Early-Stage Non-Small Cell Lung Cancer: Prevalence of Actionable Alterations in a Monocentric Consecutive Cohort
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tumor Specimens
2.2. Gene Mutations
2.3. Gene Fusions and PD-L1
2.4. Analysis of TCGA Data
2.5. Statistical Analysis
3. Results
3.1. Study Population
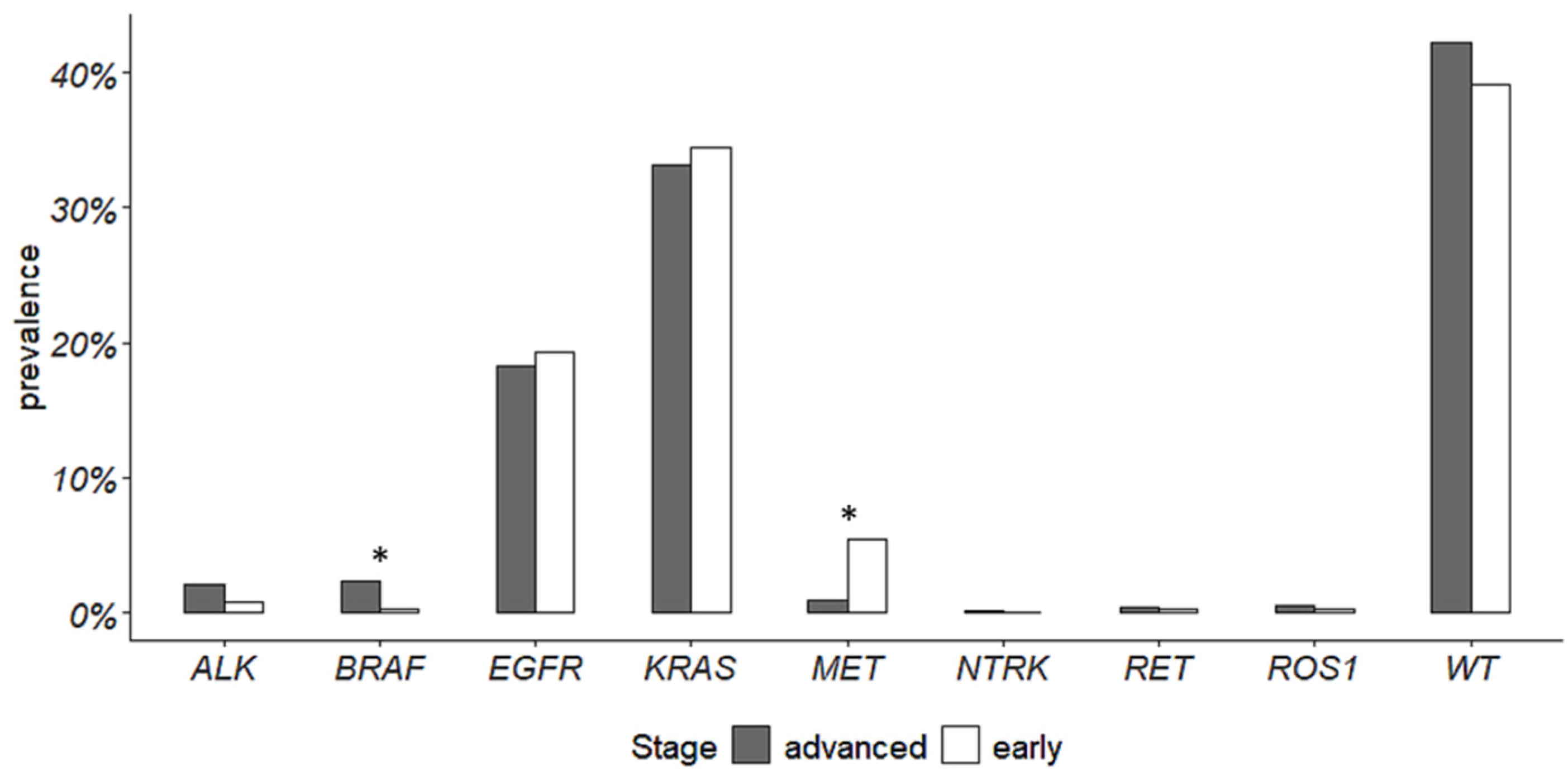
3.2. Gene Alterations
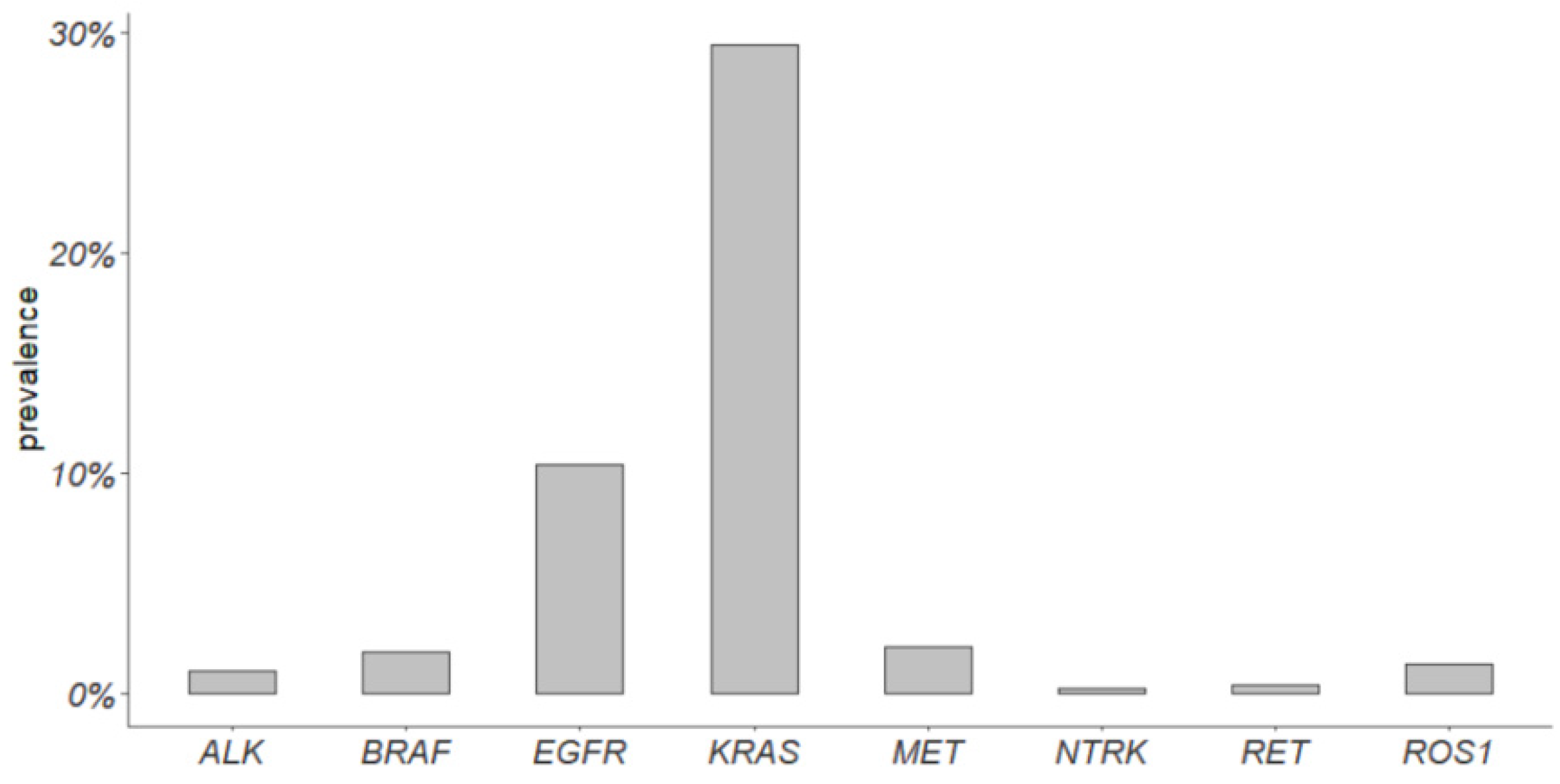
3.3. Prevalence of Actionable Alterations in Early-Stage Tumors of the LUAD TCGA Cohort
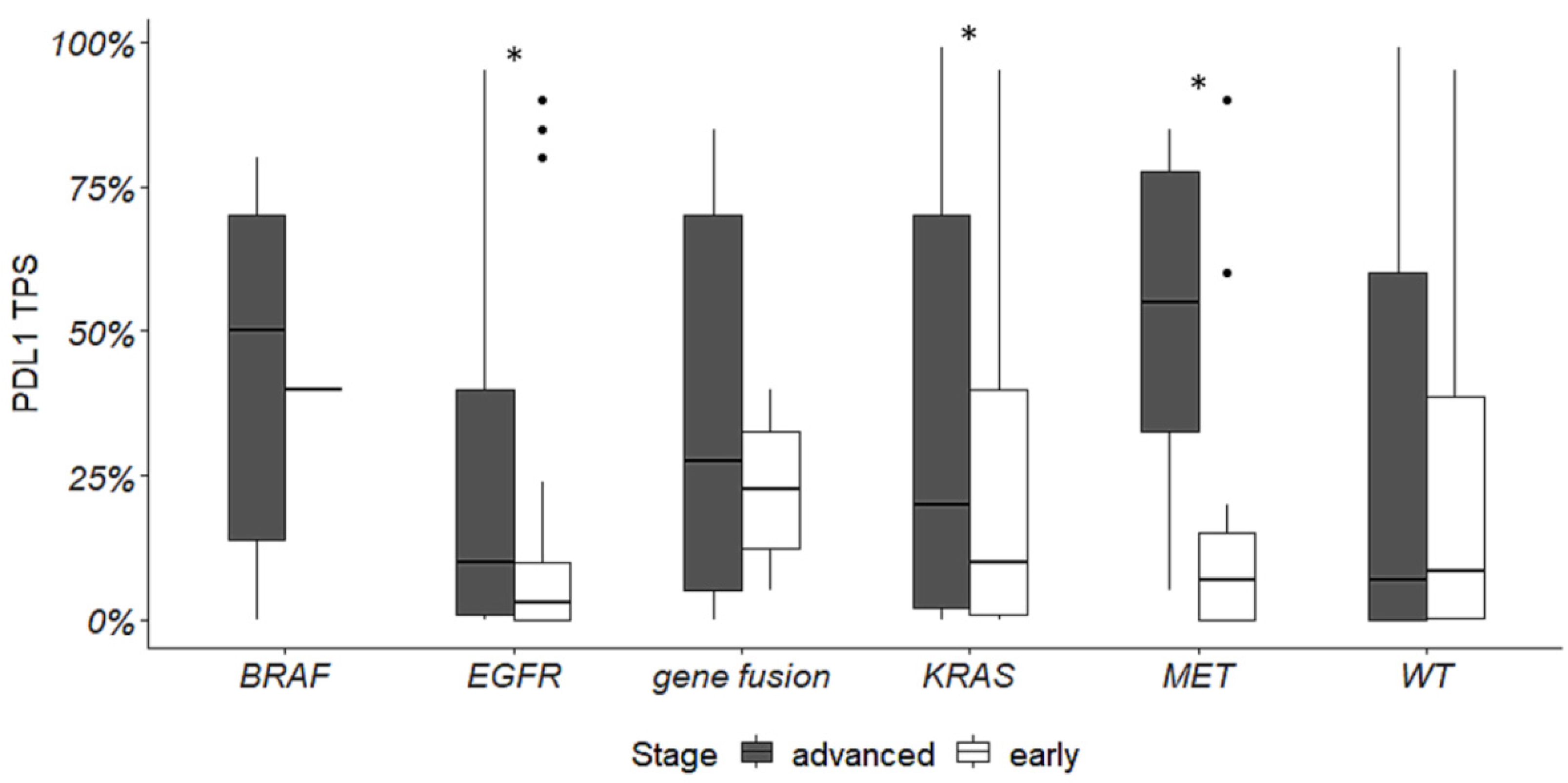
3.4. PD-L1 Expression Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Malvezzi, M.; Santucci, C.; Boffetta, P.; Collatuzzo, G.; Levi, F.; La Vecchia, C.; Negri, E. European Cancer Mortality Predictions for the Year 2023 with Focus on Lung Cancer. Ann. Oncol. 2023, 34, 410–419. [Google Scholar] [CrossRef]
- Simeone, J.C.; Nordstrom, B.L.; Patel, K.; Klein, A.B. Treatment Patterns and Overall Survival in Metastatic Non-Small-Cell Lung Cancer in a Real-World, US Setting. Future Oncol. 2019, 15, 3491–3502. [Google Scholar] [CrossRef]
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Oncogene-Addicted Metastatic Non-Small-Cell Lung Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 339–357. [Google Scholar] [CrossRef]
- Bruno, R.; Alì, G.; Poma, A.M.; Fontanini, G. Non-Small Cell Lung Cancer Molecular Characterization of Advanced Disease with Focus on Sex Differences: A Narrative Review. Precis. Cancer Med. 2021, 4, 14. [Google Scholar] [CrossRef]
