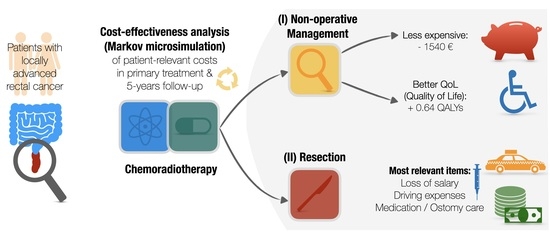
Patient-Relevant Costs for Organ Preservation versus Radical Resection in Locally Advanced Rectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
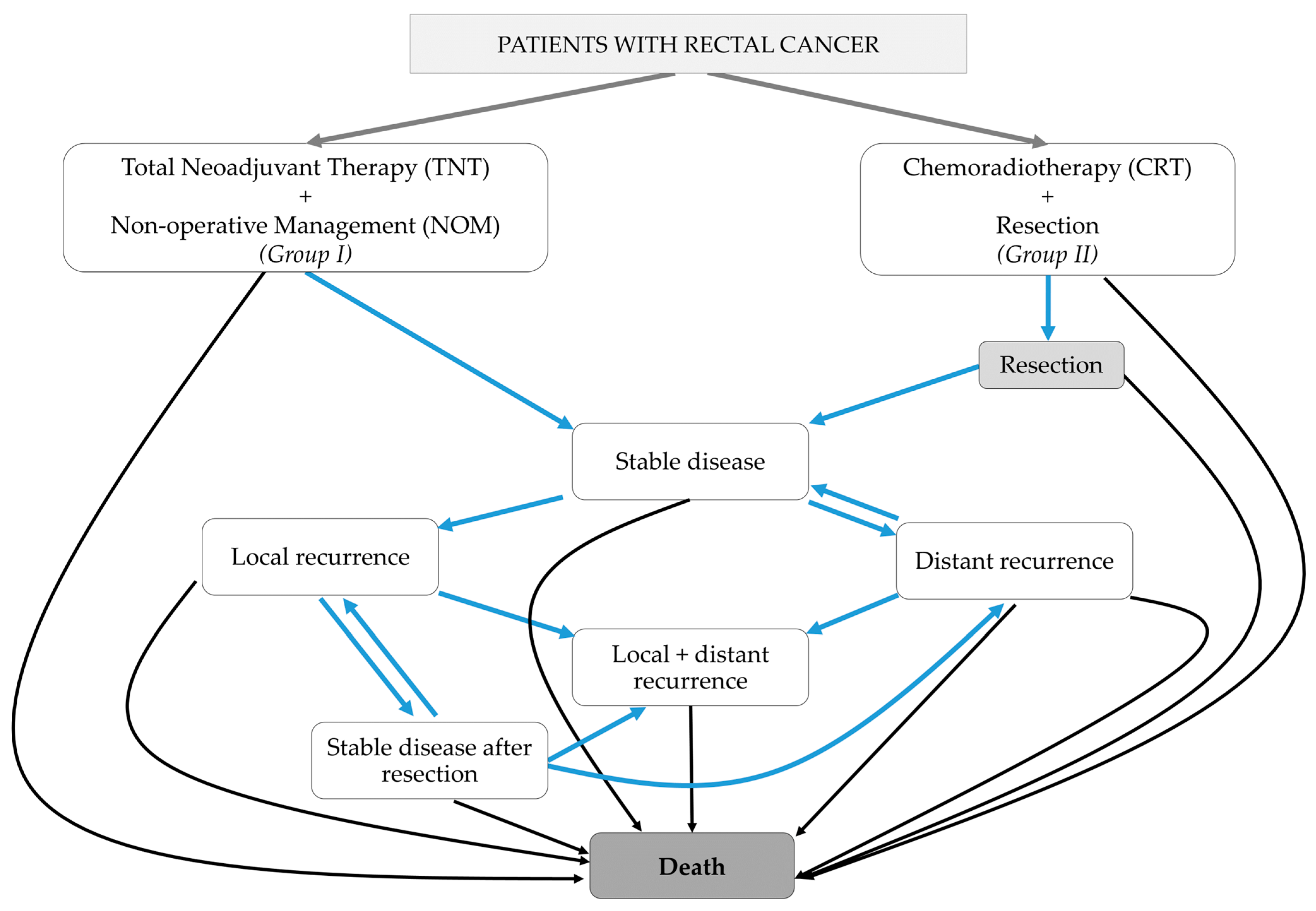
2.1. Base Case Description
2.2. Treatment Costs in the German Health Care System
2.3. Model Parameters
- A.
