Clinical and Analytical Validation of Two Methods for Ki-67 Scoring in Formalin Fixed and Paraffin Embedded Tissue Sections of Early Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Treatment
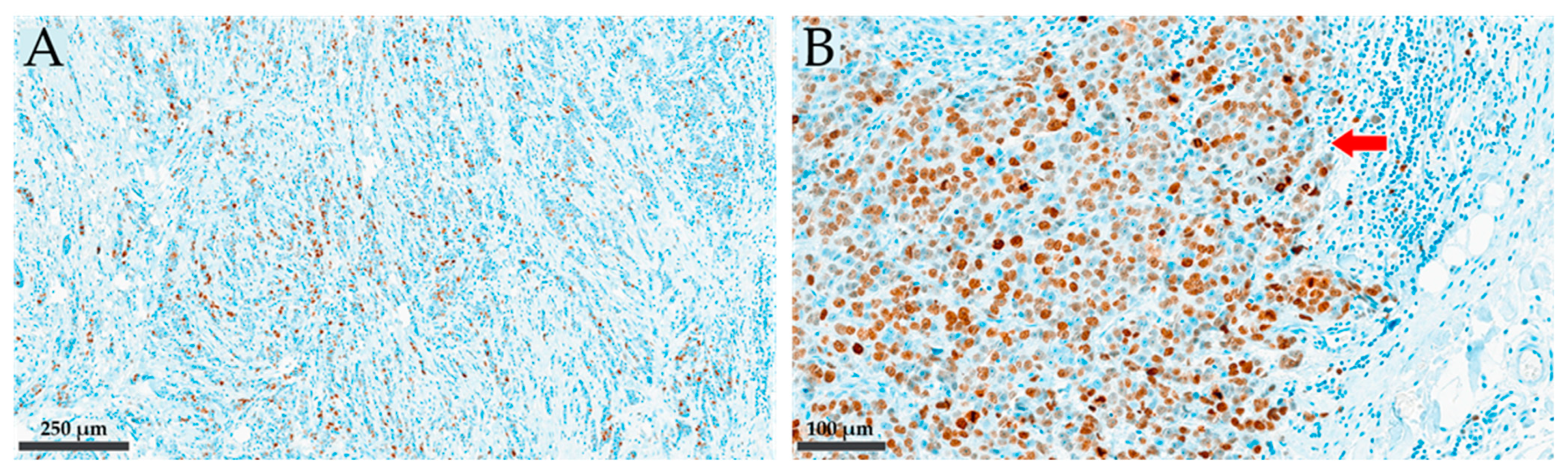
2.2. Pathohistological Examinations: IHC Staining and Scoring for Ki-67
2.3. Flow-Cytometric Determination of S Phase Fraction
2.4. Data Analysis
3. Results
3.1. Patients
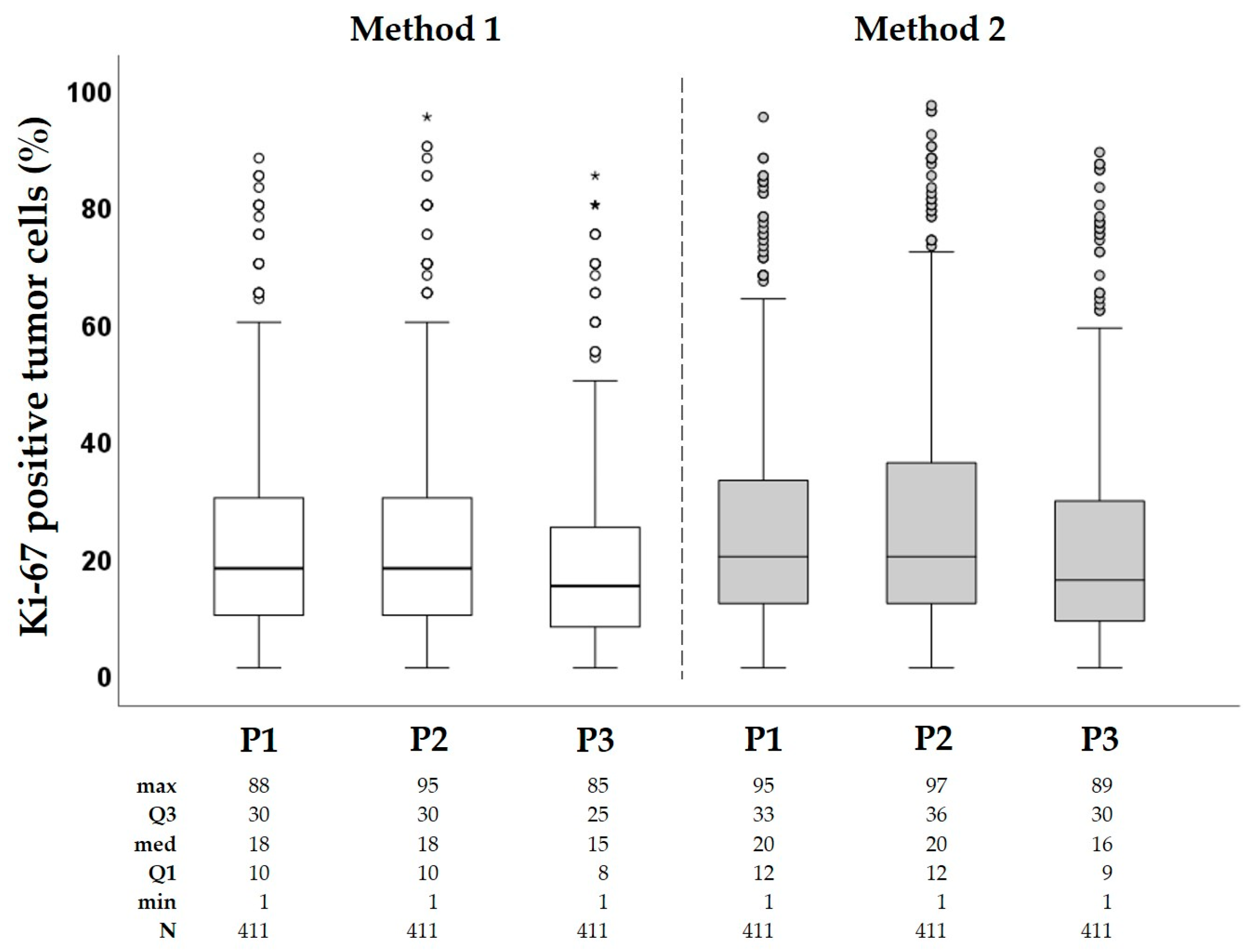
3.2. Ki-67 Scoring Results
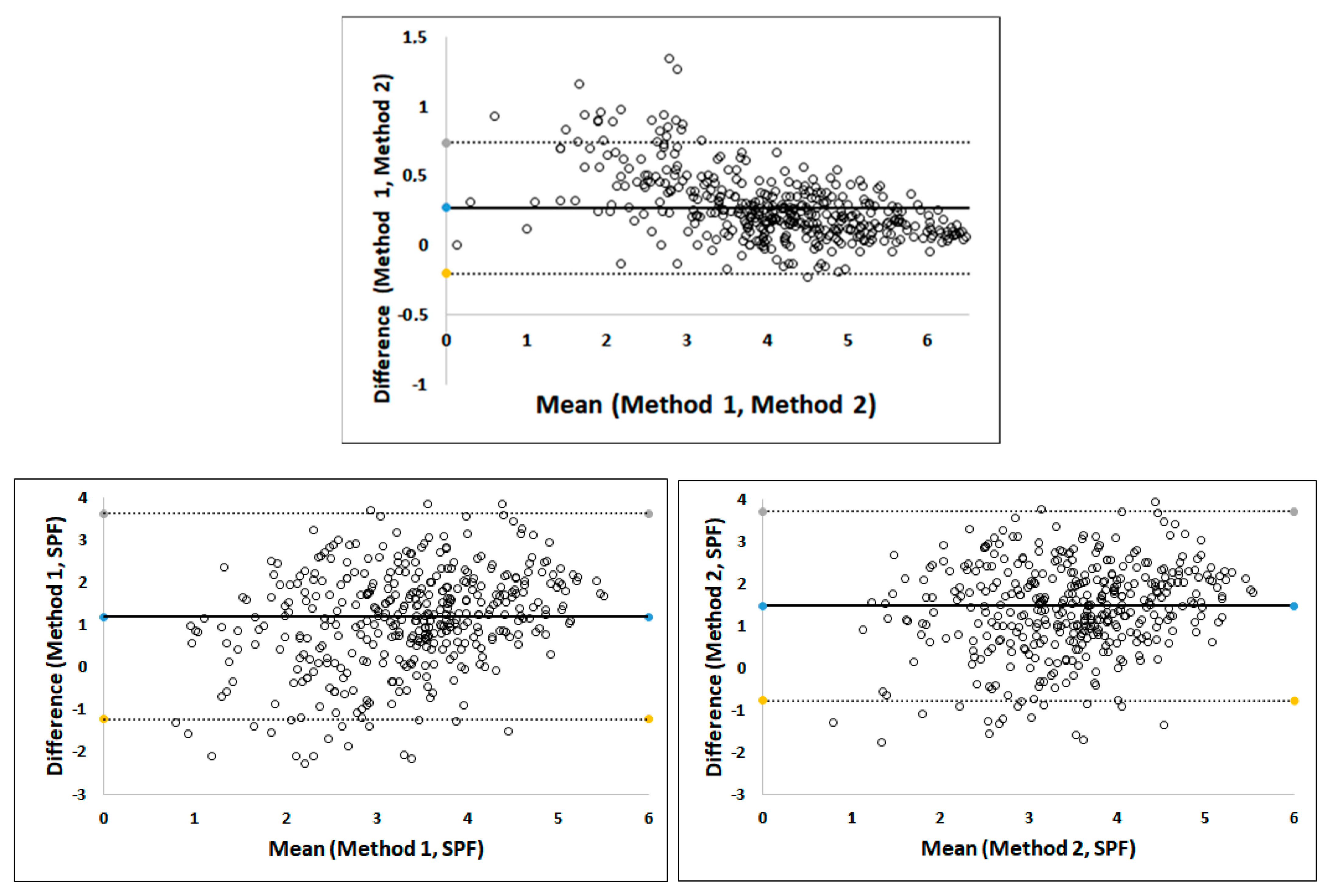
3.2.1. Agreement and Correlation for Ki-67 Scoring
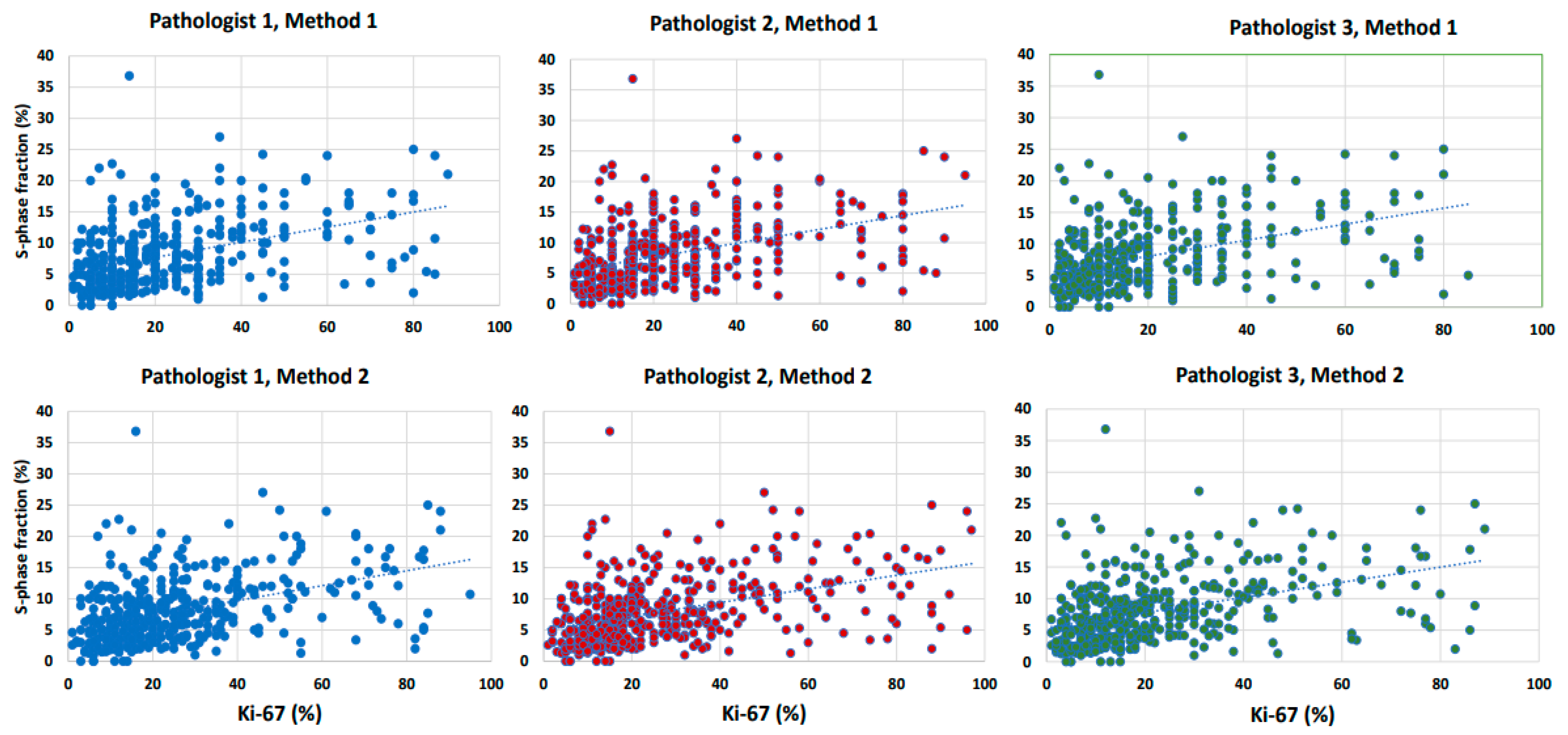
3.2.2. Agreement and Correlation between Ki-67 and SPF
3.3. Association of Clinic-Pathological Characteristics with Proliferative Indexes SPF and Ki-67
3.4. Treatment and Outcome
3.5. Survival Analysis
3.5.1. Time to Recurrences According to SPF and Ki-67 in All Patients
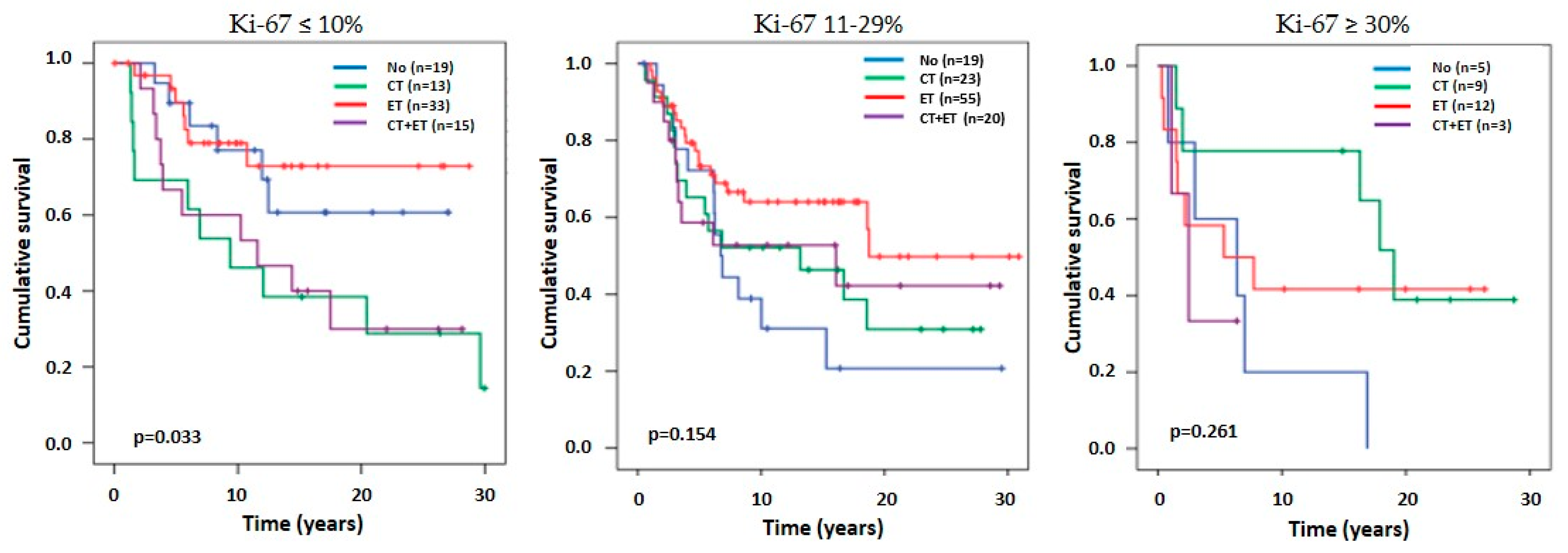
3.5.2. Time to Recurrence According to Ki-67 Groups in ER-Positive Patients
3.5.3. Univariate and Multivariate Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Epidemiology and Cancer Registry, S.C.R. Cancer in Slovenia 2020; Institute of Oncology Ljubljana: Ljubljana, Slovenia, 2023. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Beresford, M.J.; Wilson, G.D.; Makris, A. Measuring proliferation in breast cancer: Practicalities and applications. Breast Cancer Res. 2006, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, J.; Lemke, H.; Baisch, H.; Wacker, H.H.; Schwab, U.; Stein, H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984, 133, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Shak, S.; Tang, G.; Kim, C.; Baker, J.; Cronin, M.; Baehner, F.L.; Walker, M.G.; Watson, D.; Park, T.; et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 2004, 351, 2817–2826. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, C.; Wirapati, P.; Loi, S.; Harris, A.; Fox, S.; Smeds, J.; Nordgren, H.; Farmer, P.; Praz, V.; Haibe-Kains, B.; et al. Gene expression profiling in breast cancer: Understanding the molecular basis of histologic grade to improve prognosis. J. Natl. Cancer Inst. 2006, 98, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Yerushalmi, R.; Woods, R.; Ravdin, P.M.; Hayes, M.M.; Gelmon, K.A. Ki67 in breast cancer: Prognostic and predictive potential. Lancet Oncol. 2010, 11, 174–183. [Google Scholar] [CrossRef]