- McMahon, D.J.; McLaughlin, R.; Naidoo, J. Is Immunotherapy Beneficial in Patients with Oncogene-Addicted Non-Small Cell Lung Cancers? A Narrative Review. Cancers 2024, 16, 527. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; DeCamp, M.; et al. NCCN Guidelines® Insights: Non–Small Cell Lung Cancer, Version 2.2023: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2023, 21, 340–350. [Google Scholar] [CrossRef]
- Remon, J.; Soria, J.-C.; Peters, S. Early and Locally Advanced Non-Small-Cell Lung Cancer: An Update of the ESMO Clinical Practice Guidelines Focusing on Diagnosis, Staging, Systemic and Local Therapy. Ann. Oncol. 2021, 32, 1637–1642. [Google Scholar] [CrossRef]
- Lababede, O.; Meziane, M.A. The Eighth Edition of TNM Staging of Lung Cancer: Reference Chart and Diagrams. Oncologist 2018, 23, 844–848. [Google Scholar] [CrossRef]
- Sheikh, M.; Virani, S.; Robbins, H.A.; Foretova, L.; Holcatova, I.; Janout, V.; Lissowska, J.; Navratilova, M.; Mukeriya, A.; Ognjanovic, M.; et al. Survival and Prognostic Factors of Early-stage Non-small Cell Lung Cancer in Central and Eastern Europe: A Prospective Cohort Study. Cancer Med. 2023, 12, 10563–10574. [Google Scholar] [CrossRef]