- Stable disease,
- B.
- Stable disease after successful salvage for local recurrence,
- C.
- Local recurrence,
- D.
- Distant recurrence,
- E.
- Local and distant recurrence, and
- F.
- Death.
3. Results
3.1. Epidemiology of Rectal Cancer and Income Statistics
3.2. Analysis of Patients with Rectal Cancer from the “FinTox” Trial
3.3. Cost-Effectiveness Analysis
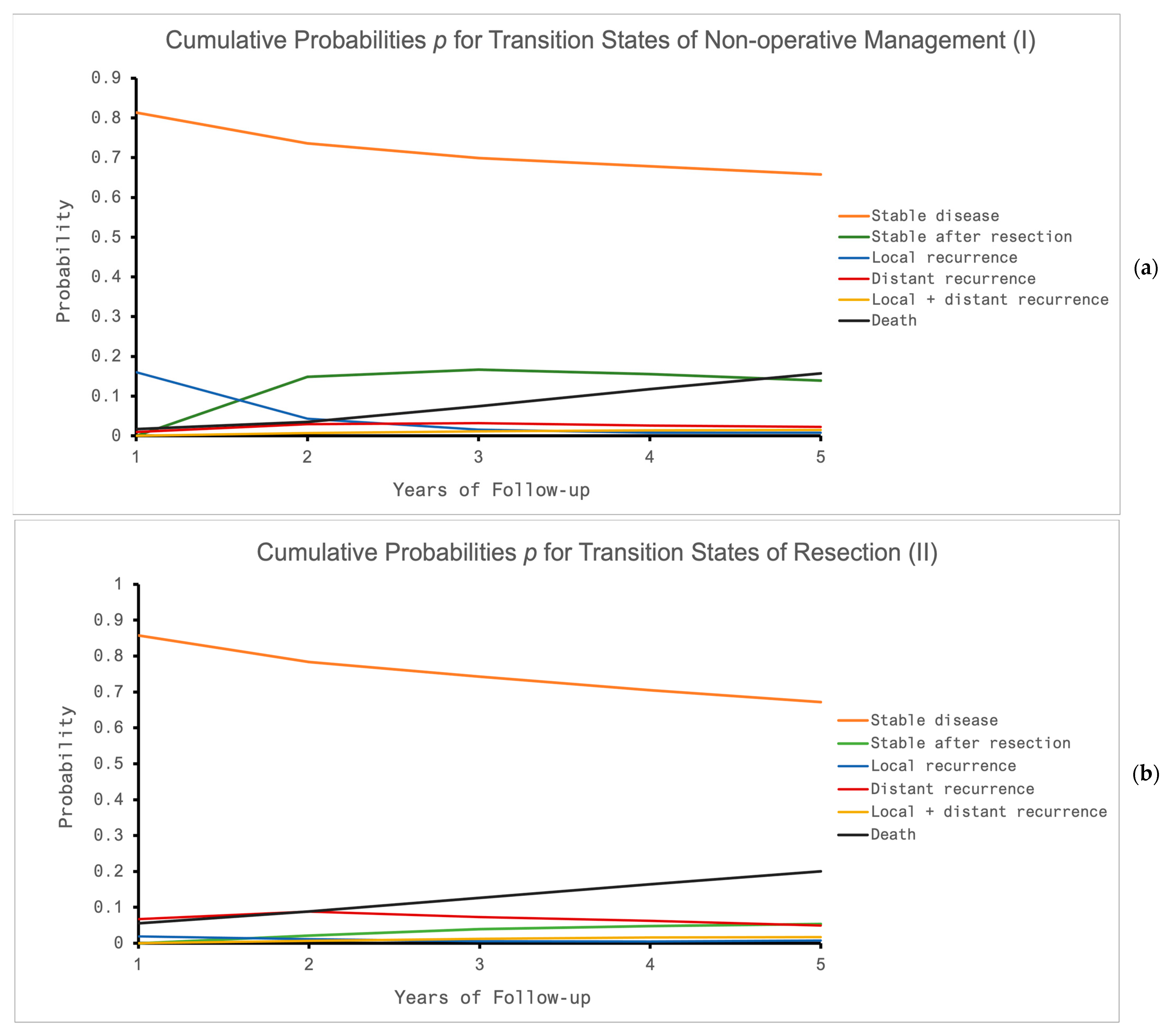
3.4. The Sensitivity of the Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APR | Abdominoperineal Resection |
| CR | Complete Response |
| CRM+ | Positive Circumferential Resection Margin |
| CRT | Chemoradiotherapy |
| DEGRO | German Society of Radiation Oncology |
| CTx | Chemotherapy |
| G-DRG | German Diagnosis-Related Groups |
| ICER | Incremental Cost-Effectiveness Ratio |
| IQR | Interquartile Range |
| N+ | Positive Lymph Nodes |
| NMB | Net Monetary Benefit |
| NOM | Non-Operative Management |
| QALY | Quality-adjusted life years |
| QoL | Quality of Life |
| RT | Radiotherapy |
| SHI | Statutory Health Insurance (German: “Gesetzliche Krankenversicherung“) |
| TME | Total Mesorectal Excision |
| TNT | Total Neoadjuvant Therapy |
| WTP | Willingness to Pay |
References
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal Cancer: Esmo Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28 (Suppl. S4), iv22–iv40. [Google Scholar] [CrossRef] [PubMed]
- Temmink, S.J.D.; Peeters, K.C.M.J.; Bahadoer, R.R.; Kranenbarg, E.M.-K.; Roodvoets, A.G.H.; Melenhorst, J.; A Burger, J.W.; Wolthuis, A.; Renehan, A.G.; Figueiredo, N.L.; et al. Watch and Wait after Neoadjuvant Treatment in Rectal Cancer: Comparison of Outcomes in Patients with and without a Complete Response at First Reassessment in the International Watch & Wait Database (IWWD). Br. J. Surg. 2023, 110, 676–684. [Google Scholar] [PubMed]
