Impact of Thrombocytopenia on Survival in Patients with Hepatocellular Carcinoma: Updated Meta-Analysis and Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction and Quality Assessment
2.4. Data Analysis
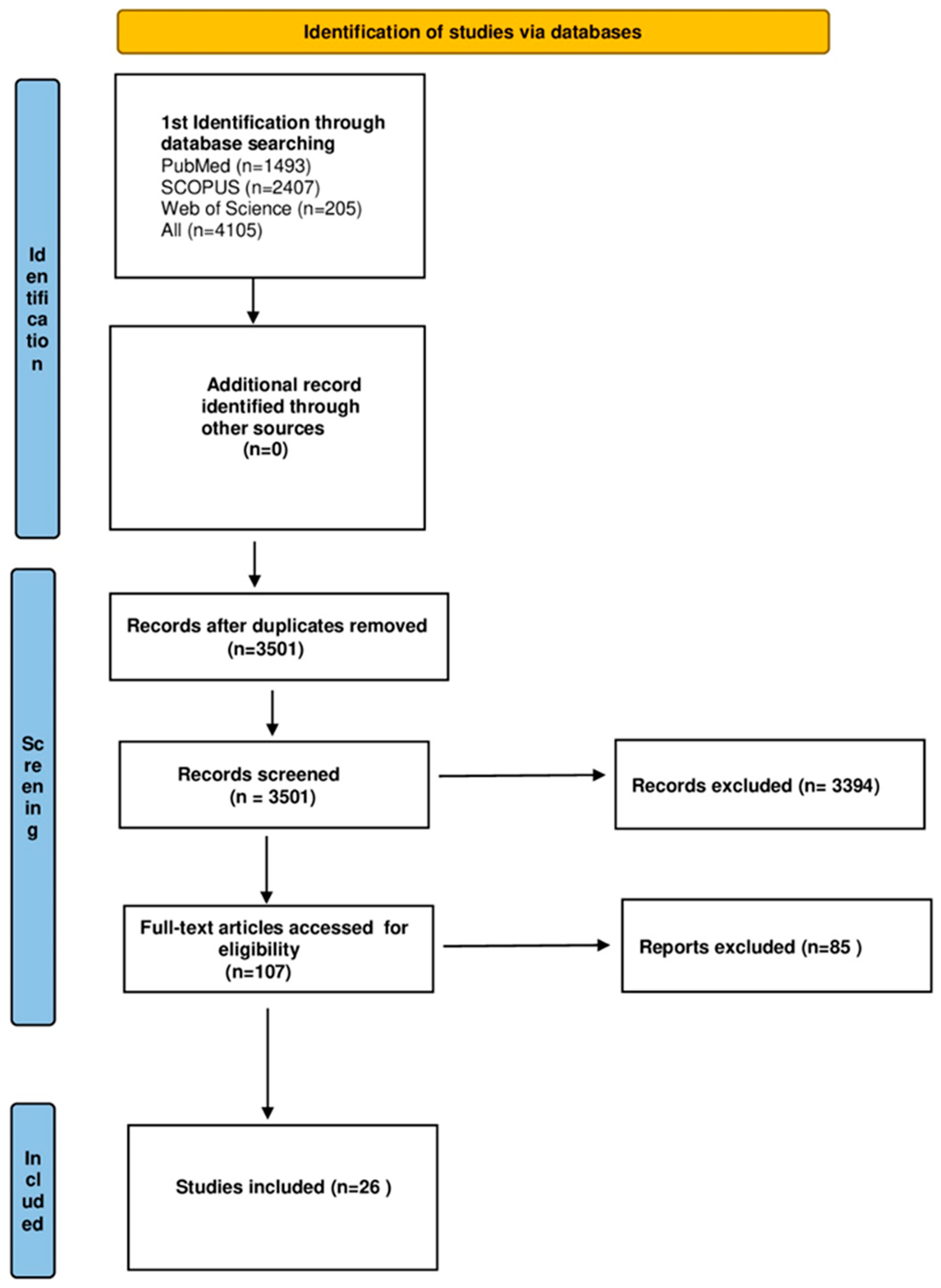
3. Results
3.1. Characteristics of the Included Studies
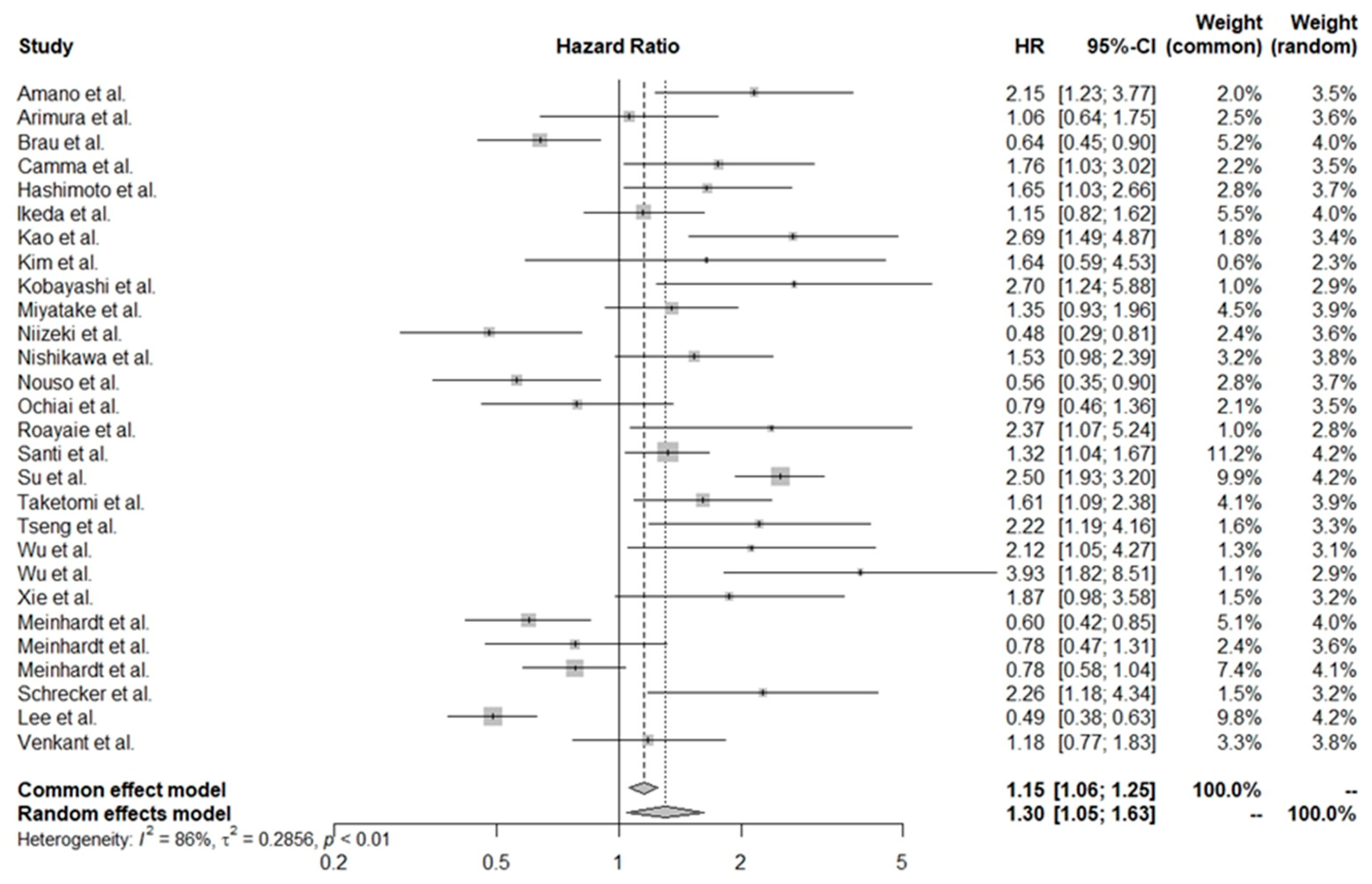
3.2. Pooled HR Values for All of the Studies
3.3. Adjusted Significance of PLTs in HCC
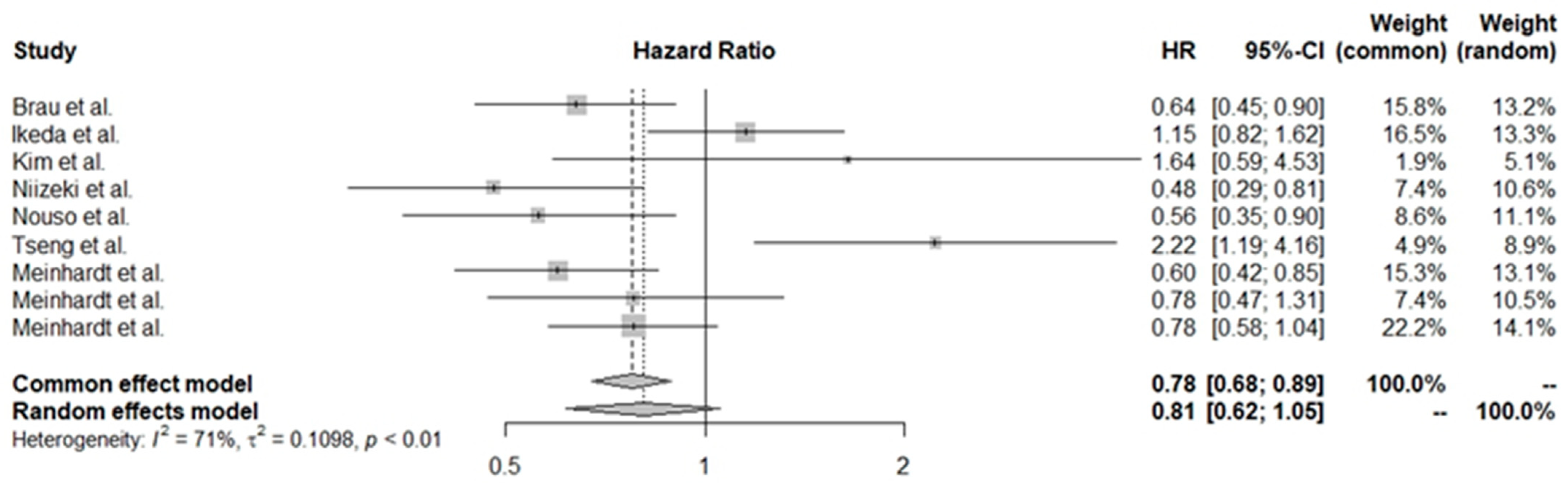
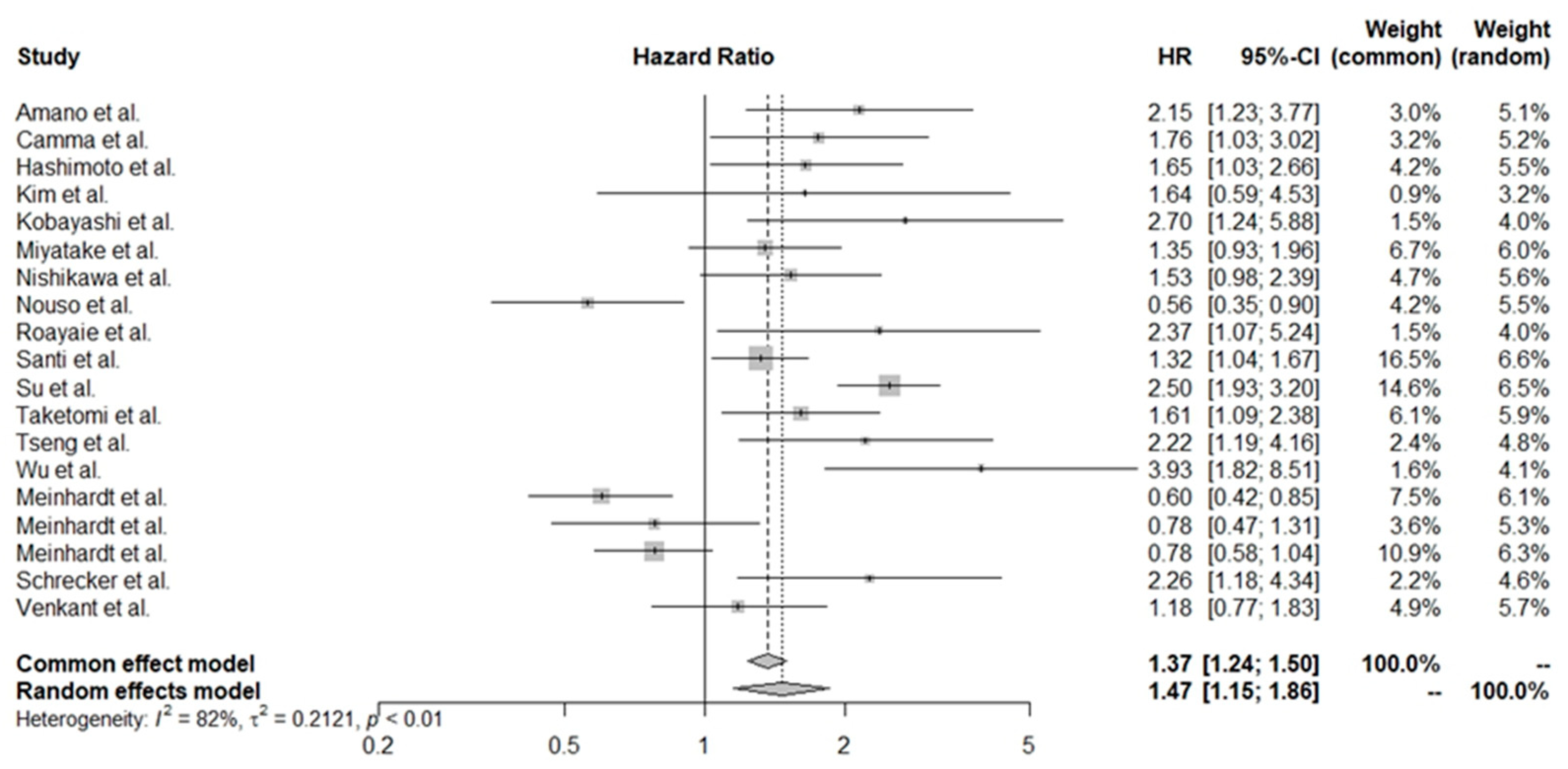
3.4. Pooled HR Values for Various Treatment Groups
3.5. Exploration of Heterogeneity
3.6. Sensitivity Analysis and Test of Publication Bias
3.7. Additional Clinicopathological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e471. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Bengtsson, B.; Widman, L.; Wahlin, S.; Stål, P.; Björkström, N.K.; Hagström, H. The risk of hepatocellular carcinoma in cirrhosis differs by etiology, age and sex: A Swedish nationwide population-based cohort study. United Eur. Gastroenterol. J. 2022, 10, 465–476. [Google Scholar] [CrossRef]
- Richani, M.; Kolly, P.; Knoepfli, M.; Herrmann, E.; Zweifel, M.; Tengg-Kobligk, H.V.; Candinas, D.; Dufour, J.-F. Treatment allocation in hepatocellular carcinoma: Assessment of the BCLC algorithm. Ann. Hepatol. 2016, 15, 82–90. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Wee, I.; Moe, F.; Sultana, R.; Ang, R.; Quek, P.; Goh, B.; Chan, C.Y.; Cheow, P.C.; Chung, A.; Raj, P.; et al. Extending surgical resection for hepatocellular carcinoma beyond Barcelona Clinic for Liver Cancer (BCLC) stage A: A novel application of the modified BCLC staging system. J. Clin. Oncol. 2023, 41, 503. [Google Scholar] [CrossRef]
- Ducreux, M.; Abou-Alfa, G.K.; Bekaii-Saab, T.; Berlin, J.; Cervantes, A.; de Baere, T.; Eng, C.; Galle, P.; Gill, S.; Gruenberger, T.; et al. The management of hepatocellular carcinoma. Current expert opinion and recommendations derived from the 24th ESMO/World Congress on Gastrointestinal Cancer, Barcelona, 2022. ESMO Open 2023, 8, 101567. [Google Scholar] [CrossRef]
- Straś, W.; Gotlib, J.; Małkowski, P.; Wasiak, D.; Śliwczyński, A.; Panczyk, M.; Tronina, O.; Brzozowska, M. Overall Survival in Patients with Hepatocellular Carcinoma Treated with Sorafenib: A Polish Experience. Med. Sci. Monit. 2021, 27, e931856. [Google Scholar] [CrossRef]
- Costantini, S.; Budillon, A. New Prognostic and Predictive Markers in Cancer Progression. Int. J. Mol. Sci. 2020, 21, 8667. [Google Scholar] [CrossRef]
- Riley, R.D.; Sauerbrei, W.; Altman, D.G. Prognostic markers in cancer: The evolution of evidence from single studies to meta-analysis, and beyond. Br. J. Cancer 2009, 100, 1219–1229. [Google Scholar] [CrossRef]
- Ruiz-Bañobre, J.; Kandimalla, R.; Goel, A. Predictive Biomarkers in Metastatic Colorectal Cancer: A Systematic Review. JCO Precis. Oncol. 2019, 3, 1–17. [Google Scholar] [CrossRef]
- Bambace, N.M.; Holmes, C.E. The platelet contribution to cancer progression. J. Thromb. Haemost. 2011, 9, 237–249. [Google Scholar] [CrossRef]