- Pignon, J.-P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.-Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung Adjuvant Cisplatin Evaluation: A Pooled Analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Herbst, R.S.; Wu, Y.-L.; John, T.; Grohe, C.; Majem, M.; Wang, J.; Kato, T.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; et al. Adjuvant Osimertinib for Resected EGFR-Mutated Stage IB-IIIA Non–Small-Cell Lung Cancer: Updated Results From the Phase III Randomized ADAURA Trial. J. Clin. Oncol. 2023, 41, 1830–1840. [Google Scholar] [CrossRef]
- Tsuboi, M.; Herbst, R.S.; John, T.; Kato, T.; Majem, M.; Grohé, C.; Wang, J.; Goldman, J.W.; Lu, S.; Su, W.-C.; et al. Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N. Engl. J. Med. 2023, 389, 137–147. [Google Scholar] [CrossRef]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant Atezolizumab after Adjuvant Chemotherapy in Resected Stage IB–IIIA Non-Small-Cell Lung Cancer (IMpower010): A Randomised, Multicentre, Open-Label, Phase 3 Trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Altorki, N.; Zhou, C.; Vallières, E.; Martínez-Martí, A.; Rittmeyer, A.; Chella, A.; Reck, M.; Goloborodko, O.; Huang, M.; et al. Overall Survival with Adjuvant Atezolizumab after Chemotherapy in Resected Stage II-IIIA Non-Small-Cell Lung Cancer (IMpower010): A Randomised, Multicentre, Open-Label, Phase III Trial. Ann. Oncol. 2023, 34, 907–919. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Paz-Ares, L.; Marreaud, S.; Dafni, U.; Oselin, K.; Havel, L.; Esteban, E.; Isla, D.; Martinez-Marti, A.; Faehling, M.; et al. Pembrolizumab versus Placebo as Adjuvant Therapy for Completely Resected Stage IB–IIIA Non-Small-Cell Lung Cancer (PEARLS/KEYNOTE-091): An Interim Analysis of a Randomised, Triple-Blind, Phase 3 Trial. Lancet Oncol. 2022, 23, 1274–1286. [Google Scholar] [CrossRef]
- Nagasaka, M.; Ou, S.I. Stage as the Sole “Biomarker” for Adjuvant Pembrolizumab in Resected Stage IB to IIIA NSCLC without Considerations for PD-L1 Expression Level, ALK/EGFR Mutational Status, and Prior Adjuvant Chemotherapy per FDA Approval Indications of PEARLS/Keynote-091? Lung Cancer Targets Ther. 2023, 14, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Bristol Myers Squibb; 4 March 2022. US Food and Drug Administration Approves Opdivo (Nivolumab) with Chemotherapy as Neoadjuvant Treatment for Certain Adult Patients with Resectable Non-Small Cell Lung Cancer. News Release. Available online: https://news.bms.com/news/details/2022/ (accessed on 26 May 2023).
- Bristol Myers Squibb. 26 May 2023. Bristol Myers Squibb Receives Positive CHMP Opinion Recommending Approval for Opdivo (Nivolumab) with Chemotherapy as Neoadjuvant Treatment of Resectable Non-Small Cell Lung Cancer at High Risk of Recurrence in Patients with Tumor Cell PD-L1 Expression ≥1% News Release. Available online: https://news.bms.com/news/details/2023 (accessed on 26 May 2023).