- van der Valk, M.J.; Hilling, D.E.; Bastiaannet, E.; Kranenbarg, E.M.K.; Beets, G.L.; Figueiredo, N.L.; Habr-Gama, A.; Perez, R.Q.; Renehan, A.G.; van de Velde, C.G.H.; et al. Long-Term Outcomes of Clinical Complete Responders after Neoadjuvant Treatment for Rectal Cancer in the International Watch &Amp; Wait Database (IWWD): An International Multicentre Registry Study. Lancet 2018, 391, 2537–2545. [Google Scholar] [PubMed]
- Baird, P.; Steinke, J.D.; Minnaar, H.S.; Stewart, A.J. Assessment of Quality of Life in Rectal Cancer with Organ-Preservation Treatment: Are We There Yet? Clin. Oncol. 2023, 35, e110–e120. [Google Scholar] [CrossRef] [PubMed]
- Hupkens, B.J.P.; Martens, M.H.; Stoot, J.H.; Berbee, M.; Melenhorst, J.; Beets-Tan, R.G.; Beets, G.L.; Breukink, S.O. Quality of Life in Rectal Cancer Patients after Chemoradiation: Watch-and-Wait Policy Versus Standard Resection—A Matched-Controlled Study. Dis. Colon Rectum 2017, 60, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.J.; Al-Najami, I.; Cunningham, C. Quality of Life after Rectal-Preserving Treatment of Rectal Cancer. Eur. J. Surg. Oncol. 2020, 46, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Gani, C.; Gani, N.; Zschaeck, S.; Eberle, F.; Schaeffeler, N.; Hehr, T.; Berger, B.; Fischer, S.G.; Claßen, J.; Zipfel, S.; et al. Organ Preservation in Rectal Cancer: The Patients’ Perspective. Front. Oncol. 2019, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Ivatury, S.J.; Durand, M.-A.; Elwyn, G. Shared Decision-Making for Rectal Cancer Treatment: A Path Forward. Dis. Colon Rectum 2019, 62, 1412–1413. [Google Scholar] [CrossRef] [PubMed]
- Borstlap, W.A.A.; van Oostendorp, S.E.; Klaver, C.E.L.; Hahnloser, D.; Cunningham, C.; Rullier, E.; Bemelman, W.A.; Tuynman, J.B.; Tanis, P.J.; The Research Committee of the European Society of Coloproctology. Organ Preservation in Rectal Cancer: A Synopsis of Current Guidelines. Color. Dis. 2018, 20, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.L.; Luo, W.Y.; Cosman, B.C.; Eisenstein, S.; Simpson, D.; Ramamoorthy, S.; Murphy, J.; Lopez, N. Cost Effectiveness of Watch and Wait Versus Resection in Rectal Cancer Patients with Complete Clinical Response to Neoadjuvant Chemoradiation. Ann. Surg. Oncol. 2022, 29, 1894–1907. [Google Scholar] [CrossRef] [PubMed]