- Lai, Q.; Vitale, A.; Manzia, T.M.; Foschi, F.G.; Levi Sandri, G.B.; Gambato, M.; Melandro, F.; Russo, F.P.; Miele, L.; Viganò, L.; et al. Platelets and Hepatocellular Cancer: Bridging the Bench to the Clinics. Cancers 2019, 11, 1568. [Google Scholar] [CrossRef]
- Midorikawa, Y.; Takayama, T.; Higaki, T.; Aramaki, O.; Teramoto, K.; Yoshida, N.; Tsuji, S.; Kanda, T.; Moriyama, M. High platelet count as a poor prognostic factor for liver cancer patients without cirrhosis. BioSci. Trends 2020, 14, 368–375. [Google Scholar] [CrossRef]
- Chaudhary, P.K.; Kim, S.; Kim, S. An Insight into Recent Advances on Platelet Function in Health and Disease. Int. J. Mol. Sci. 2022, 23, 6022. [Google Scholar] [CrossRef]
- Lesurtel, M.; Graf, R.; Aleil, B.; Walther, D.J.; Tian, Y.; Jochum, W.; Gachet, C.; Bader, M.; Clavien, P.-A. Platelet-Derived Serotonin Mediates Liver Regeneration. Science 2006, 312, 104–107. [Google Scholar] [CrossRef]
- Shaheen, A.A.M.; Myers, R.P. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C–related fibrosis: A systematic review. Hepatology 2007, 46, 912–921. [Google Scholar] [CrossRef]
- Surana, P.; Hercun, J.; Takyar, V.; Kleiner, D.E.; Heller, T.; Koh, C. Platelet count as a screening tool for compensated cirrhosis in chronic viral hepatitis. World J. Gastrointest. Pathophysiol. 2021, 12, 40–50. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network National Comprehensive Cancer Network Compendium. Available online: https://www.nccn.org/compendia-templates/compendia/biomarkers-compendium (accessed on 26 February 2024).
- Pang, Q.; Liu, S.; Wang, L.; Pan, H.; Wang, C.; Zhou, L.; Lu, Y.; Liu, H. The Significance of Platelet-Albumin-Bilirubin (PALBI) Grade in Hepatocellular Carcinoma Patients Stratified According to Platelet Count. Cancer Manag. Res. 2020, 12, 12811–12822. [Google Scholar] [CrossRef]
- Wai, C.-T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.F. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef]
- Abdel-Rahman, O. Prognostic Value of Baseline ALBI Score Among Patients With Colorectal Liver Metastases: A Pooled Analysis of Two Randomized Trials. Clin. Color. Cancer 2019, 18, e61–e68. [Google Scholar] [CrossRef]
- Tanaka, K.; Tsuji, K.; Matsui, T.; Kang, J.-H.; Sakurai, Y.; Kodama, Y.; Minami, R.; Watanabe, K.; Katanuma, A. Potential of PALBI-T score as a prognostic model for hepatocellular carcinoma in alcoholic liver disease. JGH Open 2022, 6, 36–43. [Google Scholar] [CrossRef]
- Scheiner, B.; Kirstein, M.; Popp, S.; Hucke, F.; Bota, S.; Rohr-Udilova, N.; Reiberger, T.; Müller, C.; Trauner, M.; Peck-Radosavljevic, M.; et al. Association of Platelet Count and Mean Platelet Volume with Overall Survival in Patients with Cirrhosis and Unresectable Hepatocellular Carcinoma. Liver Cancer 2019, 8, 203–217. [Google Scholar] [CrossRef]
- Huo, T.-I.; Liu, P.-H. Too Many versus Too Few Platelets in Patients with Hepatocellular Carcinoma: Good or Bad? Liver Cancer 2019, 9, 108–109. [Google Scholar] [CrossRef]
