Dexamethasone in Patients with Glioblastoma: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Screening
2.3. Data Collection
2.4. Analysis
3. Results
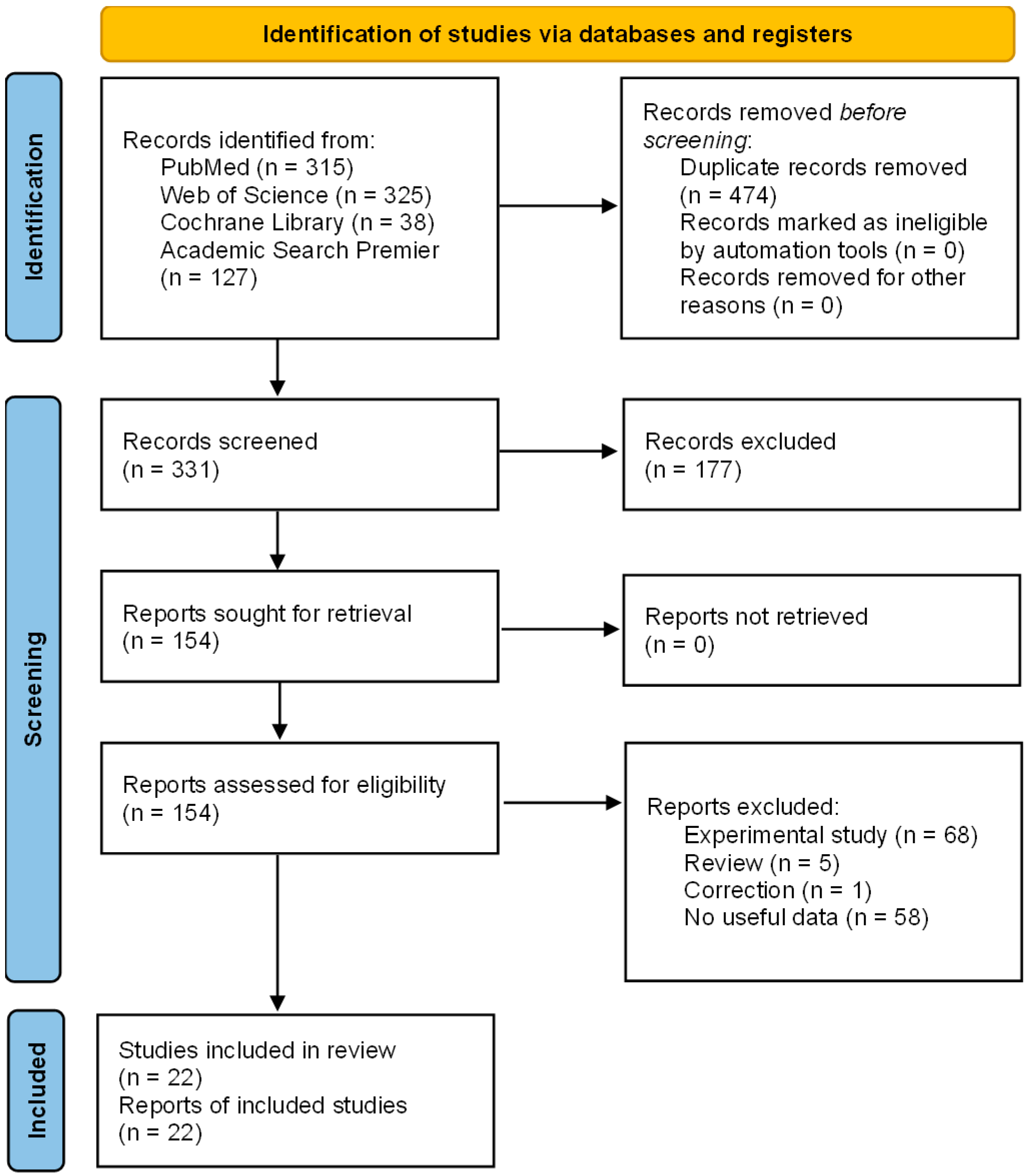
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias in Studies
3.4. Results for Individual Studies
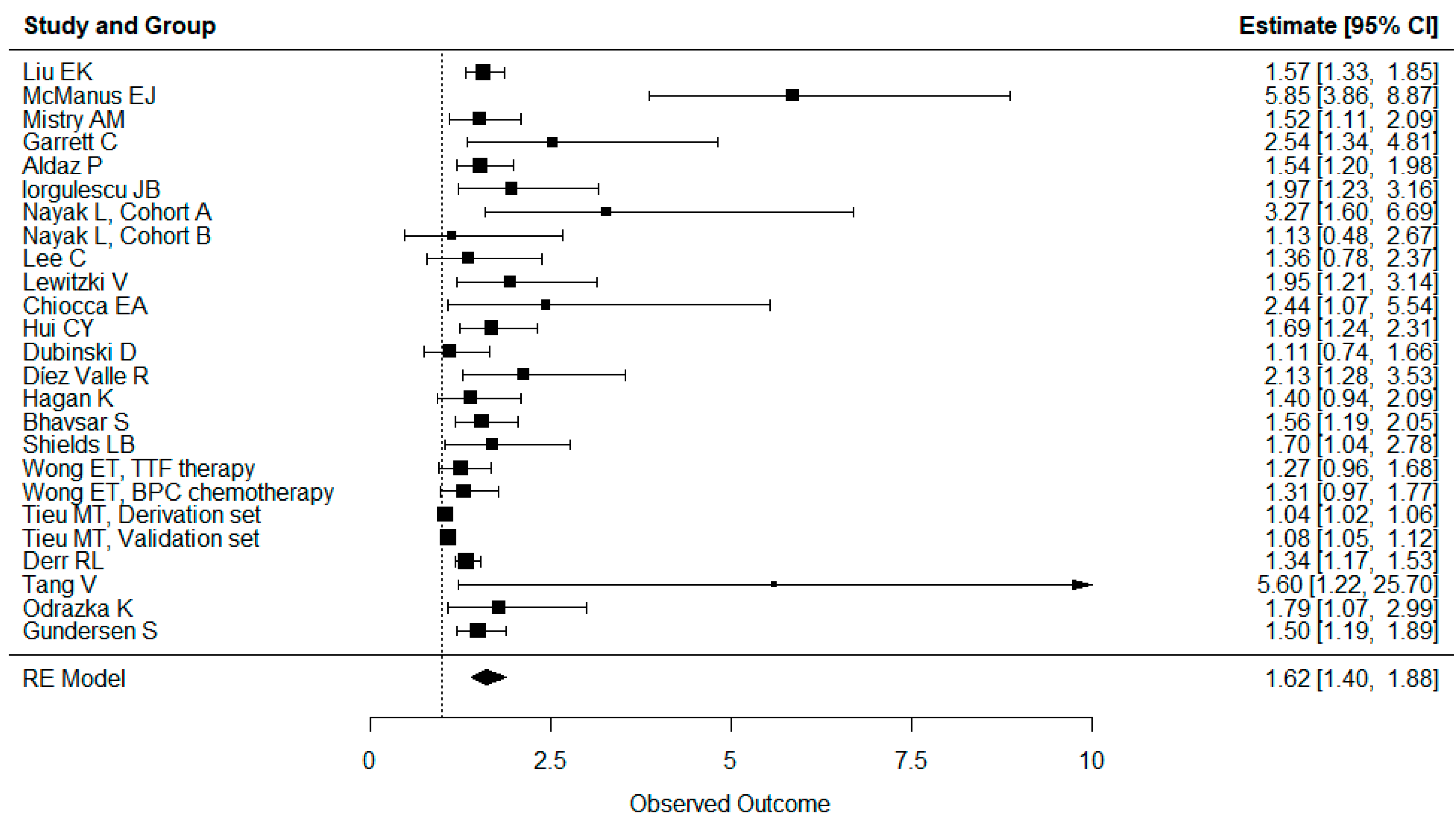
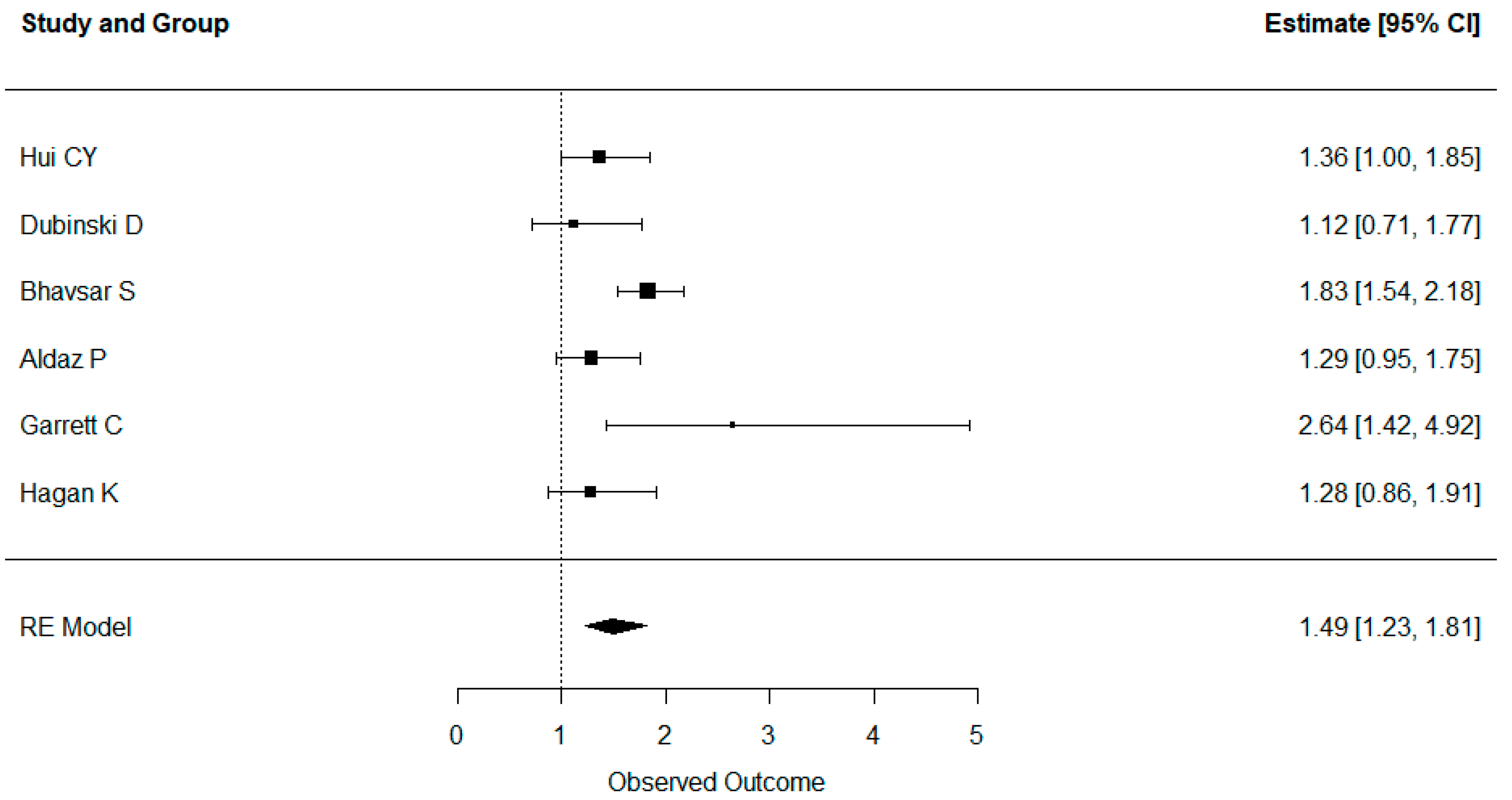
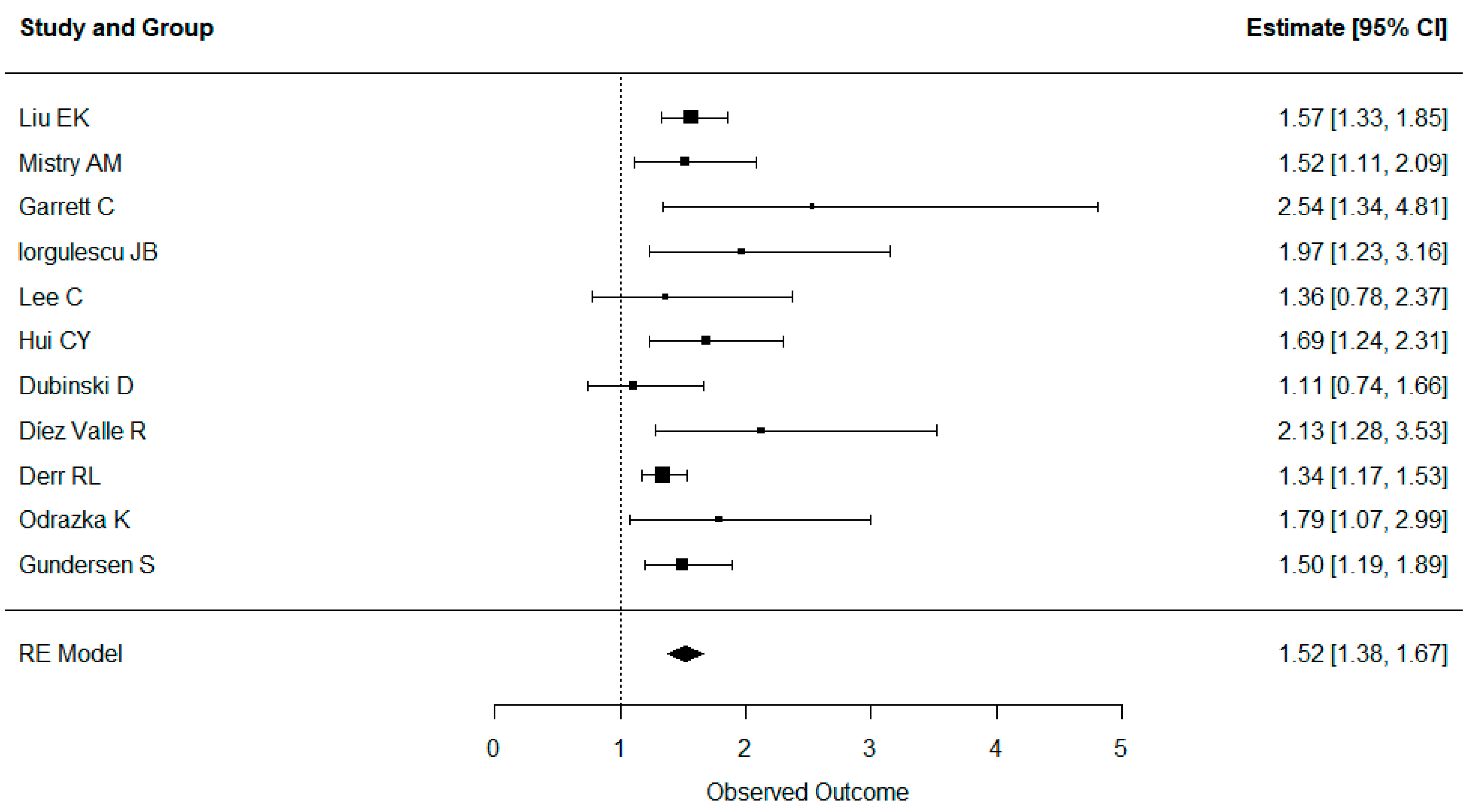
3.5. Results of Syntheses
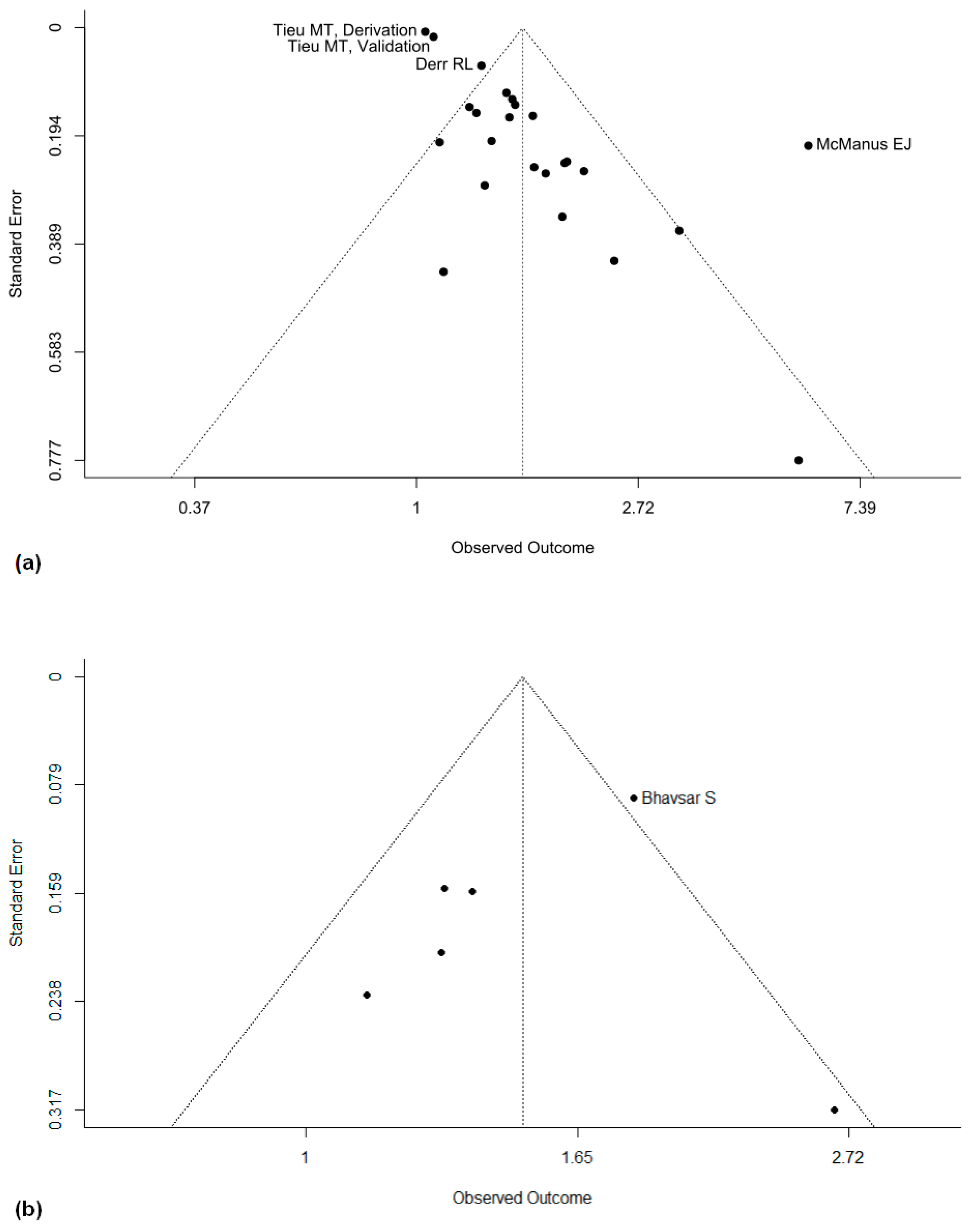
3.6. Reporting Biases
3.7. Certainty of Evidence
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncology 2019, 21 (Suppl. 5), v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Delgado-López, P.D.; Corrales-García, E.M. Survival in glioblastoma: A review on the impact of treatment modalities. Clin. Transl. Oncol. 2016, 18, 1062–1071. [Google Scholar] [CrossRef]
- Xie, Q.; Mittal, S.; Berens, M.E. Targeting adaptive glioblastoma: An overview of proliferation and invasion. Neuro-Oncology 2014, 16, 1575–1584. [Google Scholar] [CrossRef]
- Glen, A.E.; Johnston, D.B.R.; Fried, J.; Spooncer, W.W.; Hoff, D.R.; Sarett, L.H. 16-Methylated Steroids. I. 16α-Methylated Analogs of Cortisone, a New Group of Anti-Inflammatory Steroids. J. Am. Chem. Soc. 1958, 80, 3160–3161. [Google Scholar]
- Galicich, J.H.; French, L.A.; Melby, J.C. Use of dexamethasone in treatment of cerebral edema associated with brain tumors. J. Lancet 1961, 81, 46–53. [Google Scholar]
- McClelland, S., 3rd; Long, D.M. Genesis of the Use of Corticosteroids in the Treatment and Prevention of Brain Edema. Neurosurgery 2008, 62, 965–967; discussion 67–68. [Google Scholar] [CrossRef]
- Alberti, E.; Hartmann, A.; Schreckenberger, F.; Schütz, H.-J. The effect of large doses of dexamethasone on the cerebrospinal fluid pressure in patients with supratentorial tumors. J. Neurol. 1978, 217, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Palombi, L.; Marchetti, P.; Salvati, M.; Osti, M.F.; Frati, L.; Frati, A. Interventions to Reduce Neurological Symptoms in Patients with GBM Receiving Radiotherapy: From Theory to Clinical Practice. Anticancer. Res. 2018, 38, 2423–2427. [Google Scholar] [CrossRef]
