Prediction of Response to Anti-Angiogenic Treatment for Advanced Colorectal Cancer Patients: From Biological Factors to Functional Imaging
Abstract
Simple Summary
Abstract
1. Introduction
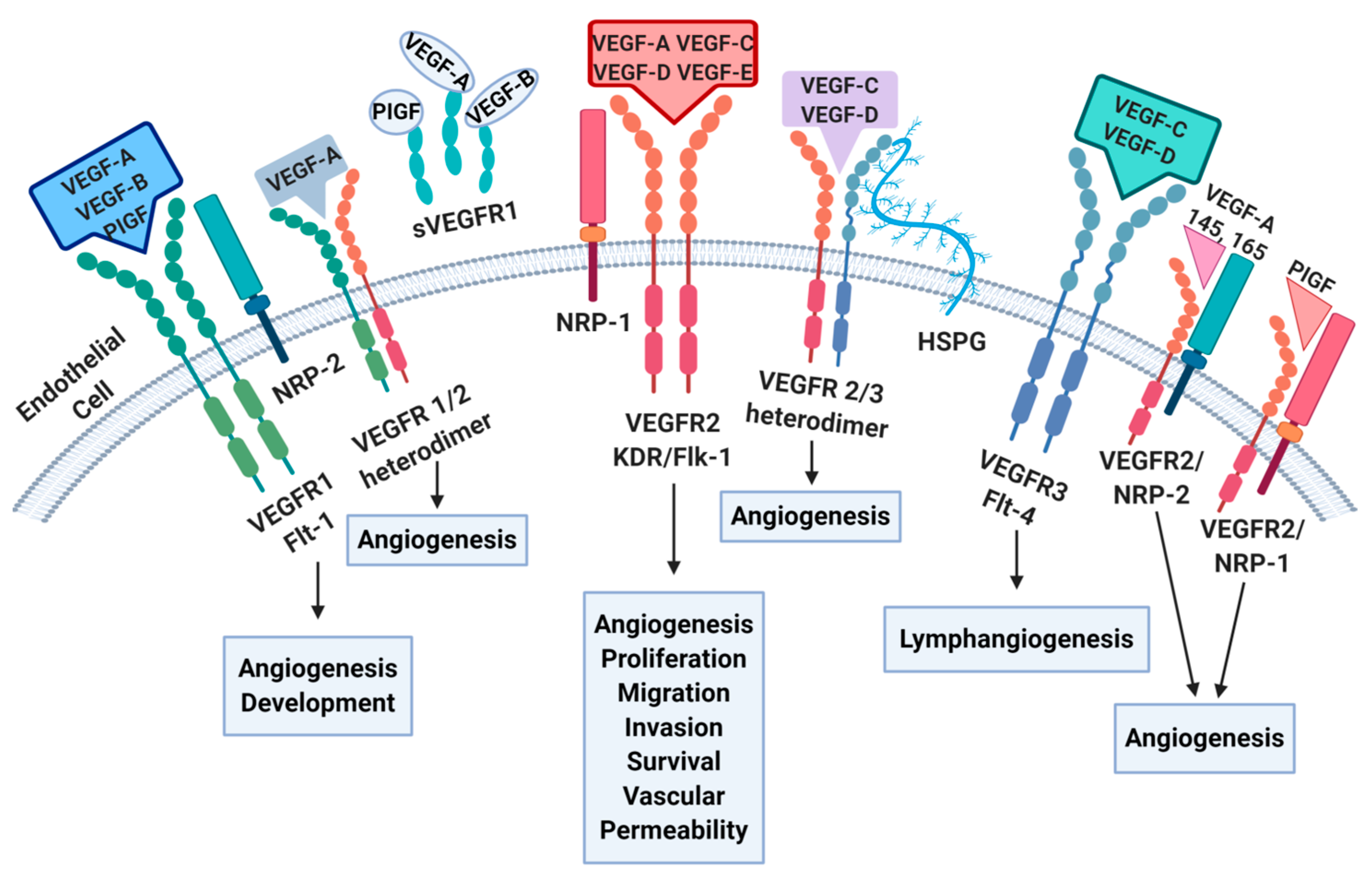
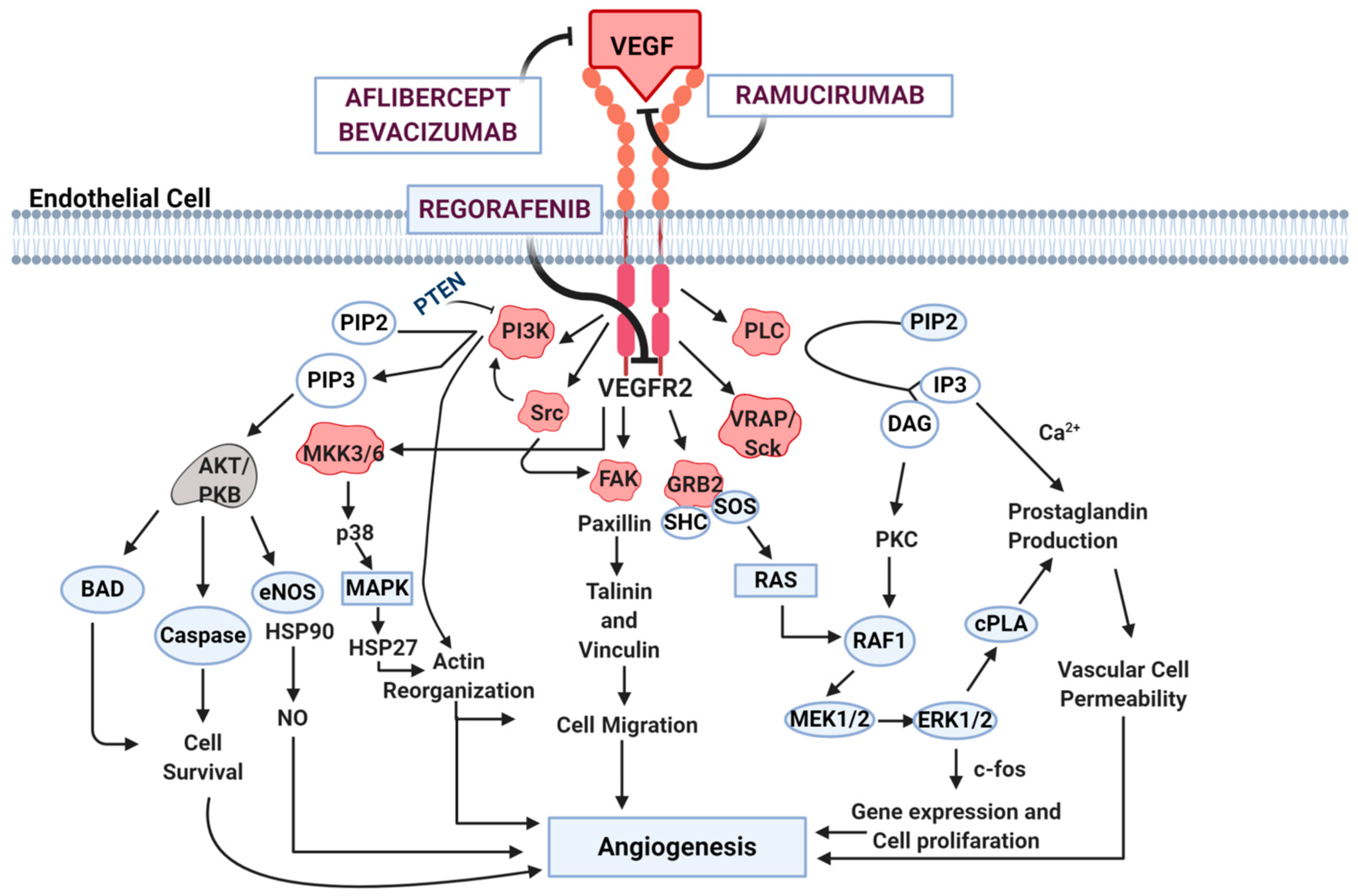
2. Angiogenesis Pathways
3. Biomarkers of Angiogenesis
3.1. Tissue-Based, Genetic Polymorphisms
3.2. Circulating Biomarkers
- In research by Duda et al. into plasmatic VEGF, PIGF, and VEGFR-1, only VEGFR-1 was related to prediction of response and tolerability to bevacizumab [53].