- Pang, Q.; Qu, K.; Zhang, J.Y.; Song, S.D.; Liu, S.S.; Tai, M.H.; Liu, H.C.; Liu, C. The Prognostic Value of Platelet Count in Patients With Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Medicine 2015, 94, e1431. [Google Scholar] [CrossRef]
- Meinhardt, G.; De Sanctis, Y.; LeBerre, M.A.; Nakajima, K. 706P—Overall survival (OS) by platelet count at baseline in patients with hepatocellular carcinoma (HCC) treated with sorafenib (SOR) in the SHARP and AP trials and regorafenib (REG) in the RESORCE trial. Ann. Oncol. 2017, 28, v240–v241. [Google Scholar] [CrossRef]
- Schwarzer, G. meta: An R package for meta-analysis. R. News 2007, 7, 40–45. [Google Scholar]
- Amano, H.; Tashiro, H.; Oshita, A.; Kobayashi, T.; Tanimoto, Y.; Kuroda, S.; Tazawa, H.; Itamoto, T.; Asahara, T.; Ohdan, H. Significance of Platelet Count in the Outcomes of Hepatectomized Patients with Hepatocellular Carcinoma Exceeding the Milan Criteria. J. Gastrointest. Surg. 2011, 15, 1173–1181. [Google Scholar] [CrossRef]
- Arimura, E.; Kotoh, K.; Nakamuta, M.; Morizono, S.; Enjoji, M.; Nawata, H. Local recurrence is an important prognostic factor of hepatocellular carcinoma. World J. Gastroenterol. 2005, 11, 5601–5606. [Google Scholar] [CrossRef]
- Bräu, N.; Fox, R.K.; Xiao, P.; Marks, K.; Naqvi, Z.; Taylor, L.E.; Trikha, A.; Sherman, M.; Sulkowski, M.S.; Dieterich, D.T.; et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: A U.S.–Canadian multicenter study. J. Hepatol. 2007, 47, 527–537. [Google Scholar] [CrossRef]
- Cammà, C.; Di Marco, V.; Orlando, A.; Sandonato, L.; Casaril, A.; Parisi, P.; Alizzi, S.; Sciarrino, E.; Virdone, R.; Pardo, S.; et al. Treatment of hepatocellular carcinoma in compensated cirrhosis with radio-frequency thermal ablation (RFTA): A prospective study. J. Hepatol. 2005, 42, 535–540. [Google Scholar] [CrossRef]
- Hashimoto, K.; Ikeda, Y.; Korenaga, D.; Tanoue, K.; Hamatake, M.; Kawasaki, K.; Yamaoka, T.; Iwatani, Y.; Akazawa, K.; Takenaka, K. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer 2005, 103, 1856–1864. [Google Scholar] [CrossRef]
- Ikeda, M.; Maeda, S.; Shibata, J.; Muta, R.; Ashihara, H.; Tanaka, M.; Fujiyama, S.; Tomita, K. Transcatheter Arterial Chemotherapy with and without Embolization in Patients with Hepatocellular Carcinoma. Oncology 2004, 66, 24–31. [Google Scholar] [CrossRef]
- Kao, W.-Y.; Chiou, Y.-Y.; Hung, H.-H.; Su, C.-W.; Chou, Y.-H.; Huo, T.-I.; Huang, Y.-H.; Wu, W.-C.; Lin, H.-C.; Lee, S.-D.; et al. Younger Hepatocellular Carcinoma Patients Have Better Prognosis After Percutaneous Radiofrequency Ablation Therapy. J. Clin. Gastroenterol. 2012, 46, 62–70. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, I.K.; Park, K.H.; Yoon, S.Y.; Oh, S.C.; Seo, J.H.; Choi, C.W.; Kim, B.S.; Shin, S.W.; Kim, Y.H.; et al. Serum Vascular Endothelial Growth Factor per Platelet Count in Hepatocellular Carcinoma: Correlations with Clinical Parameters and Survival. Jpn. J. Clin. Oncol. 2004, 34, 184–190. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ikeda, K.; Kawamura, Y.; Yatsuji, H.; Hosaka, T.; Sezaki, H.; Akuta, N.; Suzuki, F.; Suzuki, Y.; Saitoh, S.; et al. High serum des-gamma-carboxy prothrombin level predicts poor prognosis after radiofrequency ablation of hepatocellular carcinoma. Cancer 2009, 115, 571–580. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lin, Y., Jr.; Lin, C.-C.; Yen, C.-L.; Shen, C.-H.; Chang, C.-J.; Hsieh, S.-Y. Pretreatment platelet count early predicts extrahepatic metastasis of human hepatoma. Liver Int. 2015, 35, 2327–2336. [Google Scholar] [CrossRef]
- Miyatake, H.; Kobayashi, Y.; Iwasaki, Y.; Nakamura, S.-i.; Ohnishi, H.; Kuwaki, K.; Toshimori, J.; Hagihara, H.; Nouso, K.; Yamamoto, K. Effect of Previous Interferon Treatment on Outcome After Curative Treatment for Hepatitis C Virus-Related Hepatocellular Carcinoma. Dig. Dis. Sci. 2012, 57, 1092–1101. [Google Scholar] [CrossRef]
- Niizeki, T.; Sumie, S.; Torimura, T.; Kurogi, J.; Kuromatsu, R.; Iwamoto, H.; Aino, H.; Nakano, M.; Kawaguchi, A.; Kakuma, T.; et al. Serum vascular endothelial growth factor as a predictor of response and survival in patients with advanced hepatocellular carcinoma undergoing hepatic arterial infusion chemotherapy. J. Gastroenterol. 2012, 47, 686–695. [Google Scholar] [CrossRef]
- Nishikawa, H.; Osaki, Y.; Iguchi, E.; Takeda, H.; Matsuda, F.; Nakajima, J.; Sakamoto, A.; Hatamaru, K.; Saito, S.; Nasu, A.; et al. Radiofrequency ablation for hepatocellular carcinoma: The relationship between a new grading system for the ablative margin and clinical outcomes. J. Gastroenterol. 2013, 48, 951–965. [Google Scholar] [CrossRef]
- Nouso, K.; Ito, Y.M.; Kuwaki, K.; Kobayashi, Y.; Nakamura, S.; Ohashi, Y.; Yamamoto, K. Prognostic factors and treatment effects for hepatocellular carcinoma in Child C cirrhosis. Br. J. Cancer 2008, 98, 1161–1165. [Google Scholar] [CrossRef]
- OCHIAI, T.; OGINO, S.; ISHIMOTO, T.; TOMA, A.; YAMAMOTO, Y.; MORIMURA, R.; IKOMA, H.; OTSUJI, E. Prognostic Impact of Hepatectomy for Patients with Non-hepatitis B, Non-hepatitis C Hepatocellular Carcinoma. Anticancer Res. 2014, 34, 4399–4410. [Google Scholar]
- Roayaie, S.; Obeidat, K.; Sposito, C.; Mariani, L.; Bhoori, S.; Pellegrinelli, A.; Labow, D.; Llovet, J.M.; Schwartz, M.; Mazzaferro, V. Resection of hepatocellular cancer ≤2 cm: Results from two Western centers. Hepatology 2013, 57, 1426–1435. [Google Scholar] [CrossRef]
- Santi, V.; Trevisani, F.; Gramenzi, A.; Grignaschi, A.; Mirici-Cappa, F.; Poggio, P.D.; Nolfo, M.A.D.; Benvegnù, L.; Farinati, F.; Zoli, M.; et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J. Hepatol. 2010, 53, 291–297. [Google Scholar] [CrossRef]
- Schrecker, C.; Waidmann, O.; El Youzouri, H.; Trojan, J.; Schnitzbauer, A.A.; Bechstein, W.O.; Zeuzem, S.; Koch, C. Low Platelet Count Predicts Reduced Survival in Potentially Resectable Hepatocellular Carcinoma. Curr. Oncol. 2022, 29, 1475–1487. [Google Scholar] [CrossRef]