- Gani, C.; Grosse, U.; Clasen, S.; Kirschniak, A.; Goetz, M.; Rödel, C.; Zips, D. Cost Analysis of A wait-and-See Strategy after Radiochemotherapy in Distal Rectal Cancer. Strahlenther. Onkol. 2018, 194, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Wang, H.; Chang, D.T.; Pollom, E.L. Cost-Effectiveness and Quality-Adjusted Survival of Watch and Wait after Complete Response to Chemoradiotherapy for Rectal Cancer. JNCI J. Natl. Cancer Inst. 2020, 112, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.N.; Torgersen, Z.; Shashidharan, M.M.; Ternent, C.A. Cost-Effectiveness Analysis: Selective Use of Neoadjuvant Chemoradiation in Locally Advanced Rectal Cancer. Dis. Colon Rectum 2023, 66, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pascual, J.; Nuñez-Alfonsel, J.; Ielpo, B.; Lopez, M.; Quijano, Y.; de Vicente, E.; Cubillo, A.; Saborido, C.M. Watch-and-Wait Policy Versus Robotic Surgery for Locally Advanced Rectal Cancer: A Cost-Effectiveness Study (Reccoste). Surg. Oncol. 2022, 41, 101710. [Google Scholar] [CrossRef]
- Fabian, A.; Domschikowski, J.; Greiner, W.; Bockelmann, G.; Karsten, E.; Rühle, A.; Nicolay, N.H.; Grosu, A.L.; Dunst, J.; Krug, D. Financial Toxicity in Cancer Patients Treated with Radiotherapy in Germany-A Cross-Sectional Study. Strahlenther. Onkol. 2022, 198, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Fabian, A.; Rühle, A.; Domschikowski, J.; Trommer, M.; Wegen, S.; Becker, J.-N.; Wurschi, G.; Boeke, S.; Sonnhoff, M.; Fink, C.A.; et al. Financial Toxicity in Cancer Patients Undergoing Radiotherapy in a Universal Health Care System—A Prospective Multicenter Study of 1075 Patients. Radiother. Oncol. 2023, 183, 109604. [Google Scholar] [CrossRef] [PubMed]
- Statistisches Bundesamt (Destatis). Deutsches Statistisches Bundesamt (DESTATIS). Genesis-Online, Datenlizenz by 2-0. Available online: https://www-genesis.destatis.de/genesis/online (accessed on 8 January 2024).