- Cheang, M.C.; Chia, S.K.; Voduc, D.; Gao, D.; Leung, S.; Snider, J.; Watson, M.; Davies, S.; Bernard, P.S.; Parker, J.S.; et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J. Natl. Cancer Inst. 2009, 101, 736–750. [Google Scholar] [CrossRef]
- Feeley, L.P.; Mulligan, A.M.; Pinnaduwage, D.; Bull, S.B.; Andrulis, I.L. Distinguishing luminal breast cancer subtypes by Ki67, progesterone receptor or TP53 status provides prognostic information. Mod. Pathol. 2014, 27, 554–561. [Google Scholar] [CrossRef]
- Smith, I.; Robertson, J.; Kilburn, L.; Wilcox, M.; Evans, A.; Holcombe, C.; Horgan, K.; Kirwan, C.; Mallon, E.; Sibbering, M.; et al. Long-term outcome and prognostic value of Ki67 after perioperative endocrine therapy in postmenopausal women with hormone-sensitive early breast cancer (POETIC): An open-label, multicentre, parallel-group, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1443–1454. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Senn, H.J. Strategies for subtypes--dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- de Azambuja, E.; Cardoso, F.; de Castro, G., Jr.; Colozza, M.; Mano, M.S.; Durbecq, V.; Sotiriou, C.; Larsimont, D.; Piccart-Gebhart, M.J.; Paesmans, M. Ki-67 as prognostic marker in early breast cancer: A meta-analysis of published studies involving 12,155 patients. Br. J. Cancer 2007, 96, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Gazic, B.; Pizem, J.; Bracko, M.; Cufer, T.; Borstnar, S.; Pohar-Marinsek, Z.; Us-Krasovec, M. S-phase fraction determined on fine needle aspirates is an independent prognostic factor in breast cancer—A multivariate study of 770 patients. Cytopathology 2008, 19, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Colozza, M.; Azambuja, E.; Cardoso, F.; Sotiriou, C.; Larsimont, D.; Piccart, M.J. Proliferative markers as prognostic and predictive tools in early breast cancer: Where are we now? Ann. Oncol. 2005, 16, 1723–1739. [Google Scholar] [CrossRef]
- Gasparini, G.; Pozza, F.; Meli, S.; Reitano, M.; Santini, G.; Bevilacqua, P. Breast cancer cell kinetics: Immunocytochemical determination of growth fractions by monoclonal antibody Ki-67 and correlation with flow cytometric S-phase and with some features of tumor aggressiveness. Anticancer Res. 1991, 11, 2015–2021. [Google Scholar] [PubMed]
