Dermoscopy as a Tool for Identifying Potentially Metastatic Thin Melanoma: A Clinical–Dermoscopic and Histopathological Case–Control Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
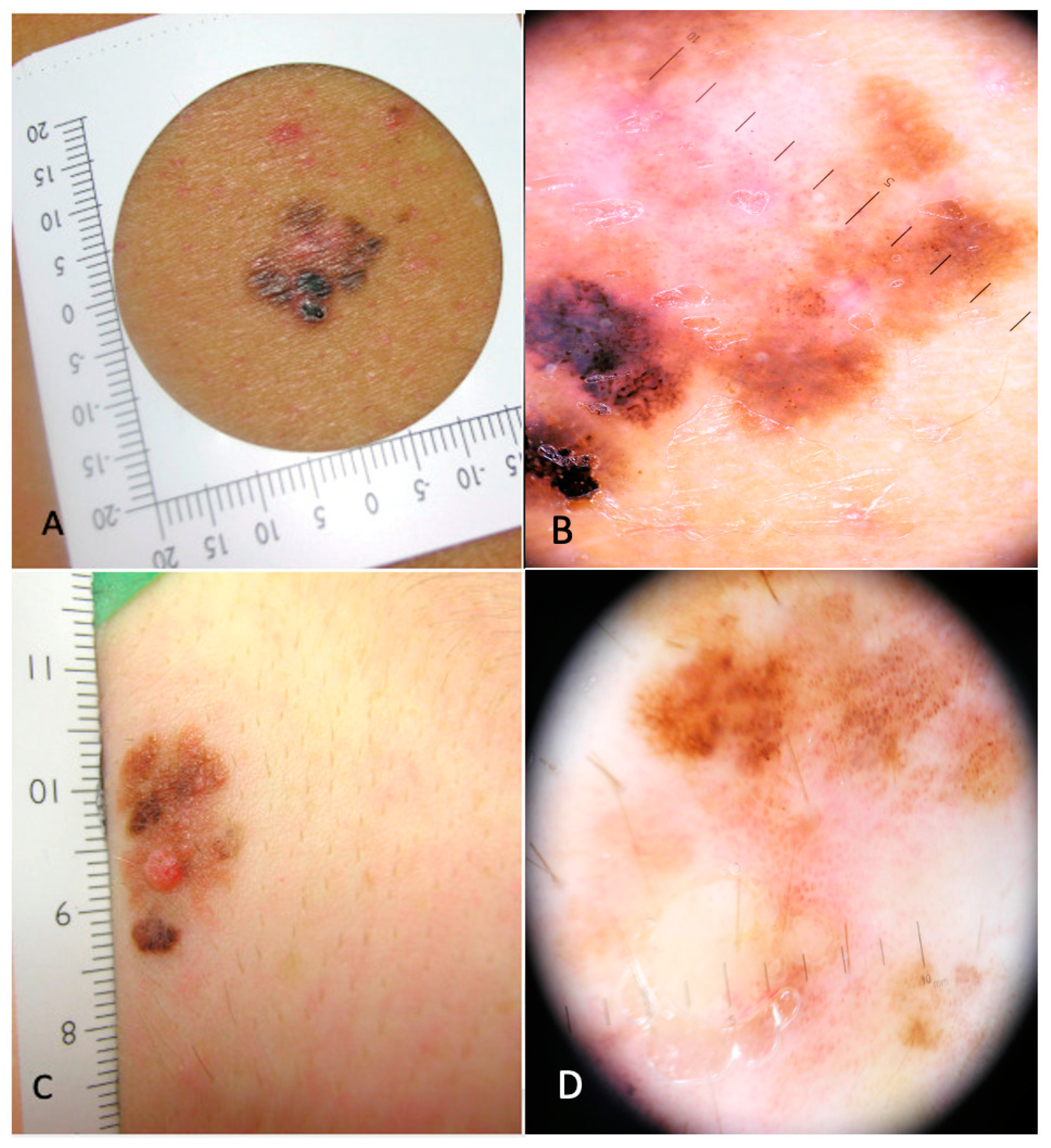
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elder, D.E. Thin melanoma. Arch. Pathol. Lab. Med. 2011, 135, 342–346. [Google Scholar] [CrossRef]
- Nazzaro, G.; Passoni, E.; Pozzessere, F.; Maronese, C.A.; Marzano, A.V. Dermoscopy Use Leads to Earlier Cutaneous Melanoma Diagnosis in Terms of Invasiveness and Size? A Single-Center, Retrospective Experience. J. Clin. Med. 2022, 11, 4912. [Google Scholar] [CrossRef]
- Karakousis, G.; Gimotty, P.A.; Bartlett, E.K.; Sim, M.S.; Neuwirth, M.G.; Fraker, D.; Czerniecki, B.J.; Faries, M.B. Thin Melanoma with Nodal Involvement: Analysis of Demographic, Pathologic, and Treatment Factors with Regard to Prognosis. Ann. Surg. Oncol. 2017, 24, 952–959. [Google Scholar] [CrossRef]
- Claeson, M.; Baade, P.; Brown, S.; Soyer, H.P.; Smithers, B.M.; Green, A.C.; Whiteman, D.C.; Khosrotehrani, K. Clinicopathological factors associated with death from thin (≤1·00 mm) melanoma. Br. J. Dermatol. 2020, 182, 927–931. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef] [PubMed]
- Mihic-Probst, D.; Shea, C.; Duncan, L.; de la Fouchardiere, A.; Landman, G.; Landsberg, J.; ven den Oord, J.; Lowe, L.; Cook, M.G.; Yun, S.J.; et al. Update on Thin Melanoma: Outcome of an International Workshop. Adv. Anat. Pathol. 2016, 23, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.E. Prognosis in Thin Melanoma Patients: Is Slightly Less than Excellent Still Okay? Ann. Surg. Oncol. 2021, 28, 6911–6914. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, C.; Kempf, W.; Mangana, J.; Cheng, P.; Emberger, M.; Lang, R.; Kaiser, A.K.; Lattmann, E.; Levesque, M.; Dummer, R.; et al. The role of cyclin D1 and Ki-67 in the development and prognostication of thin melanoma. Histopathology 2020, 77, 460–470. [Google Scholar] [CrossRef]
- Elder, D.E.; Massi, D.; Scolyer, R.A. (Eds.) WHO Classification of Skin Tumours; International Agency for Research on Cancer (IARC): Lyon, France, 2018.
- Isaksson, K.; Mikiver, R.; Eriksson, H.; Lapins, J.; Nielsen, K.; Ingvar, C.; Lyth, J. Survival in 31 670 patients with thin melanomas: A Swedish population-based study. Br. J. Dermatol. 2021, 184, 60–67. [Google Scholar] [CrossRef]