- Su, C.-W.; Chau, G.-Y.; Hung, H.-H.; Yeh, Y.-C.; Lei, H.-J.; Hsia, C.-Y.; Lai, C.-R.; Lin, H.-C.; Wu, J.-C. Impact of Steatosis on Prognosis of Patients with Early-Stage Hepatocellular Carcinoma After Hepatic Resection. Ann. Surg. Oncol. 2015, 22, 2253–2261. [Google Scholar] [CrossRef]
- Taketomi, A.; Shimada, M.; Shirabe, K.; Kajiyama, K.; Gion, T.; Sugimachi, K. Natural killer cell activity in patients with hepatocellular carcinoma. Cancer 1998, 83, 58–63. [Google Scholar] [CrossRef]
- Tseng, P.-L.; Wang, J.-H.; Tung, H.-D.; Hung, C.-H.; Kee, K.-M.; Chen, C.-H.; Chang, K.-C.; Lee, C.-M.; Changchien, C.-S.; Chen, P.-F.; et al. Optimal treatment increased survival of hepatocellular carcinoma patients detected with community-based screening. J. Gastroenterol. Hepatol. 2010, 25, 1426–1434. [Google Scholar] [CrossRef]
- Wu, W.-C.; Chiou, Y.-Y.; Hung, H.-H.; Kao, W.-Y.; Chou, Y.-H.; Su, C.-W.; Wu, J.-C.; Huo, T.-I.; Huang, Y.-H.; Lee, K.-C.; et al. Prognostic Significance of Computed Tomography Scan-derived Splenic Volume in Hepatocellular Carcinoma Treated With Radiofrequency Ablation. J. Clin. Gastroenterol. 2012, 46, 789–795. [Google Scholar] [CrossRef]
- Wu, Z.-F.; Xu, Z.; Li, W.-S.; Zhang, H.-B.; Yang, N.; Yao, X.-Q.; Liu, F.-K.; Yang, G.-S. Impact of occult hepatitis B virus infection on outcome after resection for non-B non-C hepatocellular carcinoma. J. Surg. Res. 2015, 193, 153–160. [Google Scholar] [CrossRef]
- Venkat, R.; Hannallah, J.R.; Krouse, R.S.; Maegawa, F.B. Preoperative thrombocytopenia and outcomes of hepatectomy for hepatocellular carcinoma. J. Surg. Res. 2016, 201, 498–505. [Google Scholar] [CrossRef]
- Xie, H.; Wang, H.; An, W.; Ma, W.; Qi, R.; Yang, B.; Liu, C.; Gao, Y.; Xu, B.; Wang, W. The Efficacy of Radiofrequency Ablation Combined with Transcatheter Arterial Chemoembolization for Primary Hepatocellular Carcinoma in a Cohort of 487 Patients. PLoS ONE 2014, 9, e89081. [Google Scholar] [CrossRef]
- Hatanaka, T.; Yata, Y.; Naganuma, A.; Kakizaki, S. Treatment Strategy for Intermediate-Stage Hepatocellular Carcinoma: Transarterial Chemoembolization, Systemic Therapy, and Conversion Therapy. Cancers 2023, 15, 1798. [Google Scholar] [CrossRef]
- Carr, B.I.; Guerra, V. Hepatocellular Carcinoma Size: Platelets, γ-Glutamyl Transpeptidase, and Alkaline Phosphatase. Oncology 2013, 85, 153–159. [Google Scholar] [CrossRef]
- Carr, B.I.; Guerra, V.; De Giorgio, M.; Fagiuoli, S.; Pancoska, P. Small Hepatocellular Carcinomas and Thrombocytopenia. Oncology 2012, 83, 331–338. [Google Scholar] [CrossRef]
- Carr, B.I.; Guerra, V.; Giannini, E.G.; Farinati, F.; Ciccarese, F.; Rapaccini, G.L.; Di Marco, M.; Benvegnù, L.; Zoli, M.; Borzio, F.; et al. Significance of Platelet and AFP Levels and Liver Function Parameters for HCC Size and Survival. Int. J. Biol. Markers 2014, 29, 215–223. [Google Scholar] [CrossRef]
- Berzigotti, A.; Seijo, S.; Arena, U.; Abraldes, J.G.; Vizzutti, F.; García–Pagán, J.C.; Pinzani, M.; Bosch, J. Elastography, Spleen Size, and Platelet Count Identify Portal Hypertension in Patients With Compensated Cirrhosis. Gastroenterology 2013, 144, 102–111.e101. [Google Scholar] [CrossRef]