- Villani, V.; Pace, A.; Vidiri, A.; Tanzilli, A.; Sperati, F.; Terrenato, I.; Mariantonia, C.; Casini, B.; Metro, G.; Maschio, M.; et al. Phase II study of weekly carboplatin in pretreated adult malignant gliomas. J. Neuro-Oncol. 2019, 144, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Cree, B.A. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133, 1380–1390.e2. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, A.; Feelders, R.A.; Stratakis, C.A.; Nieman, L.K. Cushing’s Syndrome. Lancet 2015, 386, 913–927. [Google Scholar] [CrossRef] [PubMed]
- Youssef, J.; Novosad, S.A.; Winthrop, K.L. Infection Risk and Safety of Corticosteroid Use. Rheum. Dis. Clin. N. Am. 2016, 42, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Alderson, P.; Roberts, I. Corticosteroids for Acute Traumatic Brain Injury. Cochrane Database Syst. Rev. 2005, 2005, Cd000196. [Google Scholar] [CrossRef] [PubMed]
- Pitter, K.L.; Tamagno, I.; Alikhanyan, K.; Hosni-Ahmed, A.; Pattwell, S.S.; Donnola, S.; Dai, C.; Ozawa, T.; Chang, M.; Chan, T.A.; et al. Corticosteroids Compromise Survival in Glioblastoma. Brain 2016, 139 Pt 5, 1458–1471. [Google Scholar] [CrossRef] [PubMed]
- Tieu, M.T.; Lovblom, L.E.; McNamara, M.G.; Mason, W.; Laperriere, N.; Millar, B.-A.; Ménard, C.; Kiehl, T.-R.; Perkins, B.A.; Chung, C. Impact of glycemia on survival of glioblastoma patients treated with radiation and temozolomide. J. Neuro-Oncol. 2015, 124, 119–126. [Google Scholar] [CrossRef]
- Shields, L.B.E.; Shelton, B.J.; Shearer, A.J.; Chen, L.; Sun, D.A.; Parsons, S.; Bourne, T.D.; LaRocca, R.; Spalding, A.C. Dexamethasone administration during definitive radiation and temozolomide renders a poor prognosis in a retrospective analysis of newly diagnosed glioblastoma patients. Radiat. Oncol. 2015, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Shen, Y.; Huang, T.; Sun, Y.; Alolga, R.N.; Zhang, G.; Ge, Y. The Prognostic Effect of Dexamethasone on Patients With Glioblastoma: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2021, 12, 727707. [Google Scholar] [CrossRef]
- Hagan, K.; Bhavsar, S.; Arunkumar, R.; Grasu, R.; Dang, A.; Carlson, R.; Cowles, C.; Arnold, B.; Potylchansky, Y.; Rahlfs, T.F.; et al. Association Between Perioperative Hyperglycemia and Survival in Patients With Glioblastoma. J. Neurosurg. Anesthesiol. 2017, 29, 21–29. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; Grade Working Group. Grade: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Aldaz, P.; Auzmendi-Iriarte, J.; Durántez, M.; Lasheras-Otero, I.; Carrasco-Garcia, E.; Zelaya, M.V.; Bragado, L.; Olías-Arjona, A.; Egaña, L.; Samprón, N.; et al. Identification of a Dexamethasone Mediated Radioprotection Mechanism Reveals New Therapeutic Vulnerabilities in Glioblastoma. Cancers 2021, 13, 361. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, S.; Hagan, K.; Arunkumar, R.; Potylchansky, Y.; Grasu, R.; Dang, A.; Carlson, R.; Cowels, C.; Arnold, B.; Rahlfs, T.; et al. Preoperative statin use is not associated with improvement in survival after glioblastoma surgery. J. Clin. Neurosci. 2016, 31, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Derr, R.L.; Ye, X.; Islas, M.U.; Desideri, S.; Saudek, C.D.; Grossman, S.A. Association Between Hyperglycemia and Survival in Patients With Newly Diagnosed Glioblastoma. J. Clin. Oncol. 2009, 27, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Dubinski, D.; Won, S.-Y.; Gessler, F.; Quick-Weller, J.; Behmanesh, B.; Bernatz, S.; Forster, M.-T.; Franz, K.; Plate, K.-H.; Seifert, V.; et al. Dexamethasone-induced leukocytosis is associated with poor survival in newly diagnosed glioblastoma. J. Neuro-Oncol. 2018, 137, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Garrett, C.; Becker, T.M.; Lynch, D.; Po, J.; Xuan, W.; Scott, K.F.; de Souza, P. Comparison of neutrophil to lymphocyte ratio and prognostic nutritional index with other clinical and molecular biomarkers for prediction of glioblastoma multiforme outcome. PLoS ONE 2021, 16, e0252614. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, S.; Lote, K.; Hannisdal, E. Prognostic Factors for Glioblastoma Multiforme: Development of a prognostic index. Acta Oncol. 1996, 35 (Suppl. 8), 123–127. [Google Scholar] [CrossRef] [PubMed]
- Schloss, M.H.; Freidberg, S.R.; Heatley, G.J.; Lo, T.C.M. Glucocorticoid Dependency as A Prognostic Factor in Radiotherapy for Cerebral Gliomas. Acta Oncol. 1989, 28, 51–55. [Google Scholar] [CrossRef]
- Hui, C.Y.; Rudra, S.; Ma, S.; Campian, J.L.; Huang, J. Impact of overall corticosteroid exposure during chemoradiotherapy on lymphopenia and survival of glioblastoma patients. J. Neuro-Oncol. 2019, 143, 129–136. [Google Scholar] [CrossRef]
- Iorgulescu, J.B.; Gokhale, P.C.; Speranza, M.C.; Eschle, B.K.; Poitras, M.J.; Wilkens, M.K.; Soroko, K.M.; Chhoeu, C.; Knott, A.; Gao, Y.; et al. Concurrent Dexamethasone Limits the Clinical Benefit of Immune Checkpoint Blockade in Glioblastoma. Clin. Cancer Res. 2021, 27, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, O.N.; Mohammad, A.; Bartek, J.; Winter, J.; Jung, M.; Stragliotto, G.; Söderberg-Nauclér, C.; Landázuri, N. Glucocorticoids promote a glioma stem cell-like phenotype and resistance to chemotherapy in human glioblastoma primary cells: Biological and prognostic significance. Int. J. Cancer 2018, 142, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Ahn, S.; Park, J.