- Martinetti et al. explored various circulating prognostic biomarkers (VEGF, PDGF, SDF-1, osteopontin, and CEA) in patients receiving bevacizumab in three randomized clinical trials. Higher levels of VEGF and SDF-1 were likely to be associated with worse prognosis, especially in terms of OS. Moreover, increasing CEA values during treatment resulted in significantly worse prognosis independent of disease extension [54].
- Two phase III studies (HORIZON II and III) evaluated baseline levels of VEGF and soluble VEGFR-2 (sVEGFR-2) as prognostic and predictive biomarkers. High baseline VEGF was associated with worse PFS in both studies and with worse OS in the HORIZON II study. However, these results were not uniformly confirmed, and further studies are necessary to clarify the role of circulating VEGF as a predictive marker [55].
- In the multicentric prospective randomized ITACa trial, VEGF-A, eNOS, EPHB4, COX2, and HIF-1α mRNA levels were evaluated at baseline and during therapy according to objective response (ORR), PFS, and OS. Reduction in eNOS and VEGF levels from baseline to the first clinical evaluation showed better OS than the others, and might suggest a response to bevacizumab for this category [56].
- Abajo et al. described higher baseline levels of epidermal growth factor (EGF) and macrophage-derived chemokine (MDC) along with lower levels of IL-10, 6, and 8 in treatment-respondent mCRC patients. Treatment exposure increased MDC and decreased IL-8 levels, suggesting that a set of inflammatory and angiogenesis-related serum markers might be associated with the efficacy of the bevacizumab-containing regimen [57].
- An exploratory preplanned analysis of serum pro-angiogenic factors (SErum aNgiogenesis-cenTRAL) was conducted in 72 mCRC patients among the participants of the phase II CENTRAL (ColorEctalavastiNTRiAlLdh) study in order to find potential serum biomarkers able to predict response to bevacizumab in combination with FOLFIRI in the first-line setting. In this study, the early increase of FGF-2 serum values was identified as a biomarker to ameliorate the selection of patients for this therapeutic strategy; indeed, patients experiencing an increase of FGF-2 during the 8-week timepoint from baseline had an improved median PFS (12.85 vs. 7.57 months) (HR: 0.73, 95%CI: 0.43–1.27, p = 0.23 and 12.98 vs. 8 months, HR: 0.78, 95%CI: 0.46–1.33, p = 0.35, respectively) [58].
- In a post hoc analysis of the VELOUR trial, VEGF-A and PIGF were significantly higher in patients pretreated with bevacizumab than in those who had not previously received anti-angiogenic agents. In patients randomized to the placebo arm, survival was shorter in case of higher levels of VEGF-A (>144 pg/mL) and PlGF (>8 pg/mL) at baseline (9.6 vs. 12.9 months and 9.7 vs. 11.7 months, respectively), suggesting that they might reflect acquired resistance to bevacizumab. Conversely, in the aflibercept group, improved OS and PFS were observed regardless of baseline VEGF-A or PlGF levels, confirming aflibercept activity even in patients with bevacizumab-induced resistance [18].
- Currently, the phase II biologically-enriched DISTINCTIVE study aims to prospectively validate VEGFR-2 plasma levels as predictive factor for the efficacy of aflibercept plus FOLFIRI in RAS wild-type mCRC patients progressing after first-line treatment with oxaliplatin, fluoropyrimidines, and anti-EGFR monoclonal antibodies [59].
3.3. Circulating Tumor Cells (CTCs) and Free Nucleic Acids
- In a study by Cohen et al., mCRC patients with high CTC count (≥3CTC/7.5 mL) showed worse PFS and OS; moreover, these CTC values were predictive of worse outcomes irrespective of the treatment received. Of note, almost 50% of patients had been treated with bevacizumab [62].
- Another clue to a potential predictive role in response to anti-angiogenic agents was provided by Rahbari et al., who demonstrated a correlation between CTC detection and circulating angiogenic factors as well as an association with lower levels of EGF and FGF [63].
- VISNU-1, an open-label multicenter randomized phase III trial, was performed in an mCRC population selected based on their baseline CTC count, with the aim of identifying those patients who might benefit most from bevacizumab plus FOLFOXIRI versus bevacizumab plus FOLFOX6. VISNU-1 considered CTC counts ≥ 3 as the cut-off for the high-risk mCRC patient population; thus, patients with at least three CTCs were randomly assigned to one of the two arms. FOLFOXIRI-bevacizumab improved PFS compared to FOLFOX-bevacizumab (12.4 months vs. 9.3 months, respectively; p = 0.0004). According to these results, bevacizumab-FOLFOXIRI might be considered an adequate treatment option for mCRC patients with ≥3 CTCs [64].
- Zhou et al. analyzed cell-free DNA from CRC patients; POLR1D amplification and expression influenced cell proliferation and induced VEGF upregulation, which has been suggested to be involved in bevacizumab resistance [68]. In another study, twenty-one mCRC patients treated with first-line bevacizumab plus chemotherapy underwent plasma collection at various timepoints; DNA was extracted and sequenced with a panel of 90 oncogenes. All patients harbored 1–6 trunk mutations in ctDNA and showed a range of 1–89% in the mutant allele frequency (MAF). MAF variations were significantly related to disease status (decreasing in case of disease remission and increasing in case of progression; p < 0.001). More specifically, improved survival was observed in patients who experienced MAF reduction to below 2% at remission [69].