- Richetta, A.G.; Valentini, V.; Marraffa, F.; Paolino, G.; Rizzolo, P.; Silvestri, V.; Zelli, V.; Carbone, A.; Di Mattia, C.; Calvieri, S.; et al. Metastases risk in thin cutaneous melanoma: Prognostic value of clinical-pathologic characteristics and mutation profile. Oncotarget 2018, 9, 32173–32181. [Google Scholar] [CrossRef]
- Kalady, M.F.; White, R.R.; Johnson, J.L.; Tyler, D.S.; Seigler, H.F. Thin melanomas: Predictive lethal characteristics from a 30-year clinical experience. Ann. Surg. 2003, 238, 528–535, discussion 535–537. [Google Scholar] [CrossRef] [PubMed]
- Podolec, K.; Bronikowska, A.; Pirowska, M.; Wojas-Pelc, A. Dermoscopic features in different dermatopathological stages of cutaneous melanomas. Postepy Dermatol. Alergol. 2020, 37, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.P.; Ikino, J.K.; Sens, M.M.; Nunes, D.H.; Di Giunta, G. Dermoscopic features of thin melanomas: A comparative study of melanoma in situ and invasive melanomas smaller than or equal to 1mm. An. Bras. Dermatol. 2013, 88, 712–717. [Google Scholar] [CrossRef]
- Ungureanu, L.; Şenilă, S.; Dănescu, S.; Rogojan, L.; Cosgarea, R. Correlation of dermatoscopy with the histopathological changes in the diagnosis of thin melanoma. Rom. J. Morphol. Embryol. 2013, 54, 315–320. [Google Scholar] [PubMed]
- Guitera, P.; Li, L.X.; Crotty, K.; Fitzgerald, P.; Mellenbergh, R.; Pellacani, G.; Menzies, S.W. Melanoma histological Breslow thickness predicted by 75-MHz ultrasonography. Br. J. Dermatol. 2008, 159, 364–369. [Google Scholar] [CrossRef]
- Argenziano, G.; Fabbrocini, G.; Carli, P.; De Giorgi, V.; Delfino, M. Epiluminescence microscopy: Criteria of cutaneous melanoma progression. J. Am. Acad. Dermatol. 1997, 37, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, H.F.; Weismann, K.; Larsen, F.G. Dermatoscopic prediction of melanoma thickness using latent trait analysis and likelihood ratios. Acta Derm. Venereol. 2001, 81, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Koga, H.; Uhara, H.; Saida, T. High-frequency 30-MHz sonography in preoperative assessment of tumor thickness of primary melanoma: Usefulness in determination of surgical margin and indication for sentinel lymph node biopsy. Int. J. Clin. Oncol. 2009, 14, 426–430. [Google Scholar] [CrossRef]
- Argenziano, G.; Fabbrocini, G.; Carli, P.; De Giorgi, V.; Delfino, M. Clinical and dermatoscopic criteria for the preoperative evaluation of cutaneous melanoma thickness. J. Am. Acad. Dermatol. 1999, 40, 61–68. [Google Scholar] [CrossRef]
