Electrochemotherapy in Kaposi’s Sarcoma Patients: From the Gold Standard Strategy to Locally Advanced Cutaneous and Subcutaneous Lesions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
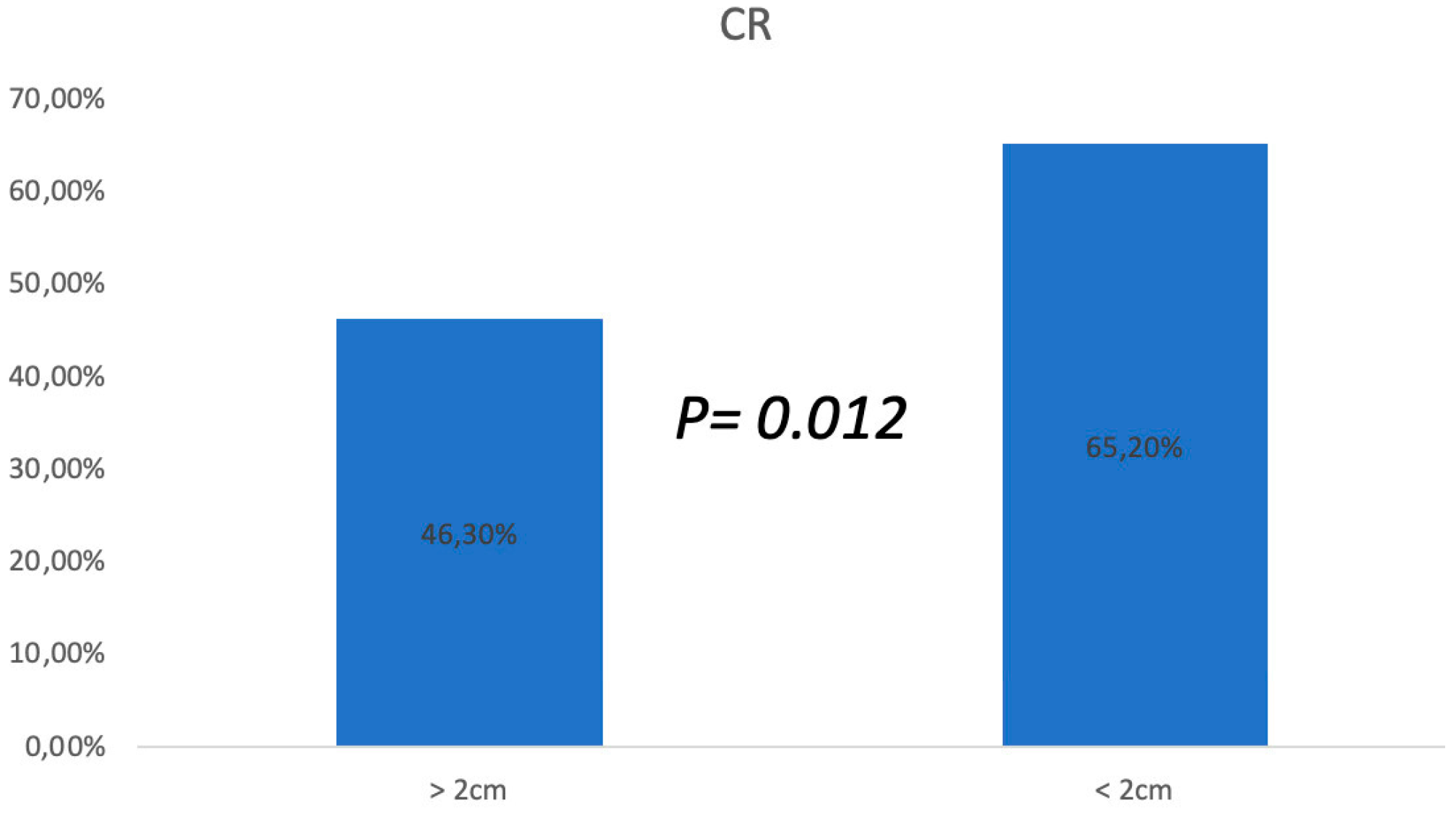
3.1. ECT Treatments
3.2. Follow-Up
3.3. Side Effects and Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Etemad, S.A.; Dewan, A.K. Kaposi Sarcoma Updates. Dermatol. Clin. 2019, 37, 505–517. [Google Scholar] [CrossRef]
- Brambilla, L.; Genovese, G.; Berti, E.; Peris, K.; Rongioletti, F.; Micali, G.; Ayala, F.; DELLA Bella, S.; Mancuso, R.; Pinton, P.C.; et al. Diagnosis and treatment of classic and iatrogenic Kaposi’s sarcoma: Italian recommendations. Ital. J. Dermatol. Venerol. 2021, 156, 356–365. [Google Scholar] [CrossRef]
- Cox, F.H.; Helwig, E.B. Kaposi’s sarcoma. Cancer 1959, 12, 289–298. [Google Scholar] [CrossRef]
- Dupin, N. Update on oncogenesis and therapy for Kaposi sarcoma. Curr. Opin. Oncol. 2020, 32, 122–128. [Google Scholar] [CrossRef]
- Chak, L.Y.; Gill, P.S.; Levine, A.M.; Meyer, P.R.; Anselmo, J.A.; Petrovich, Z. Radiation therapy for acquired immunodeficiency syndrome-related Kaposi’s sarcoma. J. Clin. Oncol. 1998, 5, 863–867. [Google Scholar] [CrossRef]
- Toschi, E.; Sgadari, C.; Monini, P.; Barillari, G.; Bacigalupo, I.; Palladino, C.; Baccarini, S.; Carlei, D.; Grosso, G.; Sirianni, M.C.; et al. Treatment of Kaposi sar- coma-an update. Anticancer. Drugs. 2002, 13, 977–987. [Google Scholar] [CrossRef]
- Campana, L.; Testori, A.; Curatolo, P.; Quaglino, P.; Mocellin, S.; Framarini, M.; Borgognoni, L.; Ascierto, P.; Mozzillo, N.; Guida, M.; et al. Treatment efficacy with electrochemotherapy: A multi-institutional prospective observational study on 376 patients with superficial tumors. Eur. J. Surg. Oncol. 2016, 42, 1914–1923. [Google Scholar] [CrossRef]
- Curatolo, P.; Quaglino, P.; Marenco, F.; Mancini, M.; Nardò, T.; Mortera, C.; Rotunno, R.; Calvieri, S.; Bernengo, M.G. Electrochemotherapy in the treatment of Kaposi sarcoma cutaneous lesions: A two-center prospective phase II trial. Ann. Surg. Oncol. 2012, 19, 192–198. [Google Scholar] [CrossRef]
- Testori, A.; Tosti, G.; Martinoli, C.; Spadola, G.; Cataldo, F.; Verrecchia, F.; Baldini, F.; Mosconi, M.; Soteldo, J.; Tedeschi, I.; et al. Electrochemotherapy for cutaneous and subcutaneous tumor lesions: A novel therapeutic approach. Dermatol. Ther. 2010, 23, 651–661. [Google Scholar] [CrossRef]