- Another prospective study of CRC patients aimed to investigate the correlation between genetic polymorphism of VEGF-A with survival using the DNA extracted from peripheral blood. In a univariate analysis, there was a significant association (OR = 0.32; p = 0.048) between genotype CC of the VEGF-A -1498C>T polymorphism and the presence of CRC liver metastasis [70].
- An open-label study by Wong et al. simultaneously analyzed ctDNA in plasma samples and tumor biopsies on different days of treatment with regorafenib in mCRC patients [71]. ctDNA was inversely correlated with PFS, and the presence of KRAS mutations was associated with shorter PFS.
- In the RAISE study, Tabernero et al. identified VEGF-D as a potential predictive biomarker for the efficacy of anti-angiogenic treatment in the second-line setting [19].
- A retrospective analysis of the CORRECT study showed how DNA mutational status obtained from the plasma of mCRC patients might be a noninvasive tool to analyze tumor genotype while at the same time finding clinically relevant mutations other than those identified in tumor tissue. Moreover, in a subgroup of patients selected according to mutational status and protein levels, regorafenib seemed to lead to improved clinical outcomes [72].
3.4. MicroRNA (miRNA)
4. Advanced Quantitative Imaging Approaches in Disease Detection and Outcome Prediction
4.1. EMVI
4.2. Radiomics
4.3. Functional Imaging
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fitzmaurice, C.; Dicker, D.; Pain, A.; Hamavid, H.; Moradi-Lakeh, M.; MacIntyre, M.F.; Allen, C.; Hansen, G.; Woodbrook, R.; Wolfe, C.; et al. The Global Burden of Cancer 2013. JAMA Oncol. 2015, 1, 505–527. [Google Scholar] [CrossRef] [PubMed]
- Bockelman, C.; Engelmann, B.E.; Kaprio, T.; Hansen, T.F.; Glimelius, B. Risk of recurrence in patients with colon cancer stage II and III: A systematic review and meta-analysis of recent literature. Acta Oncol. 2015, 54, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Schmoll, H.J.; Van Cutsem, E.; Stein, A.; Valentini, V.; Glimelius, B.; Haustermans, K.; Nordlinger, B.; van de Velde, C.J.; Balmana, J.; Regula, J.; et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann. Oncol. 2012, 23, 2479–2516. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.; Liscia, N.; Donisi, C.; Mariani, S.; Tolu, S.; Pretta, A.; Persano, M.; Pinna, G.; Balconi, F.; Pireddu, A.; et al. Molecular-Biology-Driven Treatment for Metastatic Colorectal Cancer. Cancers 2020, 12, 1214. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Ziranu, P.; Aimola, V.; Pretta, A.; Dubois, M.; Murru, R.; Liscia, N.; Cau, F.; Persano, M.; Deias, G.; Palmas, E.; et al. New Horizons in Metastatic Colorectal Cancer: Prognostic Role of CD44 Expression. Cancers 2023, 15, 1212. [Google Scholar] [CrossRef] [PubMed]
- Martini, G.; Troiani, T.; Cardone, C.; Vitiello, P.; Sforza, V.; Ciardiello, D.; Napolitano, S.; Della Corte, C.M.; Morgillo, F.; Raucci, A.; et al. Present and future of metastatic colorectal cancer treatment: A review of new candidate targets. World J. Gastroenterol. 2017, 23, 4675–4688. [Google Scholar] [CrossRef] [PubMed]
- Ziranu, P.; Lai, E.; Schirripa, M.; Puzzoni, M.; Persano, M.; Pretta, A.; Munari, G.; Liscia, N.; Pusceddu, V.; Loupakis, F.; et al. The Role of p53 Expression in Patients with RAS/BRAF Wild-Type Metastatic Colorectal Cancer Receiving Irinotecan and Cetuximab as Later Line Treatment. Target. Oncol. 2021, 16, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Ziranu, P.; Pretta, A.; Pozzari, M.; Maccioni, A.; Badiali, M.; Fanni, D.; Lai, E.; Donisi, C.; Persano, M.; Gerosa, C.; et al. CDX-2 expression correlates with clinical outcomes in MSI-H metastatic colorectal cancer patients receiving immune checkpoint inhibitors. Sci. Rep. 2023, 13, 4397. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, R.; Caporale, M.; Pietrantonio, F.; De Braud, F.; Negri, F.V.; Giuliani, F.; Pusceddu, V.; Demurtas, L.; Restivo, A.; Fontanella, C.; et al. Second-line angiogenesis inhibition in metastatic colorectal cancer patients: Straightforward or overcrowded? Crit. Rev. Oncol. Hematol. 2016, 100, 99–106. [Google Scholar] [CrossRef]
- Scartozzi, M.; Vincent, L.; Chiron, M.; Cascinu, S. Aflibercept, a New Way to Target Angiogenesis in the Second Line Treatment of Metastatic Colorectal Cancer (mCRC). Target. Oncol. 2016, 11, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, R.; Scartozzi, M.; Del Prete, M.; Fulli, A.; Faloppi, L.; Bianconi, M.; Maccaroni, E.; Cascinu, S. The “angiogenetic ladder”, step-wise angiogenesis inhibition in metastatic colorectal cancer. Cancer Treat. Rev. 2014, 40, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.; Cascinu, S.; Scartozzi, M. Are All Anti-Angiogenic Drugs the Same in the Treatment of Second-Line Metastatic Colorectal Cancer? Expert Opinion on Clinical Practice. Front. Oncol. 2021, 11, 637823. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.; Puzzoni, M.; Ziranu, P.; Cremolini, C.; Lonardi, S.; Banzi, M.; Mariani, S.; Liscia, N.; Cinieri, S.; Dettori, M.; et al. Long Term Survival With Regorafenib: REALITY (Real Life in Italy) Trial—A GISCAD Study. Clin. Color. Cancer 2021, 20, e253–e262. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Giantonio, B.J.; Catalano, P.J.; Meropol, N.J.; O’Dwyer, P.J.; Mitchell, E.P.; Alberts, S.R.; Schwartz, M.A.; Benson, A.B., III. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 2007, 25, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Bennouna, J.; Sastre, J.; Arnold, D.; Österlund, P.; Greil, R.; Van Cutsem, E.; von Moos, R.; Viéitez, J.M.; Bouché, O.; Borg, C.; et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Paccard, C.; Chiron, M.; Tabernero, J. Impact of Prior Bevacizumab Treatment on VEGF-A and PlGF Levels and Outcome Following Second-Line Aflibercept Treatment: Biomarker Post Hoc Analysis of the VELOUR Trial. Clin. Cancer Res. 2020, 26, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Hozak, R.R.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; García-Carbonero, R.; Ciuleanu, T.E.; Portnoy, D.C.; Prausová, J.; et al. Analysis of angiogenesis biomarkers for ramucirumab efficacy in patients with metastatic colorectal cancer from RAISE, a global, randomized, double-blind, phase III study. Ann. Oncol. 2018, 29, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Hozak, R.R.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.-E.; Portnoy, D.C.; Prausová, J.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cance (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar]