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- De Scordilli, M.; Michelotti, A.; Bertoli, E.; De Carlo, E.; Del Conte, A.; Bearz, A. Targeted Therapy and Immunotherapy in Early-Stage Non-Small Cell Lung Cancer: Current Evidence and Ongoing Trials. Int. J. Mol. Sci. 2022, 23, 7222. [Google Scholar] [CrossRef]
- Friedlaender, A.; Addeo, A.; Russo, A.; Gregorc, V.; Cortinovis, D.; Rolfo, C. Targeted Therapies in Early Stage NSCLC: Hype or Hope? Int. J. Mol. Sci. 2020, 21, 6329. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, B.; Raskina, K.; Lofgren, K.T.; Li, G.; Tolba, K.; Schwed, K.; Castellanos, E.; Huang, R.S.P.; Oxnard, G.R.; Schrock, A.B.; et al. Quantifying the Value of Multigene Testing in Resected Early Stage Lung Adenocarcinoma. J. Thorac. Oncol. 2023, 18, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef] [PubMed]
- Giannini, R.; Lupi, C.; Sensi, E.; Alì, G.; Proietti, A.; Boldrini, L.; Servadio, A.; Giordano, M.; Macerola, E.; Bruno, R.; et al. EGFR and KRAS Mutational Analysis in a Large Series of Italian Non-Small Cell Lung Cancer Patients: 2,387 Cases from a Single Center. Oncol. Rep. 2016, 36, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Landi, L.; Chiari, R.; Tiseo, M.; D’Incà, F.; Dazzi, C.; Chella, A.; Delmonte, A.; Bonanno, L.; Giannarelli, D.; Cortinovis, D.L.; et al. Crizotinib in MET-Deregulated or ROS1-Rearranged Pretreated Non–Small Cell Lung Cancer (METROS): A Phase II, Prospective, Multicenter, Two-Arms Trial. Clin. Cancer Res. 2019, 25, 7312–7319. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Hernández, A.; González Del Portillo, E.; Tamayo-Velasco, Á.; Figuero-Pérez, L.; Zhilina-Zhilina, S.; Fonseca-Sánchez, E.; Miramontes-González, J.P. Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer: From Current Perspectives to Future Treatments—A Systematic Review. Ann. Transl. Med. 2023, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Alì, G.; Proietti, A.; Pelliccioni, S.; Niccoli, C.; Lupi, C.; Sensi, E.; Giannini, R.; Borrelli, N.; Menghi, M.; Chella, A.; et al. ALK Rearrangement in a Large Series of Consecutive Non–Small Cell Lung Cancers: Comparison Between a New Immunohistochemical Approach and Fluorescence In Situ Hybridization for the Screening of Patients Eligible for Crizotinib Treatment. Arch. Pathol. Lab. Med. 2014, 138, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Overbeck, T.R.; Reiffert, A.; Schmitz, K.; Rittmeyer, A.; Körber, W.; Hugo, S.; Schnalke, J.; Lukat, L.; Hugo, T.; Hinterthaner, M.; et al. NTRK Gene Fusions in Non-Small-Cell Lung Cancer: Real-World Screening Data of 1068 Unselected Patients. Cancers 2023, 15, 2966. [Google Scholar] [CrossRef] [PubMed]
- Sholl, L.M.; Sun, H.; Butaney, M.; Zhang, C.; Lee, C.; Jänne, P.A.; Rodig, S.J. ROS1 Immunohistochemistry for Detection of ROS1-Rearranged Lung Adenocarcinomas. Am. J. Surg. Pathol. 2013, 37, 1441–1449. [Google Scholar] [CrossRef]
- Vingiani, A.; Lorenzini, D.; Conca, E.; Volpi, C.C.; Trupia, D.V.; Gloghini, A.; Perrone, F.; Tamborini, E.; Dagrada, G.P.; Agnelli, L.; et al. Pan-TRK Immunohistochemistry as Screening Tool for NTRK Fusions: A Diagnostic Workflow for the Identification of Positive Patients in Clinical Practice. Cancer Biomark. 2023, 38, 301–309. [Google Scholar] [CrossRef]
- Sabari, J.K.; Santini, F.; Bergagnini, I.; Lai, W.V.; Arbour, K.C.; Drilon, A. Changing the Therapeutic Landscape in Non-Small Cell Lung Cancers: The Evolution of Comprehensive Molecular Profiling Improves Access to Therapy. Curr. Oncol. Rep. 2017, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Recondo, G.; Guo, R.; Cravero, P.; Ricciuti, B.; Falcon, C.; Vokes, N.; Kris, M.G.; Leonardi, G.C.; Lamberti, G.; Nguyen, T.; et al. Clinical Characteristics, Genomic Features, and Recurrence Risk of Early-Stage MET Exon 14 Mutant Non-Small Cell Lung Cancer (NSCLC). J. Clin. Oncol. 2020, 38, 9042. [Google Scholar] [CrossRef]