- Carli, P.; de Giorgi, V.; Palli, D.; Giannotti, V.; Giannotti, B. Preoperative assessment of melanoma thickness by ABCD score of dermatoscopy. J. Am. Acad. Dermatol. 2000, 43, 459–466. [Google Scholar] [CrossRef]
- Stante, M.; De Giorgi, V.; Cappugi, P.; Giannotti, B.; Carli, P. Non-invasive analysis of melanoma thickness by means of dermoscopy: A retrospective study. Melanoma Res. 2001, 11, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Steding-Jessen, M.; Hölmich, L.R.; Chakera, A.H.; Klausen, S.; Hovaldt, H.B.; Møller, H. Thin or early melanoma, risk factors and associated mortality. Dan. Med. J. 2022, 69, A01220020. [Google Scholar] [PubMed]
- Guitart, J.; Lowe, L.; Piepkorn, M.; Prieto, V.G.; Rabkin, M.S.; Ronan, S.G.; Shea, C.R.; Tron, V.A.; White, W.; Barnhill, R.L. Histological characteristics of metastasizing thin melanomas: A case-control study of 43 cases. Arch. Dermatol. 2002, 138, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Vilmer, C.; Bailly, C.; Le Doussal, V.; Lasry, S.; Guerin, P.; Delaunay, M.M.; Mandard, A.M. Thin melanomas with unusual aggressive behavior: A report on nine cases. Melanoma Group of French Federation of Cancer Centers. J. Am. Acad. Dermatol. 1996, 34, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Maurichi, A.; Miceli, R.; Camerini, T.; Mariani, L.; Patuzzo, R.; Ruggeri, R.; Gallino, G.; Tolomio, E.; Tragni, G.; Valeri, B.; et al. Prediction of survival in patients with thin melanoma: Results from a multi-institution study. J. Clin. Oncol. 2014, 32, 2479–2485. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, E.; Gervais, M.K.; Shah, P.S.; Look Hong, N.J.; Wright, F.C. Sentinel Lymph Node Biopsy in Thin Cutaneous Melanoma: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2016, 23, 4178–4188. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Haydu, L.E.; Long, G.V.; Quinn, M.J.; Saw, R.P.; Shannon, K.; Spillane, A.J.; Stretch, J.R.; Kefford, R.F.; Thompson, J.F.; et al. Clinical and pathologic factors associated with distant metastasis and survival in patients with thin primary cutaneous melanoma. Ann. Surg. Oncol. 2012, 19, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Swetter, S.M.; Thompson, J.A.; Albertini, M.R.; Barker, C.A.; Baumgartner, J.; Boland, G.; Chmielowski, B.; Di Maio, D.; Durham, A.; Fields, R.C.; et al. NCCN Guidelines® Insights: Melanoma: Cutaneous, Version 2.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 364–376. [Google Scholar] [CrossRef]