- Jubb, A.M.; Harris, A.L. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol. 2010, 11, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, R.; Puzzoni, M.; Daniele, B.; Ferrari, D.; Lonardi, S.; Zaniboni, A.; Cavanna, L.; Rosati, G.; Pella, N.; Zampino, M.G.; et al. First-line FOLFIRI and bevacizumab in patients with advanced colorectal cancer prospectively stratified according to serum LDH: Final results of the GISCAD (Italian Group for the Study of Digestive Tract Cancers) CENTRAL (ColorEctalavastiNTRiAlLdh) trial. Br. J. Cancer 2017, 117, 1099–1104. [Google Scholar] [CrossRef][Green Version]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeong, D.; Han, Y.S.; Baek, M.J. Pivotal role of vascular endothelial growth factor pathway in tumor angiogenesis. Ann. Surg. Treat. Res. 2015, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.M.; Inder, M.K.; Real, N.C.; Stuart, G.S.; Fleming, S.B.; Mercer, A.A. The vascular endothelial growth factor (VEGF)-E encoded by orf virus regulates keratinocyte proliferation and migration and promotes epidermal regeneration. Cell. Microbiol. 2012, 14, 1376–1390. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Matsunaga, Y.; Tokunaga, Y.; Obayashi, S.; Saito, M.; Morita, T. Snake venom Vascular Endothelial Growth Factors (VEGF-Fs) exclusively vary their structures and functions among species. J. Biol. Chem. 2009, 284, 9885–9891. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci. Signal. 2009, 2, re1. [Google Scholar] [CrossRef] [PubMed]
- Kerbel, R.S. Tumor angiogenesis. N. Engl. J. Med. 2008, 358, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M.; Claesson-Welsh, L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp. Cell Res. 2006, 312, 549–560. [Google Scholar] [CrossRef]
- Harper, S.J.; Bates, D.O. VEGF-A splicing: The key to anti-angiogenic therapeutics? Nat. Rev. Cancer 2008, 8, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Soker, S.; Takashima, S.; Miao, H.Q.; Neufeld, G.; Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998, 92, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Matkar, P.N.; Jong, E.D.; Ariyagunarajah, R.; Prud’homme, G.J.; Singh, K.K.; Leong-Poi, H. Jack of many trades: Multifaceted role of neuropilins in pancreatic cancer. Cancer Med. 2018, 7, 5036–5046. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Mousa, L.; Salem, M.E.; Mikhail, S. Biomarkers of Angiogenesis in Colorectal Cancer. Biomark. Cancer 2015, 7, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Faloppi, L.; Puzzoni, M.; Casadei Gardini, A.; Silvestris, N.; Masi, G.; Marisi, G.; Vivaldi, C.; Gadaleta, C.D.; Ziranu, P.; Bianconi, M.; et al. Angiogenesis Genotyping and Clinical Outcomes in Patients with Advanced Hepatocellular Carcinoma Receiving Sorafenib: The ALICE-2 Study. Target. Oncol. 2020, 15, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Casadei Gardini, A.; Marisi, G.; Dadduzio, V.; Ielasi, L.; Vivaldi, C.; Rizzato, M.; Fornaro, L.; Lonardi, S.; Gramantieri, L.; Pecora, I.; et al. Multicentric prospective study of validation of angiogenesis-related gene polymorphisms in hepatocellular carcinoma patients treated with sorafenib: Results of INNOVATE study. Ann. Oncol. 2019, 30 (Suppl. 4), iv113. [Google Scholar] [CrossRef]
- Marisi, G.; Petracci, E.; Raimondi, F.; Faloppi, L.; Foschi, F.G.; Lauletta, G.; Iavarone, M.; Canale, M.; Valgiusti, M.; Neri, L.M.; et al. ANGPT2 and NOS3 Polymorphisms and Clinical Outcome in Advanced Hepatocellular Carcinoma Patients Receiving Sorafenib. Cancers 2019, 11, 1023. [Google Scholar] [CrossRef] [PubMed]
- Marisi, G.; Cucchetti, A.; Ulivi, P.; Canale, M.; Cabibbo, G.; Solaini, L.; Foschi, F.G.; De Matteis, S.; Ercolani, G.; Valgiusti, M.; et al. Ten years of sorafenib in hepatocellular carcinoma: Are there any predictive and/or prognostic markers? World J. Gastroenterol. 2018, 24, 4152–4163. [Google Scholar] [CrossRef] [PubMed]
- Casadei Gardini, A.; Faloppi, L.; Aprile, G.; Brunetti, O.; Caparello, C.; Corbelli, J.; Chessa, L.; Bruno, D.; Ercolani, G.; Leonetti, A.; et al. Multicenter prospective study of angiogenesis polymorphism validation in HCC patients treated with sorafenib. An INNOVATE study protocol. Tumori J. 2018, 104, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Marisi, G.; Passardi, A.; Scartozzi, M.; Silvestris, N.; Valgiusti, M.; Ulivi, P.; Faloppi, L.; Brunetti, O.; Giovanni, L.F.; Gardini, A.C. Ang-2 polymorphisms in relation to outcome in advanced HCC patients receiving sorafenib. Ann. Oncol. 2017, 28 (Suppl. 3), ii1–ii2. [Google Scholar] [CrossRef][Green Version]
- Scartozzi, M.; Faloppi, L.; Baroni, G.S.; Loretelli, C.; Piscaglia, F.; Iavarone, M.; Toniutto, P.; Fava, G.; De Minicis, S.; Mandolesi, A.; et al. VEGF and VEGFR genotyping in the prediction of clinical outcome for HCC patients receiving sorafenib: The ALICE-1 study. Int. J. Cancer 2014, 135, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Papachristos, A.; Kemos, P.; Katsila, T.; Panoilia, E.; Patrinos, G.P.; Kalofonos, H.; Sivolapenko, G.B. VEGF-A and ICAM-1 Gene Polymorphisms as Predictors of Clinical Outcome to First-Line Bevacizumab-Based Treatment in Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 5791. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, R.; Salvatore, L.; Del Prete, M.; Prochilo, T.; D’anzeo, M.; Loretelli, C.; Loupakis, F.; Aprile, G.; Maccaroni, E.; Andrikou, K.; et al. Angiogenesis genotyping and clinical outcome during regorafenib treatment in metastatic colorectal cancer patients. Sci. Rep. 2016, 6, 25195. [Google Scholar] [CrossRef] [PubMed]