- Dettmar, P.; Harbeck, N.; Thomssen, C.; Pache, L.; Ziffer, P.; Fizi, K.; Jänicke, F.; Nathrath, W.; Schmitt, M.; Graeff, H.; et al. Prognostic impact of proliferation-associated factors MIB1 (Ki-67) and S-phase in node-negative breast cancer. Br. J. Cancer 1997, 75, 1525–1533. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vielh, P.; Chevillard, S.; Mosseri, V.; Donatini, B.; Magdelenat, H. Ki67 Index and S-Phase Fraction in Human Breast Carcinomas: Comparison and Correlations with Prognostic Factors. Am. J. Clin. Pathol. 1990, 94, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Masood, S.; Bui, M.M.; Lu, L. Comparison of proliferation activity in breast carcinoma by flow cytometry analysis of S-phase and quantitative analysis of MIB-1. Ann. Clin. Lab. Sci. 1998, 28, 315–323. [Google Scholar]
- Keshgegian, A.A.; Cnaan, A. Proliferation markers in breast carcinoma. Mitotic figure count, S-phase fraction, proliferating cell nuclear antigen, Ki-67 and MIB-1. Am. J. Clin. Pathol. 1995, 104, 42–49. [Google Scholar] [CrossRef]
- González-Vela, M.C.; Garijo, M.F.; Fernández, F.; Val-Bernal, J.F. MIB1 proliferation index in breast infiltrating carcinoma: Comparison with other proliferative markers and association with new biological prognostic factors. Histol. Histopathol. 2001, 16, 399–406. [Google Scholar] [CrossRef] [PubMed]
- MacGrogan, G.; Jollet, I.; Huet, S.; Sierankowski, G.; Picot, V.; Bonichon, F.; Coindre, J.M. Comparison of quantitative and semiquantitative methods of assessing MIB-1 with the S-phase fraction in breast carcinoma. Mod. Pathol. 1997, 10, 769–776. [Google Scholar] [PubMed]
- Barzanti, F.; Dal Susino, M.; Volpi, A.; Amadori, D.; Riccobon, A.; Scarpi, E.; Medri, L.; Bernardi, L.; Naldi, S.; Aldi, M.; et al. Comparison between different cell kinetic variables in human breast cancer. Cell Prolif. 2000, 33, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.L.; Hupperets, P.S.; Arends, J.W.; Joosten-Achjanie, S.R.; Volovics, A.; Schouten, H.C.; Hillen, H.F. MIB-1 labelling index is an independent prognostic marker in primary breast cancer. Br. J. Cancer 1998, 78, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, M.; Nielsen, T.O.; A’Hern, R.; Bartlett, J.; Coombes, R.C.; Cuzick, J.; Ellis, M.; Henry, N.L.; Hugh, J.C.; Lively, T.; et al. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer working group. J. Natl. Cancer Inst. 2011, 103, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of Ki67 in Breast Cancer: Updated Recommendations from the International Ki67 in Breast Cancer Working Group. J. Natl. Cancer Inst. 2021, 113, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Polley, M.Y.; Leung, S.C.; Gao, D.; Mastropasqua, M.G.; Zabaglo, L.A.; Bartlett, J.M.; McShane, L.M.; Enos, R.A.; Badve, S.S.; Bane, A.L.; et al. An international study to increase concordance in Ki67 scoring. Mod. Pathol. 2015, 28, 778–786. [Google Scholar] [CrossRef]