- Fabian, A.; Rühle, A.; Domschikowski, J.; Trommer, M.; Wegen, S.; Becker, J.-N.; Wurschi, G.; Boeke, S.; Sonnhoff, M.; Fink, C.A.; et al. Psychosocial Distress in Cancer Patients Undergoing Radiotherapy: A Prospective National Cohort of 1042 Patients in Germany. J. Cancer Res. Clin. Oncol. 2023, 149, 9017–9024. [Google Scholar] [CrossRef] [PubMed]
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.H.; Mauskopf, J.; Loder, E. Consolidated Health Economic Evaluation Reporting Standards (Cheers)–Explanation and Elaboration: A Report of the Ispor Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013, 16, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Fabian, A.; Rühle, A.; Becker, J.-N.; Bockelmann, E.; Domschikowski, J.; Boeke, S.; Fink, C.A.; Käsmann, L.; Sonnhoff, M.; Schneider, M.; et al. Finanzielle Toxizität Bei Krebspatient*Innen Unter Strahlentherapie. Forum 2022, 37, 408–410. [Google Scholar] [CrossRef]
- Wurschi, G.W.; Knippen, S.; Ernst, T.; Schneider, C.; Helfritzsch, H.; Mothes, H.; Liebe, Y.; Huber, M.; Wittig, A. Long-Term Total Neoadjuvant Therapy Leads to Impressive Response Rates in Rectal Cancer: Results of a German Single-Center Cohort. Curr. Oncol. 2023, 30, 5366–5378. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aguilar, J.; Patil, S.; Gollub, M.J.; Kim, J.K.; Yuval, J.B.; Thompson, H.M.; Verheij, F.S.; Omer, D.M.; Lee, M.; Dunne, R.F.; et al. Organ Preservation in Patients with Rectal Adenocarcinoma Treated with Total Neoadjuvant Therapy. J. Clin. Oncol. 2022, 40, 2546–2556. [Google Scholar] [CrossRef] [PubMed]
- Rödel, C.; Liersch, T.; Becker, H.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Graeven, U.; Arnold, D.; Lang-Welzenbach, M.; Raab, H.-R.; et al. Preoperative Chemoradiotherapy and Postoperative Chemotherapy with Fluorouracil and Oxaliplatin Versus Fluorouracil Alone in Locally Advanced Rectal Cancer: Initial Results of the German Cao/Aro/Aio-04 Randomised Phase 3 Trial. Lancet Oncol. 2012, 13, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Institut für das Entgeltsystem im Krankenhaus. Fallpauschalen-Katalog Gemäß § 17b Absatz 1 Satz 4 Des Krankenhausfinanzierungsgesetzes, Katalog Ergänzender Zusatzentgelte Gemäß § 17b Absatz 1 Satz 7 Des Krankenhausfinanzierungsgesetzes, Pflegeerlöskatalog Gemäß § 17b Absatz 4 Satz 5 Des Krankenhausfinanzierungsgesetzes. In Krankenhausfinanzierungsgesetz (KHG). Available online: https://www.g-drg.de/ag-drg-system-2023/fallpauschalen-katalog/fallpauschalen-katalog-20232 (accessed on 8 January 2024).
- Anzahl Der Mitglieder Und Versicherten Der Gesetzlichen Und Privaten Krankenversicherung in Den Jahren 2017 Bis 2023 (in Millionen). Deutsches statistisches Bundesamt. Edited by Statista. 2023. Available online: https://de.statista.com/statistik/daten/studie/155823/umfrage/gkv-pkv-mitglieder-und-versichertenzahl-im-vergleich/ (accessed on 30 January 2024).
- § 3 Anspruch Auf Entgeltfortzahlung Im Krankheitsfall. In Gesetz über die Zahlung des Arbeitsentgelts an Feiertagen und im Krankheitsfall (Entgeltfortzahlungsgesetz). Edited by Bundesamt für Justiz. Available online: https://www.gesetze-im-internet.de/entgfg/__3.html (accessed on 8 January 2024).
- § 47 Höhe Und Berechnung Des Krankengeldes. In Sozialgesetzbuch (SGB) Fünftes Buch (V)—Gesetzliche Krankenversicherung, Edited by Bundesministerium für Justiz. 1988. Available online: https://www.gesetze-im-internet.de/sgb_5/__47.html (accessed on 8 January 2024).
- Zuzahlungsregelungen Der Gesetzlichen Krankenversicherung. In Informationsblatt Nr. 223-06, Edited by Bundesministerium für Gesundheit. 2018. Available online: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/A/Arzneimittelversorgung/Zuzahlungsregelungen_GKV.pdf (accessed on 8 January 2024).
- § 62 Belastungsgrenze. In Sozialgesetzbuch (SGB) Fünftes Buch (V)—Gesetzliche Krankenversicherung, Edited by Bundesamt für Justiz. 1988. Available online: https://www.gesetze-im-internet.de/sgb_5/__62.html (accessed on 8 January 2024).
- Fürstenberg, T.; Laschat, M.; Zich, K.; Klein, S.; Gierling, P.; Nolting, H.-D.; Schmidt, T. G-Drg-Begleitforschung Gemäß §17b Abs. 8 Khg. Endbericht Des Dritten Forschungszyklus (2008–2010). IGES Institut. Available online: https://www.gkv-spitzenverband.de/media/dokumente/krankenversicherung_1/krankenhaeuser/drg/drg_begleitforschung/DRG_Begleitforschung_Endbericht_3_Zyklus_2008_-_2010_2013_03.pdf (accessed on 30 January 2024).