- Garbay, J.R.; Billard, V.; Bernat, C.; Mir, L.M.; Morsli, N.; Robert, C. Successful repetitive treatments by electrochemotherapy of multiple unresectable Kaposi sarcoma nodules. Eur. J. Cancer 2006, 4 (Suppl. S4), 29–31. [Google Scholar] [CrossRef]
- Latini, A.; Bonadies, A.; Trento, E.; Bultrini, S.; Cota, C.; Solivetti, F.M.; Ferraro, C.; Ardigò, M.; Amorosi, B.; Palamara, G.; et al. Effective treatment of Kaposi’s sarcoma by electrochemotherapy and intravenous bleomycin administration. Dermatol. Ther. 2012, 25, 214–218. [Google Scholar] [CrossRef]
- Giardino, R.; Fini, M.; Bonazzi, V.; Cadossi, R.; Nicolini, A.; Carpi, A. Electrochemotherapy a novel approach to the treatment of metastatic nodules on the skin and subcutaneous tissues. Biomed. Pharmacother. 2006, 60, 458–462. [Google Scholar] [CrossRef]
- Mir, L.M.; Gehl, J.; Sersa, G.; Collins, C.G.; Garbay, J.-R.; Billard, V.; Geertsen, P.F.; Rudolf, Z.; O’sullivan, G.C.; Marty, M. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes. Eur. J. Cancer Suppl. 2006, 4, 14–25. [Google Scholar] [CrossRef]
- Solari, N.; Spagnolo, F.; Ponte, E.; Quaglia, A.; Lillini, R.; Battista, M.; Queirolo, P.; Cafiero, F. Electrochemotherapy for the management of cutaneous and subcutaneous metastasis: A series of 39 patients treated with palliative intent. J. Surg. Oncol. 2014, 109, 270–274. [Google Scholar] [CrossRef]
- Miklavcic, D.; Snoj, M.; Zupanic, A.; Kos, B.; Cemazar, M.; Kropivnik, M.; Bracko, M.; Pecnik, T.; Gadzijev, E.; Sersa, G. Towards treatment planning and treatment of deep-seated solid tumors by electrochemotherapy. Biomed. Eng. Online 2010, 9, 10. [Google Scholar] [CrossRef]
- Gehl, J. Electroporation: Theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol. Scand. 2003, 177, 437–447. [Google Scholar] [CrossRef]
- Chachoua, A.; Krigel, R.; Lafleur, F.; Ostreicher, R.; Speer, M.; Laubenstein, L.; Wernz, J.; Rubenstein, P.; Zang, E.; Friedman-Kien, A. Prognostic factors and staging classification of patients with epidemic Kaposi’s sarcoma. J. Clin. Oncol. 1989, 7, 774–780. [Google Scholar] [CrossRef]
- Brambilla, L.; Boneschi, V.; Taglioni, M.; Ferrucci, S. Staging of classic Kaposi’s sarcoma: A useful tool for therapeutic choices. Eur. J. Dermatol. 2003, 13, 83–86. [Google Scholar]
- Marty, M.; Sersa, G.; Garbay, J.R.; Gehl, J.; Collins, C.G.; Snoj, M.; Billard, V.; Geertsen, P.F.; Larkin, J.O.; Miklavcic, D.; et al. Electrochemotherapy an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur. J. Cancer Suppl. 2006, 4, 3–13. [Google Scholar] [CrossRef]