- Leung, S.C.Y.; Nielsen, T.O.; Zabaglo, L.; Arun, I.; Badve, S.S.; Bane, A.L.; Bartlett, J.M.S.; Borgquist, S.; Chang, M.C.; Dodson, A.; et al. Analytical validation of a standardized scoring protocol for Ki67: Phase 3 of an international multicenter collaboration. NPJ Breast Cancer 2016, 2, 16014. [Google Scholar] [CrossRef]
- Polley, M.Y.; Leung, S.C.; McShane, L.M.; Gao, D.; Hugh, J.C.; Mastropasqua, M.G.; Viale, G.; Zabaglo, L.A.; Penault-Llorca, F.; Bartlett, J.M.; et al. An international Ki67 reproducibility study. J. Natl. Cancer Inst. 2013, 105, 1897–1906. [Google Scholar] [CrossRef]
- Senkus, E.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rutgers, E.; Zackrisson, S.; Cardoso, F. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. S5), v8–v30. [Google Scholar] [CrossRef]
- McGuire, W.L.; Horwitz, K.B. A role for progesterone in breast cancer. Ann. N. Y. Acad. Sci. 1977, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.M.; Kelly, A.B.; Jewell, S.D.; McShane, L.M.; Clark, D.P.; Greenspan, R.; Hayes, D.F.; Hainaut, P.; Kim, P.; Mansfield, E.A.; et al. Biospecimen reporting for improved study quality (BRISQ). Cancer Cytopathol. 2011, 119, 92–101. [Google Scholar] [CrossRef] [PubMed]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br. J. Cancer 2005, 93, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Otto, F. DAPI staining of fixed cells for high-resolution flow cytometry of nuclear DNA. Methods Cell Biol. 1990, 33, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Pogacnik, A.; Us-Krasovec, M.; Bracko, M. Preparation of fine needle aspiration biopsy samples for flow cytometric analysis. Anal. Quant. Cytol. Histol. 1993, 15, 298–302. [Google Scholar] [PubMed]
- Jones, R.L.; Salter, J.; A’Hern, R.; Nerurkar, A.; Parton, M.; Reis-Filho, J.S.; Smith, I.E.; Dowsett, M. Relationship between oestrogen receptor status and proliferation in predicting response and long-term outcome to neoadjuvant chemotherapy for breast cancer. Breast Cancer Res. Treat. 2010, 119, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Agreement between methods of measurement with multiple observations per individual. J. Biopharm. Stat. 2007, 17, 571–582. [Google Scholar] [CrossRef]
- Available online: https://blogs.sas.com/content/iml/2023/04/05/interpret-spearman-kendall-corr.html (accessed on 18 January 2024).