- §5 Wegstreckenentschädigung. In Bundesreisekostengesetz (BRKG). Edited by Bundesamt für Justiz. 2005. Available online: https://www.gesetze-im-internet.de/brkg_2005/BJNR141810005.html (accessed on 8 January 2024).
- Guide to the Methods of Technology Appraisal 2013. National Institute for Health and Care Excellence (NICE). Available online: www.nice.org.uk/process/pmg9 (accessed on 8 January 2024).
- Marijnen, C.A.M.; Kapiteijn, E.; van de Velde, C.J.H.; Martijn, H.; Steup, W.H.; Wiggers, T.; Kranenbarg, E.K.; Leer, J.W.H. Acute Side Effects and Complications after Short-Term Preoperative Radiotherapy Combined with Total Mesorectal Excision in Primary Rectal Cancer: Report of a Multicenter Randomized Trial. J. Clin. Oncol. 2002, 20, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Rödel, C.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Arnold, D.; Hofheinz, R.-D.; Ghadimi, M.; Wolff, H.A.; Lang-Welzenbach, M.; et al. Oxaliplatin Added to Fluorouracil-Based Preoperative Chemoradiotherapy and Postoperative Chemotherapy of Locally Advanced Rectal Cancer (the German Cao/Aro/Aio-04 Study): Final Results of the Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2015, 16, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Diefenhardt, M.; Martin, D.; Fleischmann, M.; Hofheinz, R.-D.; Ghadimi, M.; Rödel, C.; Fokas, E. Overall Survival after Treatment Failure among Patients with Rectal Cancer. JAMA Netw. Open 2023, 6, e2340256. [Google Scholar] [CrossRef] [PubMed]
- Verheij, F.S.; Omer, D.M.; Williams, H.; Lin, S.T.; Qin, L.-X.; Buckley, J.T.; Thompson, H.M.; Yuval, J.B.; Kim, J.K.; Dunne, R.F.; et al. Long-Term Results of Organ Preservation in Patients with Rectal Adenocarcinoma Treated with Total Neoadjuvant Therapy: The Randomized Phase Ii Opra Trial. J. Clin. Oncol. 2024, 42, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, N.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Eng, C.; Das, P.; Kopetz, S.; Messick, C.; Skibber, J.M.; Chang, G.J. Impact of Recurrence and Salvage Surgery on Survival after Multidisciplinary Treatment of Rectal Cancer. J. Clin. Oncol. 2017, 35, 2631–2638. [Google Scholar] [CrossRef] [PubMed]
- Dossa, F.; Chesney, T.R.; A Acuna, S.; Baxter, N.N. A Watch-and-Wait Approach for Locally Advanced Rectal Cancer after a Clinical Complete Response Following Neoadjuvant Chemoradiation: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.; Myint, A.S.; Athanasiou, T.; Faiz, O.; Martin, A.P.; Collins, B.; Smith, F.M. Avoiding Radical Surgery in Elderly Patients with Rectal Cancer Is Cost-Effective. Dis. Colon Rectum 2017, 60, 30–42. [Google Scholar] [CrossRef]
- Bevölkerung—Zahl Der Einwohner in Deutschland Nach Relevanten Altersgruppen Am 31. Dezember 2022 (in Millionen). Statistisches Bundesamt, Edited by Statista. 2022. Available online: https://de.statista.com/statistik/daten/studie/1365/umfrage/bevoelkerung-deutschlands-nach-altersgruppen/ (accessed on 9 February 2024).