- Sibertin-Blanc, C.; Mancini, J.; Fabre, A.; Lagarde, A.; Del Grande, J.; Levy, N.; Seitz, J.-F.; Olschwang, S.; Dahan, L. Vascular Endothelial Growth Factor A c.*237C>T polymorphism is associated with bevacizumab efficacy and related hypertension in metastatic colorectal cancer. Dig. Liver Dis. 2015, 47, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Paré-Brunet, L.; Sebio, A.; Salazar, J.; Berenguer-Llergo, A.; Río, E.; Barnadas, A.; Baiget, M.; Páez, D. Genetic variations in the VEGF pathway as prognostic factors in metastatic colorectal cancer patients treated with oxaliplatin-based chemotherapy. Pharmacogenom. J. 2015, 15, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Aravantinos, G.; Isaakidou, A.; Karantanos, T.; Sioziou, A.; Theodoropoulos, G.; Pektasides, D.; Gazouli, M. Association of CD133 polymorphisms and response to bevacizumab in patients with metastatic colorectal cancer. Cancer Biomark. 2015, 15, 843–850. [Google Scholar] [CrossRef] [PubMed]
- González-Vacarezza, N.; Alonso, I.; Arroyo, G.; Martínez, J.; De Andrés, F.; Llerena, A.; Estévez-Carrizo, F. Predictive biomarkers candidates for patients with metastatic colorectal cancer treated with bevacizumab-containing regimen. Drug Metab. Pers. Ther. 2016, 31, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.D.; Stintzing, S.; Heinemann, V.; Yang, D.; Cao, S.; Sunakawa, Y.; Ning, Y.; Matsusaka, S.; Okazaki, S.; Miyamoto, Y.; et al. Impact of genetic variations in the MAPK signaling pathway on outcome in metastatic colorectal cancer patients treated with first-line FOLFIRI and bevacizumab: Data from FIRE-3 and TRIBE trials. Ann. Oncol. 2017, 28, 2780–2785. [Google Scholar] [CrossRef] [PubMed]
- Di Salvatore, M.; Pietrantonio, F.; Orlandi, A.; Del Re, M.; Berenato, R.; Rossi, E.; Caporale, M.; Guarino, D.; Martinetti, A.; Basso, M.; et al. IL-8 and eNOS polymorphisms predict bevacizumab-based first line treatment outcomes in RAS mutant metastatic colorectal cancer patients. Oncotarget 2017, 8, 16887–16898. [Google Scholar] [CrossRef] [PubMed]
- Matsusaka, S.; Hanna, D.L.; Cao, S.; Zhang, W.; Yang, D.; Ning, Y.; Sunakawa, Y.; Okazaki, S.; Berger, M.D.; Miyamato, Y.; et al. Prognostic Impact of IL6 Genetic Variants in Patients with Metastatic Colorectal Cancer Treated with Bevacizumab-Based Chemotherapy. Clin. Cancer Res. 2016, 22, 3218–3226. [Google Scholar] [CrossRef] [PubMed]
- Burgermeister, E.; Battaglin, F.; Eladly, F.; Wu, W.; Herweck, F.; Schulte, N.; Betge, J.; Härtel, N.; Kather, J.N.; Weis, C.-A.; et al. Aryl hydrocarbon receptor nuclear translocator-like (ARNTL/BMAL1) is associated with bevacizumab resistance in colorectal cancer via regulation of vascular endothelial growth factor A. EBioMedicine 2019, 45, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Duda, D.G.; Willett, C.G.; Ancukiewicz, M.; Tomaso, E.; Shah, M.; Czito, B.G.; Bentley, R.; Poleski, M.; Lauwers, G.Y.; Carroll, M.; et al. Plasma soluble VEGFR-1 is a potential dual biomarker of response and toxicity for bevacizumab with chemoradiation in locally advanced rectal cancer. Oncologist 2010, 15, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Martinetti, A.; Miceli, R.; Sottotetti, E.; Di Bartolomeo, M.; De Braud, F.; Gevorgyan, A.; Dotti, K.F.; Bajetta, E.; Campiglio, M.; Bianchi, F.; et al. Circulating biomarkers in advanced colorectal cancer patients randomly assigned to three bevacizumab-based regimens. Cancers 2014, 6, 1753–1768. [Google Scholar] [CrossRef]
- Jürgensmeier, J.M.; Schmoll, H.-J.; Robertson, J.D.; Brooks, L.; Taboada, M.; Morgan, S.R.; Wilson, D.; Hoff, P.M. Prognostic and predictive value of VEGF, sVEGFR-2 and CEA in mCRC studies comparing cediranib, bevacizumab and chemotherapy. Br. J. Cancer 2013, 108, 1316–1323. [Google Scholar] [CrossRef]
- Marisi, G.; Scarpi, E.; Passardi, A.; Nanni, O.; Ragazzini, A.; Valgiusti, M.; Gardini, A.C.; Neri, L.M.; Frassineti, G.L.; Amadori, D.; et al. Circulating VEGF and eNOS variations as predictors of outcome in metastatic colorectal cancer patients receiving bevacizumab. Sci. Rep. 2017, 7, 1293. [Google Scholar] [CrossRef] [PubMed]
- Abajo, A.; Boni, V.; Lopez, I.; Gonzalez-Huarriz, M.; Bitarte, N.; Rodriguez, J.; Zarate, R.; Bandres, E.; Garcia-Foncillas, J. Identification of predictive circulating biomarkers of bevacizumab-containing regimen efficacy in pre-treated metastatic colorectal cancer patients. Br. J. Cancer 2012, 107, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, R.; Ziranu, P.; Daniele, B.; Zizzi, A.; Ferrari, D.; Lonardi, S.; Zaniboni, A.; Cavanna, L.; Rosati, G.; Casagrande, M.; et al. From CENTRAL to SENTRAL (SErum aNgiogenesis cenTRAL): Circulating Predictive Biomarkers to Anti-VEGFR Therapy. Cancers 2020, 12, 1330. [Google Scholar] [CrossRef] [PubMed]
- Ziranu, P.; Demurtas, L.; Puzzoni, M.; Loupakis, F.; Daniele, B.; Rimassa, L.; Bilancia, D.; Lonardi, S.; Avallone, A.; Pella, N.; et al. The DISTINCTIVE study: A biologically enriched phase II study of seconD-line folfiri/aflIbercept in proSpecTIvely stratified, anti-EGFR resistaNt, metastatic coloreCTal cancer patIents with RAS Validated wild typE status-Trial in progress. Ann. Oncol. 2018, 29 (Suppl. 5), v82. [Google Scholar] [CrossRef]
