatment modality, and early reporting was a priority. Third, in addition to PD-L1, other tumor biomarkers, such as gene mutation burden and tumor microenvironment were not evaluated. Some biomarkers may help determine whether the combination of nivolumab and ipilimumab or anti-PD-(L)1 antibody alone is optimal for patients who relapse after the completion of durvalumab treatment [30]. In addition, sensitive predictive biomarkers are required to avoid ICI overdose, which could lead to the exposure of potential non-responders to unnecessary immunotoxicity. In the current study, PD-L1 expression on tumor cells was evaluated with 22C3 at diagnosis with NSCLC. Although in previous clinical trials investigating durvalumab, PD-L1 expression in tumor microenvironment was evaluated using an SP263 clone, the high correlation between PD-L1 expression data was obtained with 22C3 and SP263 [31]. Because of the popularity of 22C2 in PD-L1 testing, PD-L1 expression has usually been assessed with 22C3 in Japan.
5. Conclusions
Nivolumab plus ipilimumab was identified as a feasible and efficient treatment for patients with LA-NSCLC relapsing after CCRT–durvalumab. Nivolumab plus ipilimumab treatment may be particularly effective in patients who received durvalumab for 6 months or more. Prospective comparative trials between nivolumab plus ipilimumab therapy and cytotoxic anticancer agents with or without PD-(L)1 therapy are desirable.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16071409/s1, Figure S1: Swimmer plots in patients who received nivolumab plus ipilimumab to relapsing after concurrent chemoradiotherapy followed by consolidation durvalumab; Figure S2: Kaplan–Meier curves for time from completion of CRT to recurrence in 3 groups: within 6 months, 6–12 months, and more than 12 months; Table S1: Therapeutic profiles of CCRT followed by durvalumab consolidation; Table S2: Adverse events with durvalumab consolidation; Table S3: Comparison of adverse events with durvalumab and nivolumab plus ipilimumab.
Author Contributions
Conceptualization, A.M., S.W. and H.K.; methodology, A.M., S.W. and H.K.; data curation, A.M., S.W., T.T., Y.N., Y.S. and O.Y.; formal analysis, A.M.; visualization and writing—original draft. A.M.; supervision, S.W., H.I. and K.K. (Kunihiko Kobayashi); writing—review and editing; S.W., H.I. and K.K. (Kyoichi Kaira). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was reviewed and approved by the Institutional Review Board of Saitama Medical University International Medical Center (2022-018, 1 June 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
All study data are included in the article.
Conflicts of Interest
A.M. reports receiving personal fees from Chugai Pharmaceutical and Eli Lilly Japan outside the submitted work. S.W. reports receiving personal fees from Chugai Pharmaceutical and AstraZeneca outside the submitted work. O.Y. reports receiving personal fees from Chugai Pharmaceutical, Ono Pharmaceutical Co., Ltd., and Bristol-Myers Squibb outside the submitted work. K.K. (Kyoichi Kaira) reports receiving personal fees from Ono Pharmaceutical Co., Ltd., Chugai Pharmaceutical, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer Inc., Kyowa Kirin Co, and Takeda Pharmaceutical outside the submitted work. H.K. reports receiving personal fees from Ono Pharmaceutical Co., Ltd., Chugai Pharmaceutical, AstraZeneca, Boehringer Ingelheim, and Bristol-Myers Squibb outside the submitted work.