- Couwenberg, A.M.; Intven, M.P.M.; Burbach, J.P.M.; Emaus, M.J.M.; van Grevenstein, W.M.; Verkooijen, H.M.M. Utility Scores and Preferences for Surgical and Organ-Sparing Approaches for Treatment of Intermediate and High-Risk Rectal Cancer. Dis. Colon Rectum 2018, 61, 911–919. [Google Scholar] [CrossRef]
- Kosmala, R.; Fokas, E.; Flentje, M.; Sauer, R.; Liersch, T.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Arnold, D.; Hofheinz, R.-D.; et al. Quality of Life in Rectal Cancer Patients with or without Oxaliplatin in the Randomised Cao/Aro/Aio-04 Phase 3 Trial. Eur. J. Cancer 2021, 144, 281–290. [Google Scholar] [CrossRef]
- Brink, M.v.D.; Hout, W.B.v.D.; Stiggelbout, A.M.; Kranenbarg, E.K.; Marijnen, C.A.; van de Velde, C.J.; Kievit, J. Cost-Utility Analysis of Preoperative Radiotherapy in Patients with Rectal Cancer Undergoing Total Mesorectal Excision: A Study of the Dutch Colorectal Cancer Group. J. Clin. Oncol. 2004, 22, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Paulden, M. Calculating and Interpreting Icers and Net Benefit. Pharmacoeconomics 2020, 38, 785–807. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Blome, C.; Forschner, A.; Gutzmer, R.; Hauschild, A.; Heinzerling, L.; Livingstone, E.; Loquai, C.; Schadendorf, D.; Utikal, J.; et al. Willingness to Pay for a Cure of Low-Risk Melanoma Patients in Germany. PLoS ONE 2018, 13, e0197780. [Google Scholar] [CrossRef]
- Bock, J.-O.; Heider, D.; Matschinger, H.; Brenner, H.; Saum, K.-U.; Haefeli, W.E.; König, H.-H. Willingness to Pay for Health Insurance among the Elderly Population in Germany. Eur. J. Health Econ. 2016, 17, 149–158. [Google Scholar] [CrossRef]
- Datenbankabfrage Mit Schätzung Der Inzidenz, Prävalenz Und Des Überlebens Von Krebs in Deutschland Auf Basis Der Epidemiologischen Landeskrebsregisterdaten. Zentrum für Krebsregisterdaten im Robert Koch-Institut. 2022. Available online: https://www.krebsdaten.de/Krebs/SiteGlobals/Forms/ErgebnisAnsicht/ErgebnisAnsicht_form.html (accessed on 11 February 2024).
- Bericht ÜBer Die HöHe Des Steuerfrei Zu Stellenden Existenzminimums Von Erwachsenen Und Kindern FüR Das Jahr 2022 (13. Existenzminimumbericht). Deutscher Bundestag. Available online: https://www.bundesfinanzministerium.de/Content/DE/Downloads/Steuern/14-existenzminimumbericht.pdf?__blob=publicationFile&v=7 (accessed on 11 February 2024).
- Bibi, S.; Edilbe, M.; Rao, C. The Cost-Effectiveness of Watch and Wait for Rectal Cancer. Clin. Oncol. 2023, 35, 132–137. [Google Scholar] [CrossRef]
- Ferri, V.; Vicente, E.; Quijano, Y.; Duran, H.; Diaz, E.; Fabra, I.; Malave, L.; Ruiz, P.; Costantini, G.; Pizzuti, G.; et al. Light and Shadow of Watch-and-Wait Strategy in Rectal Cancer: Oncological Result, Clinical Outcomes, and Cost-Effectiveness Analysis. Int. J. Color. Dis. 2023, 38, 277. [Google Scholar] [CrossRef] [PubMed]
- Fabian, A.; Rühle, A.; Domschikowski, J.; Trommer, M.; Wegen, S.; Becker, J.-N.; Wurschi, G.; Boeke, S.; Sonnhoff, M.; Fink, C.A.; et al. Satisfaction with Radiotherapy Care among Cancer Patients Treated in Germany—Secondary Analysis of A large Multicenter Study. Strahlenther. Onkol. 2023. [Google Scholar] [CrossRef]
- Carlsson, E.; Forsmark, A.; Sternhufvud, C.; Scheffel, G.; Andersen, F.B.; I Persson, E. Short- and Long-Term Direct and Indirect Costs of Illness after Ostomy Creation—A Swedish Nationwide Registry Study. BMC Health Serv. Res. 2023, 23, 837. [Google Scholar] [CrossRef] [PubMed]