- Fu, M.; Feng, C.-M.; Xia, D.-Q.; Ji, Z.-M.; Xia, H.-L.; Hu, N.-N.; Leng, Z.-J.; Xie, W.; Fang, Y.; Cao, L.-J.; et al. Neoadjuvant Savolitinib Targeted Therapy Stage IIIA-N2 Primary Lung Adenocarcinoma Harboring MET Exon 14 Skipping Mutation: A Case Report. Front. Oncol. 2022, 12, 954886. [Google Scholar] [CrossRef]
- Wang, J.; Yao, W.; Wang, W.; Fan, M.; Huang, K.; Liu, Z.; Zhu, D. Complete Pathological Response and Negative Postoperative ctDNA Were Not Predictive of Discontinuation of Adjuvant Crizotinib Therapy in a Patient with Locally Advanced MET Ex14 Skipping Mutation-Positive Non-Small Cell Lung Cancer: A Case Report. Front. Oncol. 2023, 13, 1164543. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, M.; Weder, W.; Escriu, C.; Blakely, C.; He, J.; Dacic, S.; Yatabe, Y.; Zeng, L.; Walding, A.; Chaft, J.E. Neoadjuvant Osimertinib with/without Chemotherapy versus Chemotherapy Alone for EGFR-Mutated Resectable Non-Small-Cell Lung Cancer: NeoADAURA. Future Oncol. 2021, 17, 4045–4055. [Google Scholar] [CrossRef]
- Ren, S.; Yang, L.; Tong, Z.; Wang, R.; Han, W.; Yu, F.; Liu, W.; Hu, Y. Osimertinib as Neoadjuvant Therapy for Resectable Non-Small Cell Lung Cancer: A Single-Center Real-World Study. In Proceedings of the 11.01—Lung cancer, European Respiratory Society, 4 September 2022; p. 319. [Google Scholar]
- Lv, C.; Fang, W.; Wu, N.; Jiao, W.; Xu, S.; Ma, H.; Wang, J.; Wang, R.; Ji, C.; Li, S.; et al. Osimertinib as Neoadjuvant Therapy in Patients with EGFR-Mutant Resectable Stage II-IIIB Lung Adenocarcinoma (NEOS): A Multicenter, Single-Arm, Open-Label Phase 2b Trial. Lung Cancer 2023, 178, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Cortiula, F.; Naidoo, J. A Brave NEO World: Neoadjuvant Osimertinib in Resectable EGFR-Mutant NSCLC. Lung Cancer 2023, 181, 107256. [Google Scholar] [CrossRef]
- Solomon, B.J.; Ahn, J.S.; Dziadziuszko, R.; Barlesi, F.; Nishio, M.; Lee, D.H.; Lee, J.-S.; Zhong, W.-Z.; Horinouchi, H.; Mao, W.; et al. LBA2 ALINA: Efficacy and Safety of Adjuvant Alectinib versus Chemotherapy in Patients with Early-Stage ALK+ Non-Small Cell Lung Cancer (NSCLC). Ann. Oncol. 2023, 34, S1295–S1296. [Google Scholar] [CrossRef]
- Leonetti, A.; Minari, R.; Boni, L.; Gnetti, L.; Verzè, M.; Ventura, L.; Musini, L.; Tognetto, M.; Tiseo, M. Phase II, Open-Label, Single-Arm, Multicenter Study to Assess the Activity and Safety of Alectinib as Neoadjuvant Treatment in Surgically Resectable Stage III ALK-Positive NSCLC: ALNEO Trial. Clin. Lung Cancer 2021, 22, 473–477. [Google Scholar] [CrossRef]
- Goldman, J.W.; Sholl, L.M.; Dacic, S.; Fishbein, M.C.; Murciano-Goroff, Y.R.; Rajaram, R.; Szymczak, S.; Szpurka, A.M.; Chao, B.H.; Drilon, A. Case Report: Complete Pathologic Response to Neoadjuvant Selpercatinib in a Patient with Resectable Early-Stage RET Fusion-Positive Non-Small Cell Lung Cancer. Front. Oncol. 2023, 13, 1178313. [Google Scholar] [CrossRef]
- Chen, A.; Chen, D.; Li, S.; Zhao, L.; Xiao, M. Case Report: Adjuvant Crizotinib Therapy Exerted Favorable Survival Benefit in a Resectable Stage IIIA NSCLC Patient with Novel LDLR–ROS1 Fusion. Front. Oncol. 2022, 12, 837219. [Google Scholar] [CrossRef]
- Liu, C.; Lu, M.; Yang, Y.; Wang, X.; Ma, F.; Liu, X. Case Report: Major Pathologic Response Induced by Neoadjuvant Treatment Using BRAF and MEK Inhibitors in a Patient with Stage IIIA Lung Adenocarcinoma Harboring BRAF V600E-Mutation. Front. Oncol. 2022, 12, 961539. [Google Scholar] [CrossRef]