- Lim, S.H.; Becker, T.M.; Chua, W.; Caixeiro, N.J.; Ng, W.L.; Kienzle, N.; Tognela, A.; Lumba, S.; Rasko, J.E.J.; de Souza, P.; et al. Circulating tumour cells and circulating free nucleic acid as prognostic and predictive biomarkers in colorectal cancer. Cancer Lett. 2014, 346, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Groot Koerkamp, B.; Rahbari, N.N.; Buchler, M.W.; Koch, M.; Weitz, J. Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: A meta-analysis. Ann. Surg. Oncol. 2013, 20, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.J.A.; Punt, C.J.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.; Mitchell, E.; Miller, M.C.; et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 3213–3221. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, N.N.; Reissfelder, C.; Mühlbayer, M.; Weidmann, K.; Kahlert, C.; Büchler, M.W.; Weitz, J.; Koch, M. Correlation of circulating angiogenic factors with circulating tumor cells and disease recurrence in patients undergoing curative resection for colorectal liver metastases. Ann. Surg. Oncol. 2011, 18, 2182–2191. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; Vieitez, J.M.; Gomez-España, M.A.; Gil Calle, S.; Salvia, A.S.; Suárez, B.G.; Garcia-Alfonso, P.; de Castro, E.M.; Aldana, G.A.Q.; Reina-Zoilo, J.J.; et al. Randomized phase III study comparing FOLFOX + bevacizumab versus folfoxiri + bevacizumab (BEV) as 1st line treatment in patients with metastatic colorectal cancer (mCRC) with ≥ 3 baseline circulating tumor cells (bCTCs). J. Clin. Oncol. 2019, 37 (Suppl. 5), 3507. [Google Scholar] [CrossRef]
- Pretta, A.; Lai, E.; Donisi, C.; Spanu, D.; Ziranu, P.; Pusceddu, V.; Puzzoni, M.; Massa, E.; Scartozzi, M. Circulating tumour DNA in gastrointestinal cancer in clinical practice: Just a dream or maybe not? World J. Clin. Oncol. 2022, 13, 980–983. [Google Scholar] [CrossRef] [PubMed]
- Puzzoni, M.; Ziranu, P.; Demurtas, L.; Lai, E.; Mariani, S.; Liscia, N.; Soro, P.; Pretta, A.; Impera, V.; Camera, S.; et al. Why precision medicine should be applied across the continuum of care for metastatic colorectal cancer patients. Future Oncol. 2020, 16, 4337–4339. [Google Scholar] [CrossRef] [PubMed]
- Mariani, S.; Puzzoni, M.; Giampieri, R.; Ziranu, P.; Pusceddu, V.; Donisi, C.; Persano, M.; Pinna, G.; Cimbro, E.; Parrino, A.; et al. Liquid Biopsy-Driven Cetuximab Rechallenge Strategy in Molecularly Selected Metastatic Colorectal Cancer Patients. Front. Oncol. 2022, 12, 852583. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Perakis, S.O.; Ulz, P.; Mohan, S.; Riedl, J.M.; Talakic, E.; Lax, S.; Tötsch, M.; Hoefler, G.; Bauernhofer, T.; et al. Cell-free DNA analysis reveals POLR1D-mediated resistance to bevacizumab in colorectal cancer. Genome Med. 2020, 12, 20. [Google Scholar] [CrossRef]
- Yamauchi, M.; Urabe, Y.; Ono, A.; Miki, D.; Ochi, H.; Chayama, K. Serial profiling of circulating tumor DNA for optimization of anti-VEGF chemotherapy in metastatic colorectal cancer patients. Int. J. Cancer 2018, 142, 1418–1426. [Google Scholar] [CrossRef]
- do Espírito Santo, G.F.; Galera, B.B.; Duarte, E.C.; Chen, E.S.; Azis, L.; Damazo, A.S.; Saba, G.T.; de Sousa Gehrke, F.; da Silva, I.D.C.G.; Waisberg, J. Prognostic significance of vascular endothelial growth factor polymorphisms in colorectal cancer patients. World J. Gastrointest. Oncol. 2017, 9, 78–86. [Google Scholar] [CrossRef]
- Wong, A.L.A.; Lim, J.S.J.; Sinha, A.; Gopinathan, A.; Lim, R.; Tan, C.-S.; Soh, T.; Venkatesh, S.; Titin, C.; Sapari, N.S.; et al. Tumour pharmacodynamics and circulating cell free DNA in patients with refractory colorectal carcinoma treated with regorafenib. J. Transl. Med. 2015, 13, 57. [Google Scholar] [CrossRef]
- Tabernero, J.; Lenz, H.-J.; Siena, S.; Sobrero, A.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: A retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol. 2015, 16, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Kopetz, S.; Davuluri, R.; Hamilton, S.R.; Calin, G.A. MicroRNAs, ultraconserved genes and colorectal cancers. Int. J. Biochem. Cell Biol. 2010, 42, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Shivapurkar, N.; Mikhail, S.; Navarro, R.; Bai, W.; Marshall, J.; Hwang, J.; Pishvaian, M.; Wellstein, A.; He, A.R. Decrease in blood miR-296 predicts chemotherapy resistance and poor clinical outcome in patients receiving systemic chemotherapy for metastatic colon cancer. Int. J. Color. Dis. 2013, 28, 887. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stiegelbauer, V.; Perakis, S.; Deutsch, A.; Ling, H.; Gerger, A.; Pichler, M. MicroRNAs as novel predictive biomarkers and therapeutic targets in colorectal cancer. World J. Gastroenterol. 2014, 20, 11727–11735. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.F.; Kjaer-Frifeldt, S.; Christensen, R.D.; Morgenthaler, S.; Blondal, T.; Lindebjerg, J.; Sørensen, F.B.; Jakobsen, A. Redefining high-risk patients with stage II colon cancer by risk index and microRNA-21: Results from a population-based cohort. Br. J. Cancer 2014, 111, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Jin, R.; Mao, X.; Wang, J.; Yuan, J.; Zhao, G. Prognostic value of miRNA-181a/b in colorectal cancer: A meta-analysis. Biomark. Med. 2018, 12, 299–308. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.; Tong, C.W.; Wu, M.; Cho, W.C. MicroRNAs in the prognosis and therapy of colorectal cancer: From bench to bedside. World J. Gastroenterol. 2018, 24, 2949–2973. [Google Scholar] [CrossRef] [PubMed]
- Quasar Collaborative Group. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet 2007, 370, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Ågesen, T.H.; Sveen, A.; Merok, M.A.; Lind, G.E.; Nesbakken, A.; Skotheim, R.I.; Lothe, R.A. ColoGuideEx: A robust gene classifier specific for stage II colorectal cancer prognosis. Gut 2012, 61, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Labianca, R.; Nordlinger, B.; Beretta, G.D.; Brouquet, A.; Cervantes, A.; ESMO Guidelines Working Group. Primary colon cancer: ESMO Clinical Practice Guidelines for diagnosis, adjuvant treatment and follow-up. Ann. Oncol. 2010, 21 (Suppl. 5), v70–v77. [Google Scholar] [CrossRef] [PubMed]
- Ellermeier, C.; Vang, S.; Cleveland, K.; Durand, W.; Resnick, M.B.; Brodsky, A.S. Prognostic microRNA expression signature from examination of colorectal primary and metastatic tumors. Anticancer Res. 2014, 34, 3957–3967. [Google Scholar] [PubMed]