- Leung, S.C.Y.; Nielsen, T.O.; Zabaglo, L.A.; Arun, I.; Badve, S.S.; Bane, A.L.; Bartlett, J.M.S.; Borgquist, S.; Chang, M.C.; Dodson, A.; et al. Analytical validation of a standardised scoring protocol for Ki67 immunohistochemistry on breast cancer excision whole sections: An international multicentre collaboration. Histopathology 2019, 75, 225–235. [Google Scholar] [CrossRef]
- Laenkholm, A.V.; Grabau, D.; Møller Talman, M.L.; Balslev, E.; Bak Jylling, A.M.; Tabor, T.P.; Johansen, M.; Brügmann, A.; Lelkaitis, G.; Di Caterino, T.; et al. An inter-observer Ki67 reproducibility study applying two different assessment methods: On behalf of the Danish Scientific Committee of Pathology, Danish breast cancer cooperative group (DBCG). Acta Oncol. 2018, 57, 83–89. [Google Scholar] [CrossRef]
- Catteau, X.; Zindy, E.; Bouri, S.; Noel, J.C.; Salmon, I.; Decaestecker, C. Comparison Between Manual and Automated Assessment of Ki-67 in Breast Carcinoma: Test of a Simple Method in Daily Practice. Technol. Cancer Res. Treat. 2023, 22, 15330338231169603. [Google Scholar] [CrossRef] [PubMed]
- Lashen, A.; Toss, M.S.; Green, A.R.; Mongan, N.P.; Rakha, E. Ki67 assessment in invasive luminal breast cancer: A comparative study between different scoring methods. Histopathology 2022, 81, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Del Rosario Taco Sanchez, M.; Soler-Monso, T.; Petit, A.; Azcarate, J.; Lasheras, A.; Artal, C.; Gil, M.; Falo, C.; Pla, M.J.; Matias-Guiu, X. Digital quantification of KI-67 in breast cancer. Virchows Arch. 2019, 474, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.H.; Gonen, M.; Hedvat, C.; Modlin, I.M.; Klimstra, D.S. Objective quantification of the Ki67 proliferative index in neuroendocrine tumors of the gastroenteropancreatic system: A comparison of digital image analysis with manual methods. Am. J. Surg. Pathol. 2012, 36, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, E.J.; Duarte, R.; Dickens, C.; Joffe, M.; Mohanlal, R. Ki67 Immunohistochemistry Quantification in Breast Carcinoma: A Comparison of Visual Estimation, Counting, and ImmunoRatio. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 105–111. [Google Scholar] [CrossRef]
- Hida, A.I.; Bando, K.; Sugita, A.; Maeda, T.; Ueda, N.; Matsukage, S.; Nakanishi, M.; Kito, K.; Miyazaki, T.; Ohtsuki, Y.; et al. Visual assessment of Ki67 using a 5-grade scale (Eye-5) is easy and practical to classify breast cancer subtypes with high reproducibility. J. Clin. Pathol. 2015, 68, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Hida, A.I.; Oshiro, Y.; Inoue, H.; Kawaguchi, H.; Yamashita, N.; Moriya, T. Visual assessment of Ki67 at a glance is an easy method to exclude many luminal-type breast cancers from counting 1000 cells. Breast Cancer 2015, 22, 129–134. [Google Scholar] [CrossRef]
- Shim, V.C.; Baker, R.J.; Jing, W.; Puentes, R.; Agersborg, S.S.; Lee, T.K.; Gorea, I.W.; Achacoso, N.; Lee, C.; Villasenor, M.; et al. Evaluation of the international Ki67 working group cut point recommendations for early breast cancer: Comparison with 21-gene assay results in a large integrated health care system. Breast Cancer Res. Treat. 2023, 203, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Diebold, J.; Dommann-Scherrer, C.; Frick, H.; Kaup, D.; Noske, A.; Obermann, E.; Ohlschlegel, C.; Padberg, B.; Rakozy, C.; et al. How reliable is Ki-67 immunohistochemistry in grade 2 breast carcinomas? A QA study of the Swiss Working Group of Breast- and Gynecopathologists. PLoS ONE 2012, 7, e37379. [Google Scholar] [CrossRef]
- Focke, C.M.; van Diest, P.J.; Decker, T. St Gallen 2015 subtyping of luminal breast cancers: Impact of different Ki67-based proliferation assessment methods. Breast Cancer Res. Treat. 2016, 159, 257–263. [Google Scholar] [CrossRef]