- Kim, S.; Koh, J.; Kwon, D.; Keam, B.; Go, H.; Kim, Y.A.; Jeon, Y.K.; Chung, D.H. Comparative Analysis of PD-L1 Expression between Primary and Metastatic Pulmonary Adenocarcinomas. Eur. J. Cancer 2017, 75, 141–149. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Rizvi, H.; Bandlamudi, C.; Sauter, J.L.; Travis, W.D.; Rekhtman, N.; Plodkowski, A.J.; Perez-Johnston, R.; Sawan, P.; Beras, A.; et al. Clinical and Molecular Correlates of PD-L1 Expression in Patients with Lung Adenocarcinomas. Ann. Oncol. 2020, 31, 599–608. [Google Scholar] [CrossRef]
- Negrao, M.V.; Skoulidis, F.; Montesion, M.; Schulze, K.; Bara, I.; Shen, V.; Xu, H.; Hu, S.; Sui, D.; Elamin, Y.Y.; et al. Oncogene-Specific Differences in Tumor Mutational Burden, PD-L1 Expression, and Outcomes from Immunotherapy in Non-Small Cell Lung Cancer. J. Immunother. Cancer 2021, 9, e002891. [Google Scholar] [CrossRef]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.B.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune Checkpoint Inhibitors for Patients with Advanced Lung Cancer and Oncogenic Driver Alterations: Results from the IMMUNOTARGET Registry. Ann. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef]
- Su, P.-L.; Chen, J.-Y.; Chu, C.-Y.; Chen, Y.-L.; Chen, W.-L.; Lin, K.-Y.; Ho, C.-L.; Tsai, J.-S.; Yang, S.-C.; Chen, C.-W.; et al. The Impact of Driver Mutation on the Treatment Outcome of Early-Stage Lung Cancer Patients Receiving Neoadjuvant Immunotherapy and Chemotherapy. Sci. Rep. 2022, 12, 3319. [Google Scholar] [CrossRef]
- Spitaleri, G.; Trillo Aliaga, P.; Attili, I.; Del Signore, E.; Corvaja, C.; Corti, C.; Uliano, J.; Passaro, A.; De Marinis, F. MET in Non-Small-Cell Lung Cancer (NSCLC): Cross ‘a Long and Winding Road’ Looking for a Target. Cancers 2023, 15, 4779. [Google Scholar] [CrossRef]
- Sabari, J.K.; Leonardi, G.C.; Shu, C.A.; Umeton, R.; Montecalvo, J.; Ni, A.; Chen, R.; Dienstag, J.; Mrad, C.; Bergagnini, I.; et al. PD-L1 Expression, Tumor Mutational Burden, and Response to Immunotherapy in Patients with MET Exon 14 Altered Lung Cancers. Ann. Oncol. 2018, 29, 2085–2091. [Google Scholar] [CrossRef]
- Dudnik, E.; Bshara, E.; Grubstein, A.; Fridel, L.; Shochat, T.; Roisman, L.C.; Ilouze, M.; Rozenblum, A.B.; Geva, S.; Zer, A.; et al. Rare Targetable Drivers (RTDs) in Non-Small Cell Lung Cancer (NSCLC): Outcomes with Immune Check-Point Inhibitors (ICPi). Lung Cancer 2018, 124, 117–124. [Google Scholar] [CrossRef]
- Nagahashi, M.; Sato, S.; Yuza, K.; Shimada, Y.; Ichikawa, H.; Watanabe, S.; Takada, K.; Okamoto, T.; Okuda, S.; Lyle, S.; et al. Common Driver Mutations and Smoking History Affect Tumor Mutation Burden in Lung Adenocarcinoma. J. Surg. Res. 2018, 230, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Mhanna, L.; Guibert, N.; Milia, J.; Mazieres, J. When to Consider Immune Checkpoint Inhibitors in Oncogene-Driven Non-Small Cell Lung Cancer? Curr. Treat. Options Oncol. 2019, 20, 60. [Google Scholar] [CrossRef]
- Singal, G.; Miller, P.G.; Agarwala, V.; Li, G.; Kaushik, G.; Backenroth, D.; Gossai, A.; Frampton, G.M.; Torres, A.Z.; Lehnert, E.M.; et al. Association of Patient Characteristics and Tumor Genomics with Clinical Outcomes among Patients with Non–Small Cell Lung Cancer Using a Clinicogenomic Database. JAMA 2019, 321, 1391. [Google Scholar] [CrossRef]

| Clinicopathological Features | All Patients (N = 1122) | Early-Stage (N = 368) | Advanced-Stage N = 754) |
|---|---|---|---|
| Age (years), median (IQR) | 71 (63–77) | 72 (66–77) | 71 (62–78) |
| Sex, N (%) | |||
| Female | 465 (41.4) | 157 (42.7) | 308 (40.8) |
| Male | 657 (58.6) | 211 (57.3) | 446 (59.2) |
| Histological diagnosis, N (%) | |||
| ADC | 1017 (90.6) | 366 (99.5) | 651 (86.3) |
| NSCLC NOS | 99 (8.9) | 0 | 99 (13.2) |
| ADCSCC | 6 (0.5) | 2 (0.5) | 4 (0.5) |
| Materials, N (%) | |||
| Cytology/cell-blocks | 253 (22.5) | 0 | 253 (33.6) |
| Biopsies | 410 (36.5) | 3 (0.8) | 407 (54) |
| Surgical specimens | 459 (41) | 365 (99.2) | 94 (12.4) |
| Tissue, N (%) | |||