- Podrug, K.; Trkulja, V.; Zelenika, M.; Bokun, T.; Madir, A.; Kanizaj, T.F.; O’Beirne, J.; Grgurevic, I. Validation of the New Diagnostic Criteria for Clinically Significant Portal Hypertension by Platelets and Elastography. Dig. Dis. Sci. 2022, 67, 3327–3332. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. New Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Zhu, A.X.; Park, J.O.; Ryoo, B.-Y.; Yen, C.-J.; Poon, R.; Pastorelli, D.; Blanc, J.-F.; Chung, H.C.; Baron, A.D.; Pfiffer, T.E.F.; et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015, 16, 859–870. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, N.; Chen, Z.; Xu, R. Hypoxia-induced secretion of platelet-derived growth factor-BB by hepatocellular carcinoma cells increases activated hepatic stellate cell proliferation, migration and expression of vascular endothelial growth factor-A. Mol. Med. Rep. 2015, 11, 691–697. [Google Scholar] [CrossRef]
- Lv, X.; Fang, C.; Yin, R.; Qiao, B.; Shang, R.; Wang, J.; Song, W.; He, Y.; Chen, Y. Agrin para-secreted by PDGF-activated human hepatic stellate cells promotes hepatocarcinogenesis in vitro and in vivo. Oncotarget 2017, 8, 105340–105355. [Google Scholar] [CrossRef]
- Stock, P.; Monga, D.; Tan, X.; Micsenyi, A.; Loizos, N.; Monga, S.P.S. Platelet-derived growth factor receptor-α: A novel therapeutic target in human hepatocellular cancer. Mol. Cancer Ther. 2007, 6, 1932–1941. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, L.-N.; Lv, Y.; Ma, X.-Y.; Zhi, L.; Liu, C.; Ma, F.; Zhang, X.-F. Overexpression of platelet-derived growth factor receptor alpha promotes tumor progression and indicates poor prognosis in hepatocellular carcinoma. Oncotarget 2014, 5, 10307–10317. [Google Scholar] [CrossRef]
- Fatima, S.; Shi, X.; Lin, Z.; Chen, G.-q.; Pan, X.-h.; Wu, J.C.-Y.; Ho, J.W.; Lee, N.P.; Gao, H.; Zhang, G. 5-Hydroxytryptamine promotes hepatocellular carcinoma proliferation by influencing β-catenin. Mol. Oncol. 2016, 10, 195–212. [Google Scholar] [CrossRef]
- Aryal, B.; Shimizu, T.; Kadono, J.; Furoi, A.; Komokata, T.; Kitazono, I.; Koriyama, C.; Yamakuchi, M.; Hashiguchi, T.; Imoto, Y. Post-resection exhaustion of intra-platelet serotonin: Also an indicator of early hepatocellular carcinoma recurrence? J. Cancer 2017, 8, 3984. [Google Scholar] [CrossRef]
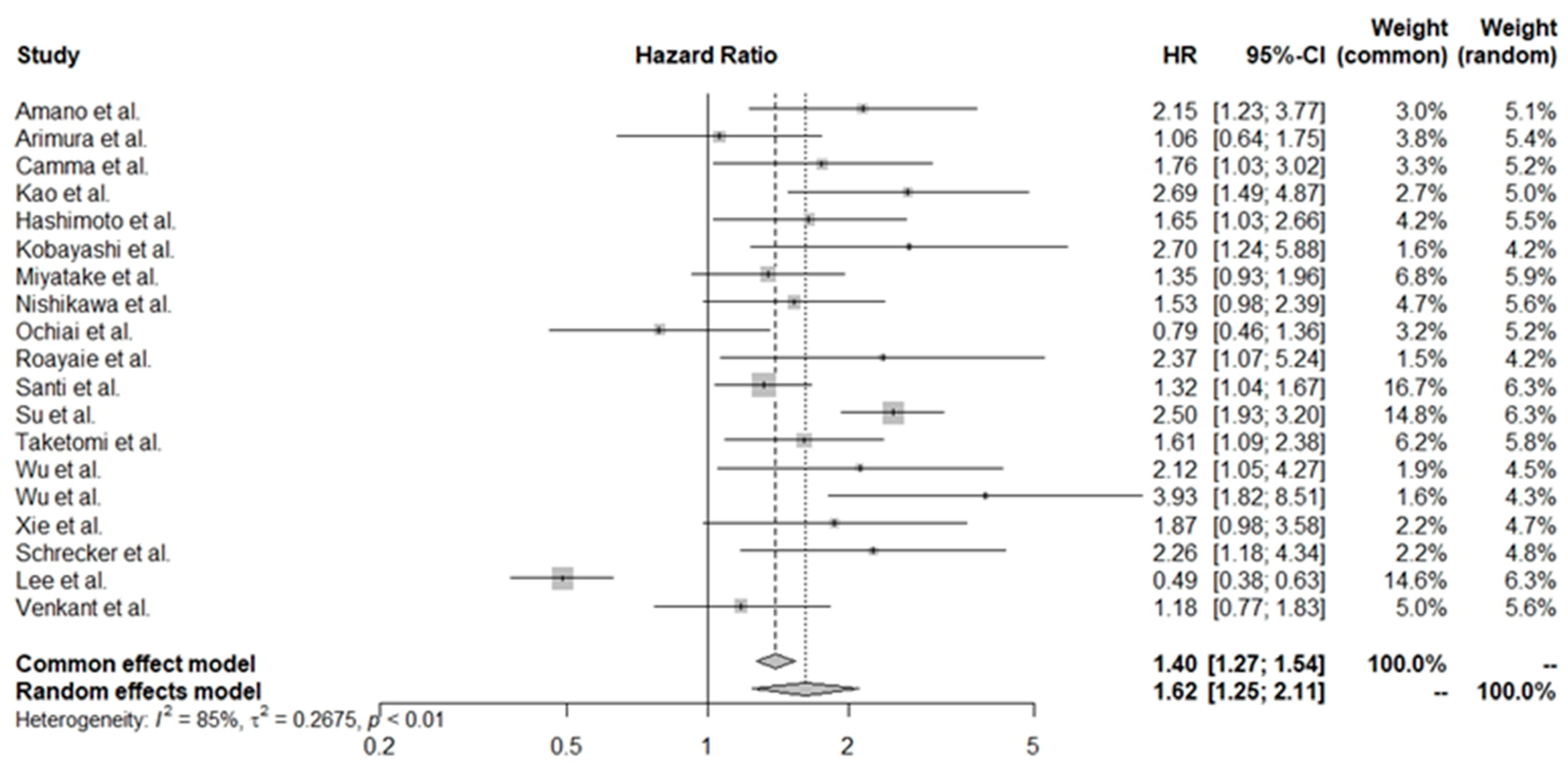
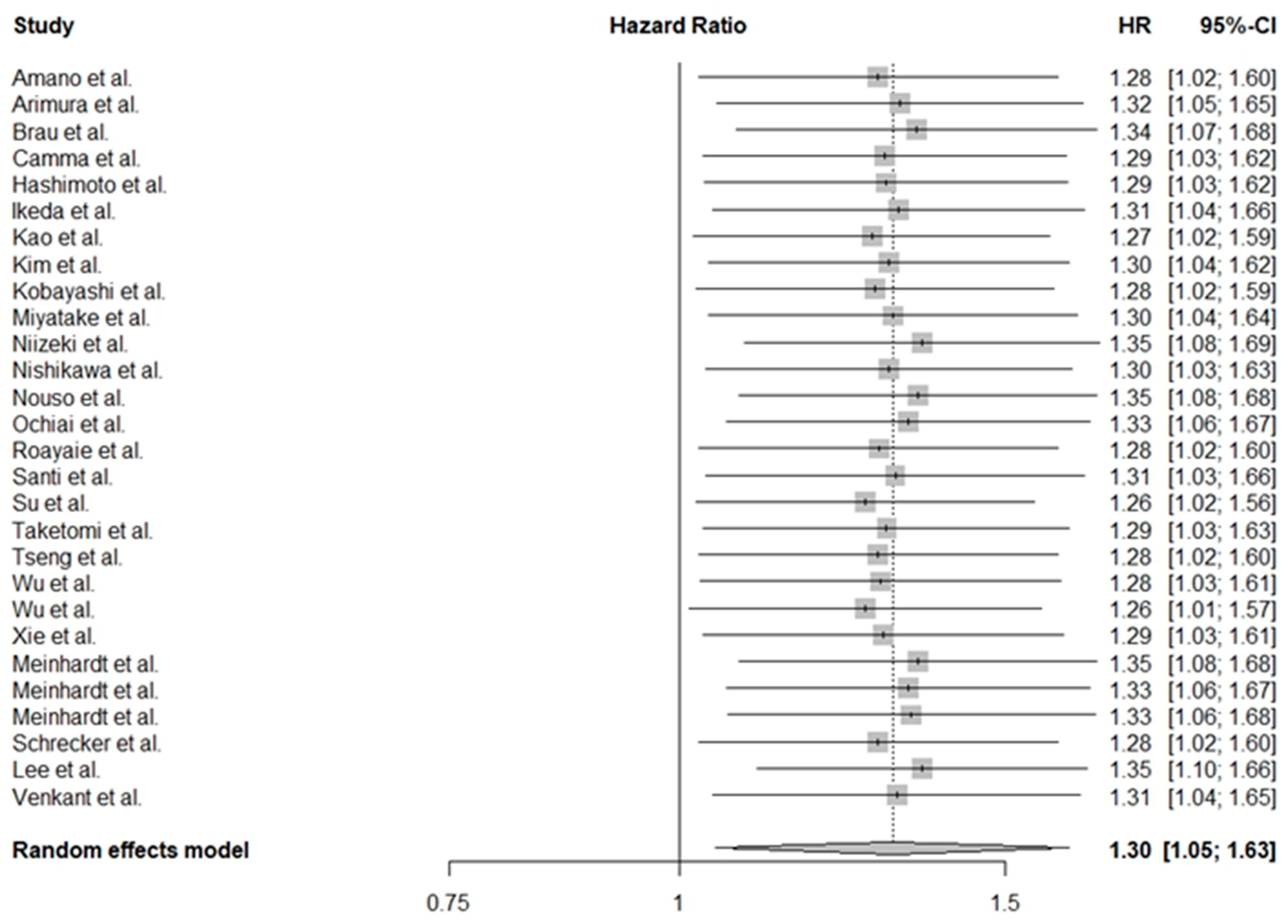
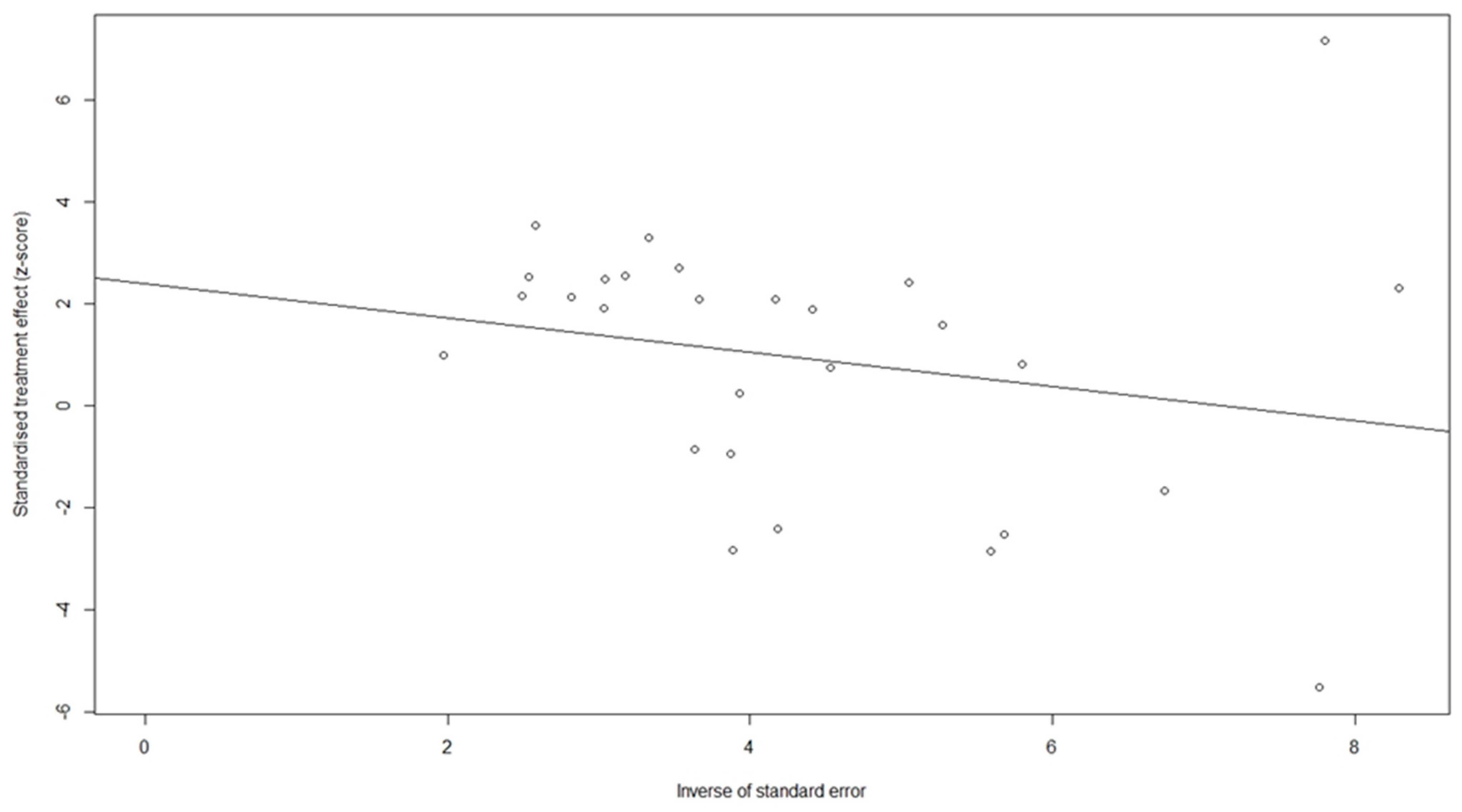
| Study | Reference | Country | Number of Patients (M/F) | Treatment | Child–Pugh (A/B/C) | PLT Cut-Off Value (109/L) | Follow-Up (Years) | NOS |
|---|---|---|---|---|---|---|---|---|
| Amano et al. | [30] | Japan | 127/24 | Surgery | 129/22 | 100 | 4.1 | 6 |
| Arimura et al. | [31] | Japan | 95/45 | PEIT/TACE | 77/48/15 | 80 | NA | 4 |
| Bräu et al. | [32] | America | 287/2 | Surgery /RFA | 113/134/42 | 100 | NA | 6 |