-S.; Song, J.H.; Hong, Y.-K.; Jeun, S.-S. Effect of Cumulative Dexamethasone Dose during Concomitant Chemoradiation on Lymphopenia in Patients with Newly Diagnosed Glioblastoma. Brain Tumor Res. Treat. 2020, 8, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Lewitzki, V.; Klement, R.J.; Kosmala, R.; Lisowski, D.; Flentje, M.; Polat, B. Accelerated hyperfractionated radiochemotherapy with temozolomide is equivalent to normofractionated radiochemotherapy in a retrospective analysis of patients with glioblastoma. Radiat. Oncol. 2019, 14, 227. [Google Scholar] [CrossRef] [PubMed]
- McManus, E.J.; Frampton, C.; Tan, A.; Phillips, M.C.L. Metabolics risk factors in a New Zealand glioblastoma cohort. Neuro-Oncol. Pract. 2022, 9, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Mistry, A.M.; Jonathan, S.V.; Monsour, M.A.; Mobley, B.C.; Clark, S.W.; Moots, P.L. Impact of postoperative dexamethasone on survival, steroid dependency, and infections in newly diagnosed glioblastoma patients. Neuro-Oncol. Pract. 2021, 8, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Molinaro, A.M.; Peters, K.; Clarke, J.L.; Jordan, J.T.; de Groot, J.; Nghiemphu, L.; Kaley, T.; Colman, H.; McCluskey, C.; et al. Randomized Phase Ii and Biomarker Study of Pembrolizumab Plus Bevacizumab Versus Pembrolizumab Alone for Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2021, 27, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Odrazka, K.; Petera, J.; Kohlova, T.; Dolezel, M.; Vaculikova, M.; Zouhar, M.; Malek, V.; Hobza, V.; Latr, I.; Nemecek, S.; et al. Prognostic Impact of Hemoglobin Level Prior to Radiotherapy on Survival in Patients with Glioblastoma. Strahlenther. Onkol. 2003, 179, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Tang, V.; Rathbone, M.; Dorsay, J.P.; Jiang, S.; Harvey, D. Rehabilitation in Primary and Metastatic Brain Tumours: Impact of Functional Outcomes on Survival. J. Neurol. 2008, 255, 820–827. [Google Scholar] [CrossRef]
- Wong, E.T.; Lok, E.; Gautam, S.; Swanson, K.D. Dexamethasone Exerts Profound Immunologic Interference on Treatment Efficacy for Recurrent Glioblastoma. Br. J. Cancer 2015, 113, 232–241. [Google Scholar] [CrossRef]