- Hieken, T.J.; Grotz, T.E.; Comfere, N.I.; Inselman, J.W.; Habermann, E.B. The effect of the AJCC 7th edition change in T1 melanoma substaging on national utilization and outcomes of sentinel lymph node biopsy for thin melanoma. Melanoma Res. 2015, 25, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Oude Ophuis, C.M.; Louwman, M.W.; Grünhagen, D.J.; Verhoef, K.; van Akkooi, A.C. Implementation of the 7th edition AJCC staging system: Effects on staging and survival for pT1 melanoma. A Dutch population based study. Int. J. Cancer 2017, 140, 1802–1808. [Google Scholar] [CrossRef]
- Gorka, E.; Fabó, D.; Gézsi, A.; Czirbesz, K.; Liszkay, G. Distance from Primary Tumor Is the Strongest Predictor for Early Onset of Brain Metastases in Melanoma. Anticancer Res. 2016, 36, 3065–3069. [Google Scholar]

| Patients | Cases | Controls | p-Value |
|---|---|---|---|
| N = 16 | N = 100 | ||
| Age at diagnosis (yr) | |||
| Median (IQR) | 49 (28–83) | 64 (27–91) | |
| ≤50 | 9 (56.3%) | 19 (19%) | 0.003 |
| >50 | 7 (43.7%) | 81 (81%) | 0.003 |
| Gender | |||
| Female | 7 (43.7%) | 45 (45%) | 0.926 |
| Male | 9 (56.3%) | 55 (55%) | 0.926 |
| Anatomical site | |||
| Torso | 7 (43.7%) | 45 (45%) | 0.926 |
| Upper limbs | 6 (37.5%) | 22 (22%) | 0.211 |
| Lower limbs | 2 (12.5%) | 25 (25%) | 0.354 |
| Head/neck | 1 (6.3%) | 8 (8%) | 1.000 |
| Histological subtype | |||
| SSM a/Low-CSD melanoma | 14 (87.5%) | 93 (93%) | 0.609 |
| LM b/High-CSD in situ melanoma | 2 (12.5%) | 7 (7%) | 0.609 |
| Breslow thickness (mm) c | |||
| Median (IQR) | 0.6 (0.2–0.8) | 0.15 (0–0.7) | |
| <0.5 | 5 (31.2%) | 81 (81%) | <0.001 |
| ≥0.5 | 9 (56.2%) | 9 (9%) | <0.001 |
| Ulceration | |||
| Present | 2 (12.5%) | 1 (1%) | 0.044 |
| Absent | 13 (81.2%) | 99 (99%) | 0.044 |
| Missing | 1 (6.3%) | 0 | |
| Mitotic rate/mm2 | |||
| 0 | 5 (31.5%) | 97 (97%) | <0.001 |
| 1 | 4 (25%) | 3 (3%) | 0.003 |
| 2–4 | 4 (25%) | 0 | <0.001 |
| Missing | 3 (18.5%) | 0 | |
| Growth phase | |||
| Vertical growth phase (VGP) | 10 (62.5%) | 27 (27%) | 0.002 |
| Radial growth phase (RGP) | 4 (25%) | 73 (73%) | 0.002 |
| Missing | 2 (12.5%) | 0 | |
| Regression | |||
| Present | 14 (87.4) | 53 (53%) | 0.003 |
| <75% ≥75% Missing | 9 (64.3%) 4 (28.5%) 1 (7.2%) | 34 (64.2%) 19 (35.8%) 0 | |
| Absent | 1 (6.3%) | 47 (47%) | 0.003 |