- DeVita, V.T.; Lawrence, T.S.; Rosenberg, S.A. Cancer: Principles and Practice of Oncology, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef]
- Pincus, T.; Bergman, M.; Sokka, T.; Roth, J.; Swearingen, C.; Yazici, Y. Visual analog scales in formats other than a 10 centimeter horizontal line to assess pain and other clinical data. J. Rheumatol. 2008, 35, 1550–1558. [Google Scholar]
- Sullivan, R.J.; Pantanowitz, L. New drug targets in Kaposi sarcoma. Expert. Opin. Ther. Targets. 2010, 14, 1355–1366. [Google Scholar] [CrossRef]
- Brambilla, L.; Miedico, A.; Ferrucci, S.; Romanelli, A.; Brambati, M.; Vinci, M.; Tedeschi, L.; Boneschi, V. Combination of vinblastine and bleomycin as first line therapy in advanced classic Kaposi’s sarcoma. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 1090–1094. [Google Scholar] [CrossRef]
- Radu, O.; Pantanowitz, L. Kaposi sarcoma. Arch. Pathol. Lab. Med. 2013, 137, 289–294. [Google Scholar] [CrossRef]
- Krigel, R.L.; Laubenstein, L.J.; Muggia, F.M. Kaposi’s sarcoma: A new staging classification. Cancer Treat. Rep. 1983, 67, 531–534. [Google Scholar]
- Fabrizio, T.; Cagiano, L.; De Terlizzi, F.; Grieco, M.P. Neoadjuvant treatment by ECT in cutaneous malignant neoplastic lesions. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 904–912. [Google Scholar] [CrossRef]
- Łapińska, Z.; Saczko, J. Novel electroporation-based treatments for breast cancer. Adv. Clin. Exp. Med. 2022, 31, 1183–1186. [Google Scholar] [CrossRef]
- Bonadies, A.; Bertozzi, E.; Cristiani, R.; Govoni, F.A.; Migliano, E. Electrochemotherapy in Skin Malignancies of Head and Neck Cancer Patients: Clinical Efficacy and Aesthetic Benefits. Acta Derm. Venereol. 2019, 99, 1246–1252. [Google Scholar] [CrossRef]
- Mali, B.; Jarm, T.; Snoj, M.; Sersa, G.; Miklavcic, D. Antitumor effectiveness of electrochemotherapy: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2013, 39, 4–16. [Google Scholar] [CrossRef]
- Di Monta, G.; Caracò, C.; Benedetto, L.; La Padula, S.; Marone, U.; Tornesello, M.; Buonaguro, F.; Simeone, E.; Ascierto, P.; Mozzillo, N. Electrochemotherapy as “new standard of care” treatment for cutaneous Kaposi’s sarcoma. Eur. J. Surg. Oncol. 2014, 40, 61–66. [Google Scholar] [CrossRef]
- Heller, R.; Jaroszeski, M.J.; Reintgen, D.S.; Puleo, C.A.; DeConti, R.C.; Gilbert, R.A.; Glass, L.F. Treatment of cutaneous and subcutaneous tumors with electrochemotherapy using intralesional bleomycin. Cancer 1998, 83, 148–157. [Google Scholar] [CrossRef]