- Brummer, A.B.; Yang, X.; Ma, E.; Gutova, M.; Brown, C.E.; Rockne, R.C. Dose-dependent thresholds of dexamethasone destabilize CAR T-cell treatment efficacy. PLOS Comput. Biol. 2022, 18, e1009504. [Google Scholar] [CrossRef]
- Linder, B.; Schiesl, A.; Voss, M.; Rödel, F.; Hehlgans, S.; Güllülü, Ö.; Seifert, V.; Kögel, D.; Senft, C.; Dubinski, D. Dexamethasone Treatment Limits Efficacy of Radiation, but Does Not Interfere With Glioma Cell Death Induced by Tumor Treating Fields. Front. Oncol. 2021, 11, 715031. [Google Scholar] [CrossRef]
- Car, M.; Šteblaj, S.; Mitrovič, G.; Dolenc-Stražar, Z.; Popović, M. Corticosteroid-Induced Immunodeficiency in a Patient with Gliomatosis Cerebri: Are Corticosteroids Indicated in All Brain Tumors? Clin. Neuropathol. 2019, 38, 189–194. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Jelluma, N.; Yang, X.; Stokoe, D.; Evan, G.I.; Dansen, T.B.; Haas-Kogan, D.A. Glucose Withdrawal Induces Oxidative Stress followed by Apoptosis in Glioblastoma Cells but not in Normal Human Astrocytes. Mol. Cancer Res. 2006, 4, 319–330. [Google Scholar] [CrossRef]
- Benfield, T.; Jensen, J.S.; Nordestgaard, B.G. Influence of diabetes and hyperglycaemia on infectious disease hospitalisation and outcome. Diabetologia 2007, 50, 549–554. [Google Scholar] [CrossRef]
- Luedi, M.M.; Singh, S.K.; Mosley, J.C.; Hassan, I.S.A.; Hatami, M.; Gumin, J.; Andereggen, L.; Sulman, E.P.; Lang, F.F.; Stueber, F.; et al. Dexamethasone-mediated oncogenicity in vitro and in an animal model of glioblastoma. J. Neurosurg. 2018, 129, 1446–1455. [Google Scholar] [CrossRef]
- Sur, P.; Sribnick, E.A.; Patel, S.J.; Ray, S.K.; Banik, N.L. Dexamethasone decreases temozolomide-induced apoptosis in human gliobastoma T98G cells. Glia 2005, 50, 160–167. [Google Scholar] [CrossRef]
- Das, A.; Banik, N.L.; Patel, S.J.; Ray, S.K. Dexamethasone protected human glioblastoma U87MG cells from temozolomide induced apoptosis by maintaining Bax:Bcl-2 ratio and preventing proteolytic activities. Mol. Cancer 2004, 3, 36. [Google Scholar] [CrossRef]
- Das, A.; Banik, N.L.; Ray, S.K. Modulatory Effects of Acetazolomide and Dexamethasone on Temozolomide Mediated Apoptosis in Human Glioblastoma T98G and U87MG Cells. Cancer Investig. 2008, 26, 352–358. [Google Scholar] [CrossRef]
- Aasland, D.; Reich, T.R.; Tomicic, M.T.; Switzeny, O.J.; Kaina, B.; Christmann, M. Repair Gene O(6) -Methylguanine-DNA Methyltransferase Is Controlled by Sp1 and up-Regulated by Glucocorticoids, but Not by Temozolomide and Radiation. J. Neurochem. 2018, 144, 139–151. [Google Scholar] [CrossRef]