| Lung | 906 (80.7) | 367 (99.7) | 539 (71.5) |
| Others | 216 (19.3) | 1 (0.3) | 215 (28.5) |
| Gene | Early-Stage NSCLC | Advanced-Stage NSCLC | ||||||
|---|---|---|---|---|---|---|---|---|
| Analyzed Cases | Wild Type | Actionable Alterations | Alteration Prevalence | Analyzed Cases | Wild Type | Actionable Alterations | Alteration Prevalence | |
| ALK fusions | 275 | 272 | 3 | 1.1% | 646 | 630 | 16 | 2.5% |
| BRAF p.(V600E) | 256 | 255 | 1 | 0.4% | 503 | 486 | 17 | 3.4% |
| EGFR * | 349 | 278 | 71 | 20.3% | 703 | 565 | 138 | 19.7% |
| KRAS ** | 299 | 172 | 127 | 42.5% | 672 | 422 | 250 | 37.2% |
| MET exon 14 skipping | 241 | 228 | 13 | 5.4% | 417 | 409 | 8 | 1.9% |
| NTRK1/2/3 fusions | 82 | 82 | 0 | 0 | 156 | 155 | 1 | 0.6% |
| RET fusions | 218 | 217 | 1 | 0.5% | 434 | 431 | 3 | 0.7% |
| ROS1 fusions | 255 | 254 | 1 | 0.4% | 573 | 569 | 4 | 0.7% |
| Early-Stage NSCLC | ||||
|---|---|---|---|---|
| EGFR | Number of Cases | Prevalence among EGFR Mutations | Prevalence among All Analyzed ES-NSCLC | |
| Exon 19 in frame deletions | 40 | 56.3% | 11.5% | |
| Exon 20 in frame insertions | 7 | 9.9% | 2% | |
| p.(L858R) | 16 | 22.5% | 4.6% | |
| Uncommon alterations | 4 | 5.6% | 1.1% | |
| Mutation type | Number of cases | |||
| p.(G719A) | 2 | |||
| p.(L861Q) | 2 | |||
| Compound mutations | 4 | |||
| Mutation type | Number of cases | 5.6% | 1.1% | |
| p.(S768I) + p.(L858R) | 1 | |||
| p.(G719S) + p.(L861Q) | 1 | |||
| p.(E709Q) + p.(L858R) | 1 | |||
| p.(E709Q) + p.(G719C) | 1 | |||
| Advanced-stage NSCLC | ||||
| EGFR | Number of cases | Prevalence among EGFR mutations | Prevalence among all analyzed advanced NSCLC | |
| Exon 19 in frame deletions | 70 | 50.7% | 9.9% | |
| Exon 20 in frame insertions | 14 | 10.1% | 2% | |
| p.(L858R) | 40 | 28.9% | 56.9% | |
| Uncommon alterations | 4 | |||
| Mutation type | Number of cases | 2.9% | 0.6% | |
| p.(G719C) | 1 | |||
| p.(G719A) | 2 | |||
| p.(S768I) | 1 | |||
| Compound mutations | 10 | |||
| Mutation type | Number of cases | 7.2% | 1.4% | |
| p.(G719C) + p.(S768I) | 1 | |||
| p.(G719A) + p.(S768I) | 1 | |||
| p.(V689L) + p.(V744M) + p.(Y827F) | 1 | |||
| p.(E709A) + p.(G719S) | 1 | |||
| p.(E709A) + p.(G719C) | 1 | |||
| p.(S768I) + p.(L858R) | 1 | |||
| p.(A871G) + p.(L858R) | 1 | |||
| p.(S768I) + p.(V744M) | 1 | |||
| p.(774M) + p.(Y827F) | 1 | |||
| p.(S768M) + p.(V744M) | 1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, R.; Poma, A.M.; Panozzi, M.; Lenzini, A.; Elia, G.; Zirafa, C.C.; Aprile, V.; Ambrogi, M.C.; Baldini, E.; Lucchi, M.; et al. Early-Stage Non-Small Cell Lung Cancer: Prevalence of Actionable Alterations in a Monocentric Consecutive Cohort. Cancers 2024, 16, 1410. https://doi.org/10.3390/cancers16071410
Bruno R, Poma AM, Panozzi M, Lenzini A, Elia G, Zirafa CC, Aprile V, Ambrogi MC, Baldini E, Lucchi M, et al. Early-Stage Non-Small Cell Lung Cancer: Prevalence of Actionable Alterations in a Monocentric Consecutive Cohort. Cancers. 2024; 16(7):1410. https://doi.org/10.3390/cancers16071410
Chicago/Turabian StyleBruno, Rossella, Anello Marcello Poma, Martina Panozzi, Alessandra Lenzini, Gianmarco Elia, Carmelina Cristina Zirafa, Vittorio Aprile, Marcello Carlo Ambrogi, Editta Baldini, Marco Lucchi, and et al. 2024. "Early-Stage Non-Small Cell Lung Cancer: Prevalence of Actionable Alterations in a Monocentric Consecutive Cohort" Cancers 16, no. 7: 1410. https://doi.org/10.3390/cancers16071410
APA StyleBruno, R., Poma, A. M., Panozzi, M., Lenzini, A., Elia, G., Zirafa, C. C., Aprile, V., Ambrogi, M. C., Baldini, E., Lucchi, M., Melfi, F., Chella, A., Sbrana, A., & Alì, G. (2024). Early-Stage Non-Small Cell Lung Cancer: Prevalence of Actionable Alterations in a Monocentric Consecutive Cohort. Cancers, 16(7), 1410. https://doi.org/10.3390/cancers16071410