References
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall survival with Durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus ipilimumab in Advanced non-small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Ciuleanu, T.E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-year survival outcomes from the PACIFIC trial: Durvalumab after chemoradiotherapy in Stage III non-small-cell lung cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef]
- Scalapino, K.J.; Daikh, D.I. CTLA-4: A key regulatory point in the control of autoimmune disease. Immunol. Rev. 2008, 223, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef]
- Egen, J.G.; Kuhns, M.S.; Allison, J.P. CTLA-4: New insights into its biological function and use in tumor immunotherapy. Nat. Immunol. 2002, 3, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Lee, J.S.; Ciuleanu, T.E.; Bernabe Caro, R.; Nishio, M.; Urban, L.; Audigier-Valette, C.; Lupinacci, L.; Sangha, R.; Pluzanski, A.; et al. Five-Year Survival Outcomes With Nivolumab Plus Ipilimumab Versus Chemotherapy as First-Line Treatment for Metastatic Non-Small-Cell Lung Cancer in CheckMate 227. J. Clin. Oncol. 2023, 41, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, I.P.; Ahmed, T.; McQuade, J.L.; Nebhan, C.A.; Park, J.J.; Versluis, J.M.; Serra-Bellver, P.; Khan, Y.; Slattery, T.; Oberoi, H.K.; et al. Ipilimumab alone or ipilimumab plus anti-PD-1 therapy in patients with metastatic melanoma resistant to anti-PD-(L)1 monotherapy: A multicentre, retrospective, cohort study. Lancet Oncol. 2021, 22, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V. The IASLC Lung Cancer Staging Project: Proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer. 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Shukuya, T.; Mori, K.; Amann, J.M.; Bertino, E.M.; Otterson, G.A.; Shields, P.G.; Morita, S.; Carbone, D.P. Relationship between overall survival and response or progression-free survival in advanced non-small cell lung cancer patients treated with anti-PD-1/PD-L1 antibodies. J. Thorac. Oncol. 2016, 11, 1927–1939. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Ariyasu, R.; Tanaka, H.; Saito, R.; Kawashima, Y.; Horiike, A.; Sakatani, T.; Tozuka, T.; Shiihara, J.; Saiki, M.; et al. Subsequent treatment for locally advanced non-small-cell lung cancer that progressed after definitive chemoradiotherapy and consolidation therapy with durvalumab: A multicenter retrospective analysis (TOPGAN 2021-02). Cancer Chemother. Pharmacol. 2023, 92, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Kaira, K.; Mori, K.; Ono, A.; Akamatsu, H.; Taira, T.; Yoshino, R.; Kenmotsu, H.; Saitoh, J.; Harada, H.; et al. Comparison of platinum combination re-challenge therapy and docetaxel monotherapy in non-small cell lung cancer patients previously treated with platinum-based chemoradiotherapy. Springerplus 2015, 4, 152. [Google Scholar] [CrossRef]
- Miyawaki, E.; Kenmotsu, H.; Shintani, Y.; Sekine, I.; Shukuya, T.; Takayama, K.; Inoue, A.; Okamoto, I.; Kiura, K.; Takahashi, K.; et al. Efficacy of platinum agents for stage III non-small-cell lung cancer following platinum-based chemoradiotherapy: A retrospective study. BMC Cancer 2022, 22, 342. [Google Scholar] [CrossRef] [PubMed]
- Amino, Y.; Kitazono, S.; Uematsu, S.; Hasegawa, T.; Yoshizawa, T.; Uchibori, K.; Yanagitani, N.; Horiike, A.; Horai, T.; Kasahara, K.; et al. Efficacy of anti-PD-1 therapy for recurrence after chemoradiotherapy in locally advanced NSCLC. Int. J. Clin. Oncol. 2020, 25, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhan, P.; Song, Y.; Liu, H.; Lv, T. Safety and efficacy of retreatment with immune checkpoint inhibitors in non-small cell lung cancer: A systematic review and meta-analysis. Transl. Lung Cancer Res. 2022, 11, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, L.; Livingstone, E.; Hassel, J.C.; Fluck, M.; Eigentler, T.; Loquai, C.; Haferkamp, S.; Gutzmer, R.; Meier, F.; Mohr, P.; et al. Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 395, 1558–1568. [Google Scholar] [CrossRef]
- Larkin, J.; Hodi, F.S.; Wolchok, J.D. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015, 373, 1270–1271. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Kishi, N.; Matsuo, Y.; Shintani, T.; Ogura, M.; Mitsuyoshi, T.; Araki, N.; Fujii, K.; Okumura, S.; Nakamatsu, K.; Kishi, T.; et al. Recurrence patterns and progression-free survival after chemoradiotherapy with or without consolidation durvalumab for stage III non-small cell lung cancer. J. Radiat. Res. 2023, 64, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, A.R.; McBride, A.; Slack, M.; Erstad, B.L.; Abraham, I. Potential immune-related adverse events associated with monotherapy and combination therapy of ipilimumab, nivolumab, and pembrolizumab for advanced melanoma: A systematic review and meta-analysis. Front. Oncol. 2020, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Kagamu, H.; Yamasaki, S.; Kitano, S.; Yamaguchi, O.; Mouri, A.; Shiono, A.; Nishihara, F.; Miura, Y.; Hashimoto, K.; Imai, H.; et al. Single-cell analysis reveals a CD4+ T-cell cluster that correlates with PD-1 blockade efficacy. Cancer Res. 2022, 82, 4641–4653. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, A.; Barberis, M.; Franco, R.; De Luca, G.; Pace, M.V.; Staibano, S.; Volante, M.; Buttitta, F.; Guerini-Rocco, E.; Righi, L.; et al. Multicenter Comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) Assays to Test PD-L1 Expression for NSCLC Patients to Be Treated with Immune Checkpoint Inhibitors. J. Thorac. Oncol. 2017, 12, 1654–1663. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).