- Ueda, S.; Mineta, T.; Nakahara, Y.; Okamoto, H.; Shiraishi, T.; Tabuchi, K. Induction of the DNA repair gene O6-methylguanine—DNA methyltransferase by dexamethasone in glioblastomas. J. Neurosurg. 2004, 101, 659–663. [Google Scholar] [CrossRef]
- Shannon, S.; Vaca, C.; Jia, D.; Entersz, I.; Schaer, A.; Carcione, J.; Weaver, M.; Avidar, Y.; Pettit, R.; Nair, M.; et al. Dexamethasone-Mediated Activation of Fibronectin Matrix Assembly Reduces Dispersal of Primary Human Glioblastoma Cells. PLoS ONE 2015, 10, e0135951. [Google Scholar] [CrossRef]
- Nair, M.; Romero, J.; Mahtabfar, A.; Meleis, A.M.; Foty, R.A.; Corbett, S.A. Dexamethasone-Mediated Upregulation of Calreticulin Inhibits Primary Human Glioblastoma Dispersal Ex Vivo. Int. J. Mol. Sci. 2018, 19, 572. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Jan, H.-J.; Lee, C.-C.; Tao, H.-Y.; Shih, Y.-L.; Wei, H.-W.; Lee, H.-M. Dexamethasone reduced invasiveness of human malignant glioblastoma cells through a MAPK phosphatase-1 (MKP-1) dependent mechanism. Eur. J. Pharmacol. 2008, 593, 1–9. [Google Scholar] [CrossRef]
- Villeneuve, J.; Galarneau, H.; Beaudet, M.; Tremblay, P.; Chernomoretz, A.; Vallières, L. Reduced Glioma Growth Following Dexamethasone or Anti-Angiopoietin 2 Treatment. Brain Pathol. 2008, 18, 401–414. [Google Scholar] [CrossRef]
- Kaup, B.; Schindler, I.; Knüpfer, H.; Schlenzka, A.; Preiβ, R.; Knüpfer, M. Time-dependent Inhibition of Glioblastoma Cell Proliferation by Dexamethasone. J. Neuro-Oncol. 2001, 51, 105–110. [Google Scholar] [CrossRef]
- Leao, D.; Craig, P.; Godoy, L.; Leite, C.; Policeni, B. Response Assessment in Neuro-Oncology Criteria for Gliomas: Practical Approach Using Conventional and Advanced Techniques. Am. J. Neuroradiol. 2020, 41, 10–20. [Google Scholar] [CrossRef]
- Kaley, T.; Nolan, C.; Carver, A.; Omuro, A. Bevacizumab for acute neurologic deterioration in patients with glioblastoma. CNS Oncol. 2013, 2, 413–418. [Google Scholar] [CrossRef]
- Kast, R.; Burns, T.C.; Halatsch, M.-E. Short review of SEC, a potential dexamethasone-sparing regimen for glioblastoma: Spironolactone, ecallantide, clotrimazole. Neurochirurgie 2021, 67, 508–515. [Google Scholar] [CrossRef]
- Abrams, D.A.; Hanson, J.A.; Brown, J.M.; Hsu, F.P.; Delashaw, J.B., Jr.; Bota, D.A. Timing of Surgery and Bevacizumab Therapy in Neurosurgical Patients with Recurrent High Grade Glioma. J. Clin. Neurosci. 2015, 22, 35–39. [Google Scholar] [CrossRef][Green Version]