- Spyratos, F.; Ferrero-Poüs, M.; Trassard, M.; Hacène, K.; Phillips, E.; Tubiana-Hulin, M.; Le Doussal, V. Correlation between MIB-1 and other proliferation markers: Clinical implications of the MIB-1 cutoff value. Cancer 2002, 94, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Skjervold, A.H.; Pettersen, H.S.; Valla, M.; Opdahl, S.; Bofin, A.M. Visual and digital assessment of Ki-67 in breast cancer tissue—A comparison of methods. Diagn. Pathol. 2022, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; Acs, B.; Lippert, M.; Hartman, J. Prognostic potential of automated Ki67 evaluation in breast cancer: Different hot spot definitions versus true global score. Breast Cancer Res. Treat. 2020, 183, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Lashen, A.G.; Wahab, N.; Miligy, I.M.; Jahanifar, M.; Toss, M.; Graham, S.; Bilal, M.; Bhalerao, A.; Atallah, N.M.; et al. AI-based intra-tumor heterogeneity score of Ki67 expression as a prognostic marker for early-stage ER+/HER2- breast cancer. J. Pathol. Clin. Res. 2024, 10, e346. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.; Orr, N.; Daley, F.; Coulson, P.; Ali, H.R.; Blows, F.; Benitez, J.; Milne, R.; Brenner, H.; Stegmaier, C.; et al. Prognostic value of automated KI67 scoring in breast cancer: A centralised evaluation of 8088 patients from 10 study groups. Breast Cancer Res. 2016, 18, 104. [Google Scholar] [CrossRef] [PubMed]
- Dy, A.; Nguyen, N.J.; Meyer, J.; Dawe, M.; Shi, W.; Androutsos, D.; Fyles, A.; Liu, F.F.; Done, S.; Khademi, A. AI improves accuracy, agreement and efficiency of pathologists for Ki67 assessments in breast cancer. Sci. Rep. 2024, 14, 1283. [Google Scholar] [CrossRef] [PubMed]
- Stuart-Harris, R.; Caldas, C.; Pinder, S.E.; Pharoah, P. Proliferation markers and survival in early breast cancer: A systematic review and meta-analysis of 85 studies in 32,825 patients. Breast 2008, 17, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Nitz, U.; Gluz, O.; Clemens, M.; Malter, W.; Reimer, T.; Nuding, B.; Aktas, B.; Stefek, A.; Pollmanns, A.; Lorenz-Salehi, F.; et al. West German Study PlanB Trial: Adjuvant Four Cycles of Epirubicin and Cyclophosphamide Plus Docetaxel Versus Six Cycles of Docetaxel and Cyclophosphamide in HER2-Negative Early Breast Cancer. J. Clin. Oncol. 2019, 37, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Nitz, U.A.; Gluz, O.; Kümmel, S.; Christgen, M.; Braun, M.; Aktas, B.; Lüdtke-Heckenkamp, K.; Forstbauer, H.; Grischke, E.M.; Schumacher, C.; et al. Endocrine Therapy Response and 21-Gene Expression Assay for Therapy Guidance in HR+/HER2- Early Breast Cancer. J. Clin. Oncol. 2022, 40, 2557–2567. [Google Scholar] [CrossRef]
- Gray, R.G.; Bradley, R.; Braybrooke, J.; Clarke, M.; Hills, R.K.; Peto, R.; Bergh, J.C.S.; Swain, S.M.; Davidson, N.E.; Francis, P.A.; et al. Effects of ovarian ablation or suppression on breast cancer recurrence and survival: Patient-level meta-analysis of 14,993 pre-menopausal women in 25 randomized trials. J. Clin. Oncol. 2023, 41, 503. [Google Scholar] [CrossRef]
- Pagani, O.; Walley, B.A.; Fleming, G.F.; Colleoni, M.; Láng, I.; Gomez, H.L.; Tondini, C.; Burstein, H.J.; Goetz, M.P.; Ciruelos, E.M.; et al. Adjuvant Exemestane With Ovarian Suppression in Premenopausal Breast Cancer: Long-Term Follow-Up of the Combined TEXT and SOFT Trials. J. Clin. Oncol. 2023, 41, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.S.; Winer, E.P.; Goldhirsch, A.; Gelber, R.D.; Gnant, M.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann. Oncol. 2015, 26, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Han, G.; Mani, N.; Beruti, S.; Nerenberg, M.; Rimm, D.L. Loss of antigenicity with tissue age in breast cancer. Lab. Investig. 2016, 96, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.N.; Acs, B.; Warrell, J.; Bai, Y.; Gaule, P.; Martinez-Morilla, S.; Vathiotis, I.; Shafi, S.; Moutafi, M.; Gerstein, M.; et al. A new tool for technical standardization of the Ki67 immunohistochemical assay. Mod. Pathol. 2021, 34, 1261–1270. [Google Scholar] [CrossRef]
- Johansson, H.J.; Socciarelli, F.; Vacanti, N.M.; Haugen, M.H.; Zhu, Y.; Siavelis, I.; Fernandez-Woodbridge, A.; Aure, M.R.; Sennblad, B.; Vesterlund, M.; et al. Breast cancer quantitative proteome and proteogenomic landscape. Nat. Commun. 2019, 10, 1600. [Google Scholar] [CrossRef]


| Characteristic n (%) | All Patients n = 411 (100) | Low SPF (<6.7%) 219 (47) | High SPF (≥6.7%) 218 (53) | p-Value | Low Ki-67 1–10% 122 (29.7) | Ki-67 11–29% 193 (47.0) | High Ki-67 ≥30% 96 (23.3) | p-Value |
|---|---|---|---|---|---|---|---|---|
| Age median (IQR) | 58.9 (46.7–67.2) | 60.5 (47.7–68.6) | 58.3 (46.7–65.4) | 0.075 | 58.9 (47.7–68.3) | 60.5 (49.2–67.2) | 54.8 (44.4–65.4) | 0.042 1 0.023 2 |