- Slaby, O.; Svoboda, M.; Fabian, P.; Smerdova, T.; Knoflickova, D.; Bednarikova, M.; Nenutil, R.; Vyzula, R. Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology 2007, 72, 397–402. [Google Scholar] [CrossRef]
- Shibuya, H.; Iinuma, H.; Shimada, R.; Horiuchi, A.; Watanabe, T. Clinicopathological and prognostic value of microRNA-21 and microRNA-155 in colorectal cancer. Oncology 2010, 79, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Drusco, A.; Nuovo, G.J.; Zanesi, N.; Di Leva, G.; Pichiorri, F.; Volinia, S.; Fernandez, C.; Antenucci, A.; Costinean, S.; Bottoni, A.; et al. MicroRNA profiles discriminate among colon cancer metastasis. PLoS ONE 2014, 9, e96670. [Google Scholar] [CrossRef] [PubMed]
- Aharonov, R.; Weissmann-Brenner, A.; Kushnir, M.; Yanai, G.L.; Gibori, H.; Purim, O.; Kundel, Y.; Morgenstern, S.; Halperin, M.; Niv, Y.; et al. Tumor microRNA-29a expression and the risk of recurrence in stage II colon cancer. Int. J. Oncol. 2012, 40, 2097–2103. [Google Scholar] [CrossRef]
- Tang, W.; Zhu, Y.; Gao, J.; Fu, J.; Liu, C.; Liu, Y.; Song, C.; Zhu, S.; Leng, Y.; Wang, G.; et al. MicroRNA-29a promotes colorectal cancer metastasis by regulating matrix metalloproteinase 2 and E-cadherin via KLF4. Br. J. Cancer 2014, 110, 450–458. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, K.H.; Shin, S.J.; Lee, K.Y.; Kim, T.I.; Kim, N.K.; Rha, S.Y.; Roh, J.K.; Ahn, J.B. p16 Hypermethylation and KRAS Mutation Are Independent Predictors of Cetuximab Plus FOLFIRI Chemotherapy in Patients with Metastatic Colorectal Cancer. Cancer Res. Treat. 2016, 48, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Kokelaar, R.F.; Jones, H.G.; Williamson, J.; Williams, N.; Griffiths, A.P.; Beynon, J.; Jenkins, G.J.; Harris, D.A. DNA hypermethylation as a predictor of extramural vascular invasion (EMVI) in rectal cancer. Cancer Biol. Ther. 2018, 19, 214–221. [Google Scholar] [CrossRef]
- Hinganu, D.; Hinganu, M.V.; Bulimar, V.; Andronic, D. Correlation criteria between extramural invasion of blood vessels and immunohistochemical markers in the processes of neovasculogenesis. Rev. Chim. 2018, 69, 371–374. [Google Scholar] [CrossRef]
- Koutrafouri, V.; Leondiadis, L.; Avgoustakis, K.; Livaniou, E.; Czarnecki, J.; Ithakissios, D.S.; Evangelatos, G.P. Effect of thymosin peptides on the chick chorioallantoic membrane angiogenesis model. Biochim. Biophys. Acta 2001, 1568, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Nemolato, S.; Restivo, A.; Cabras, T.; Coni, P.; Zorcolo, L.; Orrù, G.; Cau, F.; Gerosa, C.; Fanni, D.; Messana, I.; et al. Thymosin β-4 in colorectal cancer is localized predominantly at the invasion front in tumor cells undergoing epithelial mesenchymal transition. Cancer Biol. Ther. 2012, 13, 191–197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wijnhoven, B.P.; Dinjens, W.N.; Pignatelli, M. E-cadherin-catenin cell-cell adhesion complex and human cancer. Br. J. Surg. 2000, 87, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Kim, J.H.; Park, S.J.; Han, J.K. Prediction of the therapeutic response after FOLFOX and FOLFIRI treatment for patients with liver metastasis from colorectal cancer using computerized CT texture analysis. Eur. J. Radiol. 2016, 85, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Q.; Liang, C.-H.; He, L.; Tian, J.; Liang, C.-S.; Chen, X.; Ma, Z.-L.; Liu, Z.-Y. Development and Validation of a Radiomics Nomogram for Preoperative Prediction of Lymph Node Metastasis in Colorectal Cancer. J. Clin. Oncol. 2016, 34, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yao, Q.; Xiao, J.; Li, M.; Yang, J.; Hou, W.; Du, M.; Chen, K.; Qu, Y.; Li, L.; et al. Contrast-enhanced CT radiomics for predicting lymph node metastasis in pancreatic ductal adenocarcinoma: A pilot study. Cancer Imaging 2020, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Huang, Y.; He, L.; Chen, X.; Ma, Z.; Dong, D.; Tian, J.; Liang, C.; Liu, Z. The development and validation of a CT-based radiomics signature for the preoperative discrimination of stage I-II and stage III-IV colorectal cancer. Oncotarget 2016, 7, 31401–31412. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Kim, K.; Im, S.; Hwang, S.S.; Kim, K. Prediction of KRAS Mutation in Rectal Cancer Using MRI. Anticancer Res. 2016, 36, 4799–7804. [Google Scholar] [CrossRef] [PubMed]
- van Griethuysen, J.J.M.; Lambregts, D.M.; Trebeschi, S.; Lahaye, M.J.; Bakers, F.C.; Vliegen, R.F.A.; Beets, G.L.; Aerts, H.J.W.L.; Beets-Tan, R.G.H. Radiomics performs comparable to morphologic assessment by expert radiologists for prediction of response to neoadjuvant chemoradiotherapy on baseline staging MRI in rectal cancer. Abdom. Radiol. (NY) 2020, 45, 632–642. [Google Scholar]
- Qu, H.; Zhai, H.; Zhang, S.; Chen, W.; Zhong, H.; Cui, X. Dynamic radiomics for predicting the efficacy of antiangiogenic therapy in colorectal liver metastases. Front. Oncol. 2023, 13, 992096. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhang, Y.; Dong, D.; Li, C.; Liang, X.; Zhang, C.; Wan, L.; Zhao, X.; Xu, K.; Zhou, C.; et al. Novel Radiomic Signature as a Prognostic Biomarker for Locally Advanced Rectal Cancer. J. Magn. Reson. Imaging 2018, 48, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Griffin, N.; Addley, H.; Sala, E.; Shaw, A.S.; Grant, L.A.; Eldaly, H.; Davies, S.E.; Prevost, T.; Alexander, G.J.; Lomas, D.J. Vascular invasion in hepatocellular carcinoma: Is there a correlation with MRI? Br. J. Radiol. 2012, 85, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Segal, E.; Sirlin, C.B.; Ooi, C.; Adler, A.S.; Gollub, J.; Chen, X.; Chan, B.K.; Matcuk, G.R.; Barry, C.T.; Chang, H.Y.; et al. Decoding global gene expression programs in liver cancer by noninvasive imaging. Nat. Biotechnol. 2007, 25, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Wang, D.S.; Kim, H.J.; Sirlin, C.B.; Chan, M.G.; Korn, R.L.; Rutman, A.M.; Siripongsakun, S.; Lu, D.; Imanbayev, G.; et al. A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology 2015, 62, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Biswas, M.; Kuppili, V.; Godia, E.C.; Suri, H.S.; Edla, D.R.; Omerzu, T.; Laird, J.R.; Khanna, N.N.; Mavrogeni, S.; et al. The present and future of deep learning in radiology. Eur. J. Radiol. 