| Patients n° | Sex, Age | Localization | Clinical Response | Response | Stage |
|---|---|---|---|---|---|
| 1 | F, 85 | Right foot | Present | CR | I |
| 2 | F, 74 | Lower limb | Present | CR at second ECT | I |
| 3 | F, 63 | Lower limbs bilateral Foot | Present | PR after third ECT | II |
| 4 | F, 55 | Foot | Present | CR | I |
| 5 | F, 70 | Lower limbs bilateral Foot | Present | CR | I |
| 6 | M, 72 | Foot | Present | CR at second ECT | II |
| 7 | M, 68 | Foot | Present | CR | I |
| 8 | M, 60 | Foot | Present | CR | I |
| 9 | M, 80 | Lower limbs | Present | CR | I |
| 10 | M, 75 | Right foot | Present | CR | I |
| 11 | M, 71 | Bilateral foot | Present | PR after third ECT | II |
| 12 | M, 78 | Lower limbs | Present | CR | I |
| 13 | M, 77 | Lower limbs | Present | CR at second ECT | I |
| 14 | M, 68 | Foot | Present | CR | I |
| 15 | M, 70 | Lower limbs | Present | PR after third ECT | II |
| 16 | M, 76 | Left limb | Present | CR | I |
| 17 | M,74 | Right limb | Present | CR | I |
| 18 | M, 84 | Foot | Present | CR at second ECT | I |
| 19 | M, 69 | Bilateral foot | Present | PR after third ECT | II |
| 20 | M, 71 | Excluded for comorbidities |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rullo, V.; Castellaneta, F.; D’Antonio, S.; De Rosa, A.; Grieco, M.P.; Fabrizio, T. Electrochemotherapy in Kaposi’s Sarcoma Patients: From the Gold Standard Strategy to Locally Advanced Cutaneous and Subcutaneous Lesions. Cancers 2024, 16, 1295. https://doi.org/10.3390/cancers16071295
Rullo V, Castellaneta F, D’Antonio S, De Rosa A, Grieco MP, Fabrizio T. Electrochemotherapy in Kaposi’s Sarcoma Patients: From the Gold Standard Strategy to Locally Advanced Cutaneous and Subcutaneous Lesions. Cancers. 2024; 16(7):1295. https://doi.org/10.3390/cancers16071295
Chicago/Turabian StyleRullo, Vincenzo, Francesco Castellaneta, Santolo D’Antonio, Anna De Rosa, Michele Pio Grieco, and Tommaso Fabrizio. 2024. "Electrochemotherapy in Kaposi’s Sarcoma Patients: From the Gold Standard Strategy to Locally Advanced Cutaneous and Subcutaneous Lesions" Cancers 16, no. 7: 1295. https://doi.org/10.3390/cancers16071295
APA StyleRullo, V., Castellaneta, F., D’Antonio, S., De Rosa, A., Grieco, M. P., & Fabrizio, T. (2024). Electrochemotherapy in Kaposi’s Sarcoma Patients: From the Gold Standard Strategy to Locally Advanced Cutaneous and Subcutaneous Lesions. Cancers, 16(7), 1295. https://doi.org/10.3390/cancers16071295