| Missing | 1 (6.3%) | 0 | |
| Pathological Stage (AJCC 8th Ed.) c | |||
| pTx | 2 (12.5%) | 0 | 0.016 |
| pTis | 0 | 58 (58%) | <0.001 |
| pT1a | 8 (50%) | 41 (41%) | 0.368 |
| pT1b | 5 (31.2%) | 1 (1%) | <0.001 |
| Missing | 1 (6.3%) | 0 | |
| SLNB d | |||
| Not performed | 12 (75%) | 99 (99%) | |
| Negative | 4 (25%) | 1 (1%) |
| Patients | Cases N = 16 | Controls N = 100 | p-Value |
|---|---|---|---|
| Clinical–dermoscopic features | |||
| ≥3 colors | 12 (80%) | 3 (3%) | <0.001 |
| Diameter > 10 mm | 11 (73.3%) | 29 (29%) | 0.002 |
| White patch | 11 (73.3%) | 43 (43%) | 0.055 |
| Atypical vascular patterns | 10 (66.5%) | 15 (15%) | <0.001 |
| Blue-gray areas | 9 (60%) | 27 (27%) | 0.038 |
| Absence of pigment network | 9 (60%) | 15 (15%) | 0.001 |
| Blue-white veil | 3 (20%) | 5 (5%) | 0.079 |
| Regression structures (white patch or blue-gray areas) | 16 (100%) | 45 (45%) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Giorgi, V.; Silvestri, F.; Cecchi, G.; Venturi, F.; Zuccaro, B.; Perillo, G.; Cosso, F.; Maio, V.; Simi, S.; Antonini, P.; et al. Dermoscopy as a Tool for Identifying Potentially Metastatic Thin Melanoma: A Clinical–Dermoscopic and Histopathological Case–Control Study. Cancers 2024, 16, 1394. https://doi.org/10.3390/cancers16071394
De Giorgi V, Silvestri F, Cecchi G, Venturi F, Zuccaro B, Perillo G, Cosso F, Maio V, Simi S, Antonini P, et al. Dermoscopy as a Tool for Identifying Potentially Metastatic Thin Melanoma: A Clinical–Dermoscopic and Histopathological Case–Control Study. Cancers. 2024; 16(7):1394. https://doi.org/10.3390/cancers16071394
Chicago/Turabian StyleDe Giorgi, Vincenzo, Flavia Silvestri, Giovanni Cecchi, Federico Venturi, Biancamaria Zuccaro, Gabriella Perillo, Federica Cosso, Vincenza Maio, Sara Simi, Pietro Antonini, and et al. 2024. "Dermoscopy as a Tool for Identifying Potentially Metastatic Thin Melanoma: A Clinical–Dermoscopic and Histopathological Case–Control Study" Cancers 16, no. 7: 1394. https://doi.org/10.3390/cancers16071394
APA StyleDe Giorgi, V., Silvestri, F., Cecchi, G., Venturi, F., Zuccaro, B., Perillo, G., Cosso, F., Maio, V., Simi, S., Antonini, P., Pillozzi, S., Antonuzzo, L., Massi, D., & Doni, L. (2024). Dermoscopy as a Tool for Identifying Potentially Metastatic Thin Melanoma: A Clinical–Dermoscopic and Histopathological Case–Control Study. Cancers, 16(7), 1394. https://doi.org/10.3390/cancers16071394