| Cammá et al. | [33] | Italy | 131/71 | RFA | 165/37 | 100 | 1.25 | 8 |
| Hashimoto et al. | [34] | Japan | 120/29 | Surgery | NA | 120 | 3.5 | 9 |
| Ikeda et al. | [35] | Japan | 122/46 | TACE | 86/82 | 75 | 2.8 | 5 |
| Kao et al. | [36] | Taiwan | 162/69 | RFA | 226/32 | 100 | 2.375 | 6 |
| Kim et al. | [37] | Korea | 39/13 | TACE | 16/23/13 | 150 | 0.4 | 6 |
| Kobayashi et al. | [38] | Japan | 146/53 | Surgery | 199 | 100 | 3.25 | 7 |
| Lee et al. | [39] | Taiwan | 1245/415 | Surgery /RFA/TACE | 830/349/149 | 118 | 5 | 7 |
| Miyatake et al. | [40] | Japan | 260/135 | Surgery | 317/74/4 | 100 | 3.5 | 7 |
| Meinhardt et al. | [28] | America | 221 | Systemic treatment | A major | 150 | NA | 4 |
| 95 | ||||||||
| 318 | ||||||||
| Niizeki et al. | [41] | Japan | 56/15 | TACE | 43/28 | 120 | NA | 5 |
| Nishikawa et al. | [42] | Japan | 217/151 | RFA | 70/162/100 | 100 | 3 | 8 |
| Nouso et al. | [43] | Japan | 116/41 | RFA/TACE | 157(C) | 80 | NA | 7 |
| Ochiai et al. | [44] | Japan | 208/76 | Surgery | 273/11 | 110 | 3 | 6 |
| Roayaie et al. | [45] | America | 95/37 | Surgery | 132 | 150 | 3.2 | 7 |
| Santi et al. | [46] | Italy | 457/192 | Surgery/RFA/TACE | 477/172 | 100 | 3.3 | 9 |
| Schrecker et al. | [47] | Germany | 96/32 | Surgery | 126/2 | 100 | 4.6 | 8 |
| Su et al. | [48] | Taiwan | 152/36 | Surgery | A major | 100 | 5.8 | 8 |
| Taketomi et al. | [49] | Japan | 167/43 | Surgery | 158/52 | 150 | 2.4 | 7 |
| Tseng et al. | [50] | Taiwan | 48/34 | Surgery /RFA/TACE | NA | 100 | NA | 6 |
| Wu et al. | [51] | Taiwan | 104/57 | RFA | A major | 100 | 3.2 | 4 |
| Wu et al. | [52] | China | 79/7 | Surgery | A major | 100 | NA | 7 |
| Venkant et al. | [53] | America | 1411/686 | Surgery | NA | 150 | NA | 7 |
| Xie et al. | [54] | China | 408/79 | RFA/TACE | NA | 97 | NA | 6 |
| Study | Age (Years) | Race | Follow-Up | Disease Stage (BCLC) | Viral Infection | Alcohol Intake |
|---|---|---|---|---|---|---|
| Meinhardt et al. [28] | NA | NA | NA | NA | NA | NA |
| Amano et al. [30] | >18 | NA | 4.1 ± 3.1 years | NA | HCV | NA |
| Arimura et al. [31] | 63.3 ± 8.54 | NA | NA | NA | NA | NA |
| Bräu et al. [32] | 52.2 (±8.0)–63.9 (±11.0) | White, Latino, Black, Asian | NA | A-D | HIV, HCV, HBV | + |
| Cammà et al. [33] | 66.8 (±8.2) 67.4 (±6.9) | NA | 15 months | A-B | HCV, HBV | + |
| Hashimoto et al. [34] | 61.7 | N/A | 42.1 months | N/A | HCV, HBV | N/A |
| Ikeda et al. [35] | 63 (45–80) | N/A | 2.8 years | N/A | HCV, HBV | + |
| Kao et al. [36] | >18 | N/A | 28.5 ± 18.7 months | N/A | HCV, HBV | N/A |
| Kim et al. [37] | 57 (35–80) | N/A | 5 months | N/A | HCV, HBV | + |
| Kobayashi et al. [38] | 62 (29–80)–67 (38–87) | N/A | 3.3 years | N/A | HCV, HBV | + |
| Lee et al. [39] | N/A | N/A | 5 years | A | HCV, HBV | N/A |
| Miyatake et al. [40] | 58 | N/A | 1.3 | N/A | HCV | N/A |
| Niizeki et al. [41] | 65 | N/A | N/A | N/A | HCV, HBV | N/A |
| Nishikawa et al. [42] | 69.9 ± 9.0 | N/A | N/A | N/A | HCV, HBV | N/A |
| Nouso et al. [43] | 63 | N/A | N/A | N/A | HCV, HBV | N/A |