| Age n (%) | 0.704 | 0.087 | ||||||
| <50 years | 123 (29.9) | 56 (45.5) | 67 (54.5) | 36 (29.3) | 50 (40.6) | 37 (30.1) | ||
| ≥50 years | 288 (70.1) | 137 (47.6) | 151 (52.4) | 86 (29.9) | 143 (49.6) | 59 (20.5) | ||
| Histology n (%) | 0.077 | 0.010 | ||||||
| NST | 350 (85.2) | 158 (45.1) | 192 (54.9) | 94 (26.9) | 170 (48.6) | 86 (24.6) | ||
| ILC and other | 61 (14.8) | 35 (57.4) | 26 (42.6) | 28 (45.9) | 23 (37.7) | 10 (16.4) | ||
| Grade n (%) | <0.0001 | <0.0001 | ||||||
| Grade 1 | 42 (10.2) | 33 (78.6) | 9 (21.4) | 31 (73.8) | 11 (26.2) | 0 (0) | ||
| Grade 2 | 170 (41.4) | 104 (61.2) | 66 (38.8) | 76 (44.7) | 88 (51.8) | 6 (3.5) | ||
| Grade 3 | 199 (48.4) | 56 (28.1) | 143 (71.9) | 15 (7.6) | 94 (47.2) | 90 (45.2) | ||
| LVI n (%) | 0.011 | 0.339 | ||||||
| Absent | 218 (68.3) | 111 (50.9) | 107 (49.1) | 74 (33.9) | 101 (46.3) | 43 (19.7) | ||
| Present | 191 (31.7) | 36 (36.6) | 65 (64.4) | 26 (25.7) | 53 (52.5) | 22 (21.8) | ||
| Tumour stage n (%) | 0.038 | 0.010 | ||||||
| pT1 (≤20 mm) | 154 (37.5) | 85 (55.2) | 69 (44.8) | 55 (35.7) | 73 (47.4) | 26 (16.9) | ||
| pT2 (>20≤50) | 221 (53.8) | 93 (22.1) | 128 (57.9) | 53 (24) | 110 (49.8) | 58 (26.2) | ||
| pT3 (>50 mm) | 35 (8.5) | 15 (42.9) | 20 (57.1) | 13 (37.1) | 10 (28.6) | 12 (34.3) | ||
| Missing | 1 (0.2) | |||||||
| Lymph nodes n (%) | 0.260 | 0.559 | ||||||
| pN0 (negative) | 227 (55.2) | 116 (51.1) | 111 (48.9) | 63 (27.7) | 108 (47.6) | 56 (24.7) | ||
| pN1 (1–3 positive) | 100 (24.3) | 44 (44.0) | 56 (56.0) | 35 (35.0) | 44 (44.0) | 21 (21.0) | ||
| pN2 (4–9 positive) | 49 (11.9) | 20 (40.8) | 29 (59.2) | 15 (39.6) | 26 (53.1) | 8 (16.3) | ||
| pN3 (≥10 positive) | 35 (8.5) | 13 (37.1) | 22 (62.9) | 9 (25.7) | 15 (42.9) | 11 (31.4) | ||
| ER n (%) | <0.0001 | <0.0001 | ||||||
| ER negative | 126 (31.3) | 38 (30.2) | 88 (69.8) | 24 (19.0) | 44 (35.0) | 58 (46.0) | ||
| ER positive | 276 (68.7) | 15 1 (54.7) | 125 (45.3) | 96 (34.8) | 145 (52.5) | 35 (12.7) |
| Intraclass Correlation | 95% Confidence Interval | F-Test with True Value 0 | |||||
|---|---|---|---|---|---|---|---|
| Single Measures | Lower Bound | Upper Bound | Value | df1 | df2 | Sig. | |
| Method 1 | 0.962 | 0.926 | 0.978 | 110.934 | 410 | 410 | 0.000 |
| Method 2 | 0.946 | 0.888 | 0.969 | 79.217 | 410 | 820 | 0.000 |
| Type of Treatment | All Patients (n = 411) | Low SPF (<6.7%) (n = 193) | High SPF (≥6.7%) (n = 218) | p-Value | Low Ki-67 1–10% 122 (29.7) | Intermediate Ki-67 11–29% 193 (47.0) | High Ki-67 ≥30% 96 (23.3) | p-Value |
|---|---|---|---|---|---|---|---|---|
| Type of surgery | 0.780 | 0.864 | ||||||
| Mastectomy | 274 (66.7) | 130 (47.4) | 144 (52.6) | 83 (30.3) | 129 (47.1) | 62 (22.6) | ||
| BCS | 137 (33.3) | 63 (46) | 74 (54) | 39 (28.5) | 64 (46.7) | 34 (24.8) | ||
| Adjuvant RT | 148 (36) | 57 (38.5) | 91 (61.5) | 0.010 | 42 (28.4) | 65 (43.9) | 41 (27.7) | 0.293 |
| Adjuvant ET | 224 (55.7) | 116 (51.8) | 108 (42.8) | 0.001 | 69 (30.8) | 115 (51.3) | 40 (17.9) | <0.0001 |
| ER negative | 50 (22.3) | 16 (32) | 34 (68) | 9 (18) | 20 (40) | 21 (42) | ||
| ER positive | 174 (77.7) | 100 (57.5) | 74 (42.5) | 60 (34.5) | 95 (54.6) | 19 (10.9) | ||
| Adjuvant CMF | 207 (50.4) | 86 (41.5) | 121 (58.5) | 0.001 | 54 (26.5) | 92 (45.1) | 58 (28.4) | <0.0001 |
| ER negative | 84 (41.2) | 23 (27.4) | 62 (72.6) | 15 (17.9) | 26 (31) | 43 (51.1) | ||
| ER positive | 120 (58.8) | 62 (51.7) | 58 (48.3) | 39 (32.5) | 66 (55) | 15 (12.5) | ||
| Events | ||||||||
| Relapse | 221 (53.1) | 99 (44.8) | 122 (55.2) | 0.213 | 56 (25.3) | 112 (50.2) | 53 (24.0) | 0.104 |
| Death | 316 (76.9) | 151 (47.8) | 165 (52.2) | 0.540 | 92 (29.1) | 155 (49.1) | 69 (21.8) | 0.249 |
| Time to Progression | Relapse-Free Survival | Overall Survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable HR (95%CI) | p-Value | Multivariable HR (95% CI) | p-Value | Univariable HR (95% CI) | p-Value | Multivariable HR (95% CI) | p-Value | Univariable HR (95% CI) | p-Value | Multivariable HR (95% CI) | p-Value | |
| Age < 50 years | 1.00 | 0.214 | / | / | 1.00 | <0.0001 | 1.00 | <0.0001 | 1.00 | <0.0001 | 1.00 | <0.0001 |