- Gordon, C.R.; Rojavin, Y.; Patel, M.; Zins, J.E.; Grana, G.; Kann, B.; Simons, R.; Atabek, U. A Review on Bevacizumab and Surgical Wound Healing: An Important Warning to All Surgeons. Ann. Plast. Surg. 2009, 62, 707–709. [Google Scholar] [CrossRef]
| First Author | Year | PMID | Groups | n (Patients) | Survival Data | Dexamethasone Dosing | Adjusted Variables | ROBINS-I Risk of Bias Assessment |
|---|---|---|---|---|---|---|---|---|
| Liu EK | 2023 | 36382106 | 89 | OS | Time-weighted dexamethasone dose (mg/day) | Age, KPS, MGMT, blood glucose, methylation subclass, subclass glucose interaction | Moderate | |
| McManus EJ | 2022 | 35096403 | 170 | OS | More or less than 10 mg/d | None | Serious | |
| Mistry AM | 2021 | 34594571 | 360 | OS | More or less than 200 mg total in first 3 postoperative weeks | Age, KPS, IDH, MGMT, temozolomide, RT, tumor volume, EOR, blood glucose | Moderate | |
| Garrett C | 2021 | 34138894 | 87 | OS and PFS | Daily dexamethasone or not | None for OS, age, ECOG, gender, BMI, IDH, temozolomide, previous surgery, EOR for PFS | Moderate | |
| Aldaz P | 2021 | 33478100 | 285 | OS and PFS | Dexamethasone yes or no postoperatively | None | Serious | |
| Iorgulescu JB | 2021 | 33239433 | 181 | OS | Low (1 and 2.5 mg/kg/d) or high (10 mg/kg/d) doses | Age, KPS, MGMT, tumor volume, EOR | Moderate | |
| Nayak L | 2021 | 33199490 | Cohorts A and B | 80 | OS | Any vs. no baseline dexamethasone use in patients with recurrent glioblastoma | None | Serious |
| Lee C | 2020 | 32648384 | 125 | OS | More or less than 2 mg/d after initiation of radiochemotharapy | Age, sex, ECOG, EOR | Moderate | |
| Lewitzki V | 2019 | 31831026 | 152 | OS | Any vs. no dexamethasone during radiotherapy | MGMT, recurrence, salvage therapy | Serious | |
| Chiocca EA | 2019 | 31413142 | 31 | OS | More or less than 20 mg within 2 weeks postoperatively after recurrent resection | None | Serious | |
| Hui CY | 2019 | 30864102 | 319 | OS and PFS | More or less than 4 mg/d during radiochemotherapy | Age, KPS, sex, race, EOR, MGMT, RT | Moderate | |
| Dubinski D | 2018 | 29349612 | 113 | OS and PFS | 12 mg dexamethasone vs. none preoperatively | None | Serious | |
| Díez Valle R | 2018 | 29107723 | 131 | OS | Dexamethasone yes or no at 2 months postoperatively | Age, sex, KPS, MGMT, EOR, time to surgery | Moderate | |