| Ochiai et al. [44] | 63.9 | N/A | 36 months | N/A | HCV, HBV | + |
| Roayaie et al. [45] | 63.1 ± 10.5 | N/A | 37.5 months | 0 | HCV, HBV | + |
| Santi et al. [46] | 67 | N/A | 38.6 ± 32.8 months | N/A | HCV, HBV | + |
| Schrecker et al. [47] | 65 (34–81) | N/A | 55.1 months | 0-B | N/A | N/A |
| Su et al. [48] | 61.5 (52.0–70.75) | N/A | 69.8 months | N/A | HCV, HBV | N/A |
| Taketomi et al. [49] | 60.7 ± 7.9 | N/A | 26.6 ± 22.0 months | N/A | N/A | N/A |
| Tseng et al. [50] | 65.8 ± 9.6 | N/A | 4 years | 0-B | HCV, HBV | N/A |
| Wu et al. [51] | 67.5 ± 11.4 | N/A | 38.1 ± 20.8 months | N/A | HCV, HBV | N/A |
| Wu et al. [52] | >18 | N/A | 7 years | I–IV * | HBV | N/A |
| Venkat et al. [53] | 64 | Caucasian, African–American | N/A | N/A | N/A | N/A |
| Xie et al. [54] | 52 ± 7.3–69 ± 3.7 | N/A | N/A | N/A | HCV, HBV | N/A |
| Covariates | Subgroup | No | HR | Heterogeneity | ||
|---|---|---|---|---|---|---|
| Ps | I2 | Pa | ||||
| Curative | No | 9 | 0.7754 [0.6760; 0.8894] | 0.115 | 70.5% | <0.001 |
| Yes | 19 | 1.4006 [1.2717; 1.5426] | <0.001 | 84.7% | ||
| Child–Pugh | ≤50% | 4 | 0.9099 [0.5312; 1.5587] | 0.731 | 78.5% | 0.349 |
| >50% | 23 | 1.3921 [1.0847; 1.7865] | 0.009 | 87% | ||
| No Data | 1 | 1.1800 [0.7656; 1.8187] | 0.453 | 0% | ||
| HCV | ≤50% | 10 | 1.2986 [0.8032; 2.0995] | 0.286 | 92.4% | 0.883 |
| >50% | 14 | 1.2797 [0.9778; 1.6748] | 0.019 | 79.7% | ||
| No Data | 4 | 1.4110 [1.0576; 1.8825] | 0.072 | 32% | ||
| PLT | ≤100 | 17 | 1.6004 [1.2515; 2.0465] | <0.001 | 80.1% | 0.028 |
| 101–149 | 5 | 0.8207 [0.4778; 1.4099] | 0.474 | 84.0% | ||
| 150 | 6 | 1.0251 [0.7137; 1.4724] | 0.893 | 77.4% | ||
| Number of patients | ≤200 | 15 | 1.5324 [1.1127; 2.1105] | 0.009 | 82.2% | 0.127 |
| >200 | 13 | 1.1016 [0.8341; 1.4548] | 0.495 | 85.8% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraj, L.; Chmiel, P.; Gryziak, M.; Grabowska-Derlatka, L.; Szymański, Ł.; Wysokińska, E. Impact of Thrombocytopenia on Survival in Patients with Hepatocellular Carcinoma: Updated Meta-Analysis and Systematic Review. Cancers 2024, 16, 1293. https://doi.org/10.3390/cancers16071293
Kraj L, Chmiel P, Gryziak M, Grabowska-Derlatka L, Szymański Ł, Wysokińska E. Impact of Thrombocytopenia on Survival in Patients with Hepatocellular Carcinoma: Updated Meta-Analysis and Systematic Review. Cancers. 2024; 16(7):1293. https://doi.org/10.3390/cancers16071293
Chicago/Turabian StyleKraj, Leszek, Paulina Chmiel, Maciej Gryziak, Laretta Grabowska-Derlatka, Łukasz Szymański, and Ewa Wysokińska. 2024. "Impact of Thrombocytopenia on Survival in Patients with Hepatocellular Carcinoma: Updated Meta-Analysis and Systematic Review" Cancers 16, no. 7: 1293. https://doi.org/10.3390/cancers16071293
APA StyleKraj, L., Chmiel, P., Gryziak, M., Grabowska-Derlatka, L., Szymański, Ł., & Wysokińska, E. (2024). Impact of Thrombocytopenia on Survival in Patients with Hepatocellular Carcinoma: Updated Meta-Analysis and Systematic Review. Cancers, 16(7), 1293. https://doi.org/10.3390/cancers16071293