| Age ≥ 50 years | 1.20 (0.90–1.61) | 1.80 (1.40–2.32) | 1.83 (1.36–2.47) | 2.25 (1.72–2.94) | 2.29 (1.66–3.15) | |||||||
| Histology | ||||||||||||
| NST | 1.00 | 0.506 | / | / | 1.00 | 0.753 | / | / | 1.00 | 0.592 | / | / |
| ILC + other | 1.13 (0.79–1.60) | 1.05 (0.78–1.41) | 0.92 (0.67–1.25) | |||||||||
| Grade | ||||||||||||
| Grade 1 | 1.00 | 0.082 | 1.00 | 0.518 | 1.00 | 0.206 | / | / | 1.00 | 0.077 | 1.00 | 0.397 |
| Grade 2 | 1.50 (0.89–2.52) | 0.126 | 1.23 (0.63–2.41) | 0.539 | 1.41 (0.96–2.06) | 0.077 | 1.55 (1.04–2.30) | 0.032 | 1.27 (0.78 (2.09) | 0.340 | ||
| Grade 3 | 1.75 (1.05–2.91) | 0.031 | 1.41 (0.72–2.74) | 0.316 | 1.29 (0.89–1.88) | 0.180 | 1.55 (1.04–2.29) | 0.030 | 1.40 (0.85–2.30) | 0.185 | ||
| Tumor stage | ||||||||||||
| pT1 (≤20 mm) | 1.00 | <0.0001 | 1.00 | 0.898 | 1.00 | <0.0001 | 1.00 | 0.489 | 1.00 | 0.001 | 1.00 | 0.772 |
| pT2 (21–50 mm) | 1.49 (1.11–1.99) | 0.008 | 1.04 (0.74–1.48) | 0.809 | 1.42 (1.13–1.80) | 0.003 | 1.07 (0.81–1.41) | 0.635 | 1.37 (1.08–1.74) | 0.010 | 1.00 (0.75–1.33) | 0.994 |
| pT3 (>50 mm) | 2.73 (1.74–4.28) | <0.0001 | 0.92 (0.50–1.70) | 0.797 | 2.60 (1.75–3.82) | <0.0001 | 1.39 (0.81–2.38) | 0.232 | 1.98 (1.33–2.95) | 0.001 | 1.20 (0.71–2.02) | 0.505 |
| Nodal stage | ||||||||||||
| pN0 (negative) | 1.00 | <0.001 | 1.00 | <0.0001 | 1.00 | <0.0001 | 1.00 | <0.0001 | 1.00 | <0.0001 | 1.00 | <0.0001 |
| pN1 (1–3 positive) | 1.19 (0.86–1.65) | 0.294 | 1.23 (0.83–1.82) | 0.310 | 1.05 (0.80–1.36) | 0.747 | 1.81 (0.86–1.62) | 0.301 | 1.04 (0.79–1.37) | 0.792 | 1.31 (0.95–1.81) | 0.102 |
| pN2 (4–9 positive) | 2.29 (1.56–3.37) | <0.0001 | 2.20 (1.40–3.46) | 0.001 | 2.33 (1.68–3.24) | <0.0001 | 2.13 (1.47–3.09) | <0.0001 | 2.69 (1.92–3.77) | <0.0001 | 2.72 (1.85–4.00) | <0.0001 |
| pN3 (≥10 positive) | 3.98 (2.61–6.08) | <0.0001 | 3.75 (2.20–6.37) | <0.0001 | 43.21 (2.20–4.68) | <0.0001 | 2.52 (1.57 (4.07) | <0.0001 | 3.35 (2.92–4.89) | <0.0001 | 2.84 1.79–4.53) | <0.0001 |
| LVI | ||||||||||||
| No | 1.00 | <0.0001 | 1.00 | <0.0001 | 1.00 | <0.0001 | 1.00 | 0.003 | 1.00 | 0.007 | 1.00 | 0.022 |
| Yes | 2.37 (1.74–3.23) | 1.98 (1.42–2.76) | 1.70 (1.31–2.21) | 1.54 (1.16–2.04) | 1.45 (1.1–1.89) | 1.40 (1.05–1.88) | ||||||
| ER negative | 1.00 | 0.917 | / | / | 1.00 | 0.571 | / | / | 1.00 | 0.729 | / | / |
| ER positive | 0.99 (0.74–1.31) | 1.07 (0.84–1.36) | 1.04 (0.82–1.33) | |||||||||
| Low SPF (<6.7%) | 1.00 | 0.232 | / | / | 1.00 | 0.530 | / | / | 1.00 | 0.409 | / | / |
| High SPF (≥6.7%) | 1.18 (0.90–1.53) | 1.07 (0.86–1.33) | 1.10 (0.88–1.37) | |||||||||
| Ki-67 | ||||||||||||
| Low (≤5%) | 1.00 | 0.324 | / | / | 1.00 | 0.623 | / | / | 1.00 | 0.760 | / | / |
| Intermediate (6–29%) | 1.38 (0.90–2.12) | 0.136 | 1.08 (0.78–1.49) | 0.649 | 1.05 (0.76–1.46) | 0.760 | ||||||
| High (≥30%) | 1.35 (0.84–2.18) | 0.214 | 0.95 (0.65–1.38) | 0.784 | 0.95 (0.65–1.39) | 0.794 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đokić, S.; Gazić, B.; Grčar Kuzmanov, B.; Blazina, J.; Miceska, S.; Čugura, T.; Grašič Kuhar, C.; Jeruc, J. Clinical and Analytical Validation of Two Methods for Ki-67 Scoring in Formalin Fixed and Paraffin Embedded Tissue Sections of Early Breast Cancer. Cancers 2024, 16, 1405. https://doi.org/10.3390/cancers16071405
Đokić S, Gazić B, Grčar Kuzmanov B, Blazina J, Miceska S, Čugura T, Grašič Kuhar C, Jeruc J. Clinical and Analytical Validation of Two Methods for Ki-67 Scoring in Formalin Fixed and Paraffin Embedded Tissue Sections of Early Breast Cancer. Cancers. 2024; 16(7):1405. https://doi.org/10.3390/cancers16071405
Chicago/Turabian StyleĐokić, Snežana, Barbara Gazić, Biljana Grčar Kuzmanov, Jerca Blazina, Simona Miceska, Tanja Čugura, Cvetka Grašič Kuhar, and Jera Jeruc. 2024. "Clinical and Analytical Validation of Two Methods for Ki-67 Scoring in Formalin Fixed and Paraffin Embedded Tissue Sections of Early Breast Cancer" Cancers 16, no. 7: 1405. https://doi.org/10.3390/cancers16071405
APA StyleĐokić, S., Gazić, B., Grčar Kuzmanov, B., Blazina, J., Miceska, S., Čugura, T., Grašič Kuhar, C., & Jeruc, J. (2024). Clinical and Analytical Validation of Two Methods for Ki-67 Scoring in Formalin Fixed and Paraffin Embedded Tissue Sections of Early Breast Cancer. Cancers, 16(7), 1405. https://doi.org/10.3390/cancers16071405