2019, 114, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Capirci, C.; Rampin, L.; Erba, P.A.; Galeotti, F.; Crepaldi, G.; Banti, E.; Gava, M.; Fanti, S.; Mariani, G.; Muzzio, P.C.; et al. Sequential FDG-PET/CT reliably predicts response of locally advanced rectal cancer to neo-adjuvant chemo-radiation therapy. Eur. J. Nucl. Med. 2007, 34, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Kalff, V.; Duong, C.; Drummond, E.G.; Matthews, J.P.; Hicks, R.J. Findings on 18F-FDG PET scans after neoadjuvant chemoradiation provides prognostic stratification in patients with locally advanced rectal carcinoma subsequently treated by radical surgery. J. Nucl. Med. 2006, 47, 14–22. [Google Scholar] [PubMed]
- Lee, S.T.; Tebbutt, N.; Gan, H.K.; Liu, Z.; Sachinidis, J.; Pathmaraj, K.; Scott, A.M. Evaluation of 18F-FMISO PET and 18F-FDG PET Scans in Assessing the Therapeutic Response of Patients With Metastatic Colorectal Cancer Treated With Anti-Angiogenic Therapy. Front. Oncol. 2021, 11, 606210. [Google Scholar] [CrossRef]
- Ono, K.; Ochiai, R.; Yoshida, T.; Kitagawa, M.; Omagari, J.; Kobayashi, H.; Yamashita, Y. Comparison of diffusion-weighted MRI and 2-[fluorine-18]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) for detecting primary colorectal cancer and regional lymph node metastases. J. Magn. Reson. Imaging 2009, 29, 336–340. [Google Scholar] [CrossRef]
- Hein, P.A.; Kremser, C.; Judmaier, W.; Griebel, J.; Pfeiffer, K.-P.; Kreczy, A.; Hug, E.B.; Lukas, P.; DeVries, A.F. Diffusion-weighted magnetic resonance imaging for monitoring diffusion changes in rectal carcinoma during combined, preoperative chemoradiation: Preliminary results of a prospective study. Eur. J. Radiol. 2003, 45, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Goh, V.; Halligan, S.; Wellsted, D.M.; Bartram, C.I. Can perfusion CT assessment of primary colorectal adenocarcinoma blood flow at staging predict for subsequent metastatic disease? A pilot study. Eur. Radiol. 2009, 19, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Hayano, K.; Shuto, K.; Koda, K.; Yanagawa, N.; Okazumi, S.; Matsubara, H. Quantitative measurement of blood flow using perfusion CT for assessing clinicopathologic features and prognosis in patients with rectal cancer. Dis. Colon Rectum 2009, 52, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Bellomi, M.; Petralia, G.; Sonzogni, A.; Zampino, M.G.; Rocca, A. CT perfusion for the monitoring of neoadjuvant chemotherapy and radiation therapy in rectal carcinoma: Initial experience. Radiology 2007, 244, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Sahani, D.V.; Kalva, S.P.; Hamberg, L.M.; Hahn, P.F.; Willett, C.G.; Saini, S.; Mueller, P.R.; Lee, T.-Y. Assessing tumor perfusion and treatment response in rectal cancer with multisection CT: Initial observations. Radiology 2005, 234, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.-S.; Kim, S.H.; Park, H.-J.; Park, M.-S.; Kim, K.W.; Kim, W.H.; Kim, N.K.; Lee, J.M.; Cho, H.J. Correlations of dynamic contrast-enhanced magnetic resonance imaging with morphologic, angiogenic, and molecular prognostic factors in rectal cancer. Yonsei Med. J. 2013, 54, 123–130. [Google Scholar] [CrossRef]
- Masi, G.; Salvatore, L.; Boni, L.; Loupakis, F.; Cremolini, C.; Fornaro, L.; Schirripa, M.; Cupini, S.; Barbara, C.; Safina, V.; et al. Continuation or reintroduction of bevacizumab beyond progression to first-line therapy in metastatic colorectal cancer: Final results of the randomized BEBYP trial. Ann. Oncol. 2015, 26, 724–730. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Tabernero, J.; Lakomy, R.; Prenen, H.; Prausová, J.; Macarulla, T.; Ruff, P.; van Hazel, G.A.; Moiseyenko, V.; Ferry, D.; et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J. Clin. Oncol. 2012, 30, 3499–3506. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.-E.; Portnoy, D.C.; Van Cutsem, E.; Grothey, A.; et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015, 16, 499–508. [Google Scholar] [PubMed]
- Li, J.; Qin, S.; Xu, R.; Yau, T.C.C.; Ma, B.; Pan, H.; Xu, J.; Bai, Y.; Chi, Y.; Wang, L.; et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015, 16, 619–629. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrias, G.; Lai, E.; Ziranu, P.; Mariani, S.; Donisi, C.; Liscia, N.; Saba, G.; Pretta, A.; Persano, M.; Fanni, D.; et al. Prediction of Response to Anti-Angiogenic Treatment for Advanced Colorectal Cancer Patients: From Biological Factors to Functional Imaging. Cancers 2024, 16, 1364. https://doi.org/10.3390/cancers16071364
Corrias G, Lai E, Ziranu P, Mariani S, Donisi C, Liscia N, Saba G, Pretta A, Persano M, Fanni D, et al. Prediction of Response to Anti-Angiogenic Treatment for Advanced Colorectal Cancer Patients: From Biological Factors to Functional Imaging. Cancers. 2024; 16(7):1364. https://doi.org/10.3390/cancers16071364
Chicago/Turabian StyleCorrias, Giuseppe, Eleonora Lai, Pina Ziranu, Stefano Mariani, Clelia Donisi, Nicole Liscia, Giorgio Saba, Andrea Pretta, Mara Persano, Daniela Fanni, and et al. 2024. "Prediction of Response to Anti-Angiogenic Treatment for Advanced Colorectal Cancer Patients: From Biological Factors to Functional Imaging" Cancers 16, no. 7: 1364. https://doi.org/10.3390/cancers16071364
APA StyleCorrias, G., Lai, E., Ziranu, P., Mariani, S., Donisi, C., Liscia, N., Saba, G., Pretta, A., Persano, M., Fanni, D., Spanu, D., Balconi, F., Loi, F., Deidda, S., Restivo, A., Pusceddu, V., Puzzoni, M., Solinas, C., Massa, E., ... Scartozzi, M. (2024). Prediction of Response to Anti-Angiogenic Treatment for Advanced Colorectal Cancer Patients: From Biological Factors to Functional Imaging. Cancers, 16(7), 1364. https://doi.org/10.3390/cancers16071364