| Hagan K | 2016 | 27438798 | 162 | OS and PFS | Dexamethasone yes or no preoperatively | Age, ASA, blood glucose | Serious | |
| Bhavsar S | 2016 | 27396375 | 841 | OS and PFS | Dexamethasone yes or no perioperatively | Age, BMI, sex, ASA, Charlson CI | Moderate | |
| Shields LB | 2015 | 26520780 | 73 | OS | Dexamethasone yes or no during radiotherapy | None | Serious | |
| Wong ET | 2015 | 26125449 | TTF therapy and BPC chemotherapy | 35 | OS | Dexamethasone over or less than 4.1 mg/d in recurrent glioblastoma | None | Serious |
| Tieu MT | 2015 | 26015297 | Derivation and validation sets | 359 | OS | Mean TWM dexamethasone dose per mg during radiotherapy | Age, ECOG, blood glucose, BMI, EOR | Moderate |
| Derr RL | 2009 | 19139429 | 191 | OS | Mean daily dexamethasone dose per 10 mg/d | Age, KPS, blood glucose | Moderate | |
| Tang V | 2008 | 18500499 | 18 | OS | More or less than 8 mg/d upon admission in rehabilitation | FMI | Serious | |
| Odrazka K | 2003 | 14628127 | 85 | OS | More or less than 2 mg/d before radiotherapy | Age, ECOG, EOR | Moderate | |
| Gundersen S | 1996 | 9073058 | 495 | OS | Dexamethasone yes or no on admission | KPS, age, resection | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheffler, P.; Fung, C.; Momjian, S.; Koessinger, D.; Häni, L.; Neidert, N.; Straehle, J.; Volz, F.; Schnell, O.; Beck, J.; et al. Dexamethasone in Patients with Glioblastoma: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 1393. https://doi.org/10.3390/cancers16071393
Scheffler P, Fung C, Momjian S, Koessinger D, Häni L, Neidert N, Straehle J, Volz F, Schnell O, Beck J, et al. Dexamethasone in Patients with Glioblastoma: A Systematic Review and Meta-Analysis. Cancers. 2024; 16(7):1393. https://doi.org/10.3390/cancers16071393
Chicago/Turabian StyleScheffler, Pierre, Christian Fung, Shahan Momjian, Dominik Koessinger, Levin Häni, Nicolas Neidert, Jakob Straehle, Florian Volz, Oliver Schnell, Jürgen Beck, and et al. 2024. "Dexamethasone in Patients with Glioblastoma: A Systematic Review and Meta-Analysis" Cancers 16, no. 7: 1393. https://doi.org/10.3390/cancers16071393
APA StyleScheffler, P., Fung, C., Momjian, S., Koessinger, D., Häni, L., Neidert, N., Straehle, J., Volz, F., Schnell, O., Beck, J., & El Rahal, A. (2024). Dexamethasone in Patients with Glioblastoma: A Systematic Review and Meta-Analysis. Cancers, 16(7), 1393. https://doi.org/10.3390/cancers16071393