- Meisner, S.; Lehur, P.-A.; Moran, B.; Martins, L.; Jemec, G.B.E. Peristomal Skin Complications Are Common, Expensive, and Difficult to Manage: A Population Based Cost Modeling Study. PLoS ONE 2012, 7, e37813. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.C.; Dixon, S.W.; White, P.; Williams, A.C.; Thomas, M.G.; Messenger, D.E. Demographic Trends in the Incidence of Young-Onset Colorectal Cancer: A Population-Based Study. Br. J. Surg. 2020, 107, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.; Nassar, N.; Lee, C.Y.Y.; Suen, M.K.; Al Zahrani, S.; Gladman, M.A. Young-Onset Colorectal Cancer in New South Wales: A Population-Based Study. Med. J. Aust. 2016, 205, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.R.; Ruan, Y.; Shaw, E.; De, P.; Heitman, S.J.; Hilsden, R.J. Increasing Colorectal Cancer Incidence Trends among Younger Adults in Canada. Prev. Med. 2017, 105, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, J.; Davidson, C.; Hall, C.; Pearson, J.; Eglinton, T.; Wakeman, C.; Frizelle, F. Population-Based Study Demonstrating an Increase in Colorectal Cancer in Young Patients. J. Br. Surg. 2017, 104, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; De, P. Trends in Colorectal Cancer Incidence and Related Lifestyle Risk Factors in 15–49-Year-Olds in Canada, 1969–2010. Cancer Epidemiol. 2016, 42, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. JNCI J. Natl. Cancer Inst. 2017, 109, djw322. [Google Scholar] [CrossRef]
- Vuik, F.E.; Nieuwenburg, S.A.; Bardou, M.; Lansdorp-Vogelaar, I.; Dinis-Ribeiro, M.; Bento, M.J.; Zadnik, V.; Pellisé, M.; Esteban, L.; Kaminski, M.F.; et al. Increasing Incidence of Colorectal Cancer in Young Adults in Europe over the Last 25 Years. Gut 2019, 68, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
| Parameters: (Time Frame) | NOM after TNT (I) | Resection after CRT (II) | ||
|---|---|---|---|---|
| Value | Citation | Value | Citation | |
| Overall grade 3 or 4 toxicity | 0.32 | Garcia-Aguilar et al. 2022 [22] | 0.23 | Rödel et al. 2012 [23] |
| Perioperative death | 0.04 * | Marijnen et al. 2002 [33] | 0.04 | Marijnen et al. 2002 [33] |
| Local recurrence | ||||
| (2 years) | 0.21 | Van der Valk et al. 2018 [3] | - | |
| (3 years) | - | 0.03 | Rödel et al. 2015 [34] | |
| (5 years) | 0.24 | Van der Valk et al. 2018 [3] | 0.05 | Diefenhardt et al. 2023 [35] |
| Distant recurrence | Van der Valk et al. 2018 [3] | |||
| (3 years) | 0.04 | Van der Valk et al. 2018 [3], | 0.19 | Rödel et al. 2015 [34] |
| (5 years) | 0.08 | Verheij et al. 2023 [36] | 0.21 | Rödel et al. 2015 [34] |
| Overall Survival | ||||
| (3 years) | 0.95 | Van der Valk et al. 2018 [3] | 0.89 | Rödel et al. 2015 [34] |
| (5 years) | 0.85 | Van der Valk et al. 2018 [3] | 0.79 | Rödel et al. 2015 [34] |
| Distant recurrence when local recurrence occurred | 0.18 | Van der Valk et al. 2018 [3] | 0.17 | Diefenhardt et al. 2023 [35] |
| Local recurrence when distant recurrence occurred | 0.54 | Van der Valk et al. 2018 [3] | 0.36 | Diefenhardt et al. 2023 [35] |
| Local recurrence after salvage resection | ||||
| (5 years) | 0.13 | Ikoma et al. 2017 [37] | 0.13 | Ikoma et al. 2017 [37] |
| Distant recurrence after local recurrence | ||||
| (3 years) | 0.11 | Van der Valk et al. 2018 [3] | 0.11 | Van der Valk et al. 2018 [3] |
| Salvage for local recurrence | 0.94 | Dossa et al. 2019 [38] | 0.59 | Ikoma et al. 2017 [37] |
| Salvage for distant recurrence | 0.57 | Ikoma et al. 2017 [37] | 0.57 | Ikoma et al. 2017 [37] |
| Mortality after local recurrence and salvage | ||||
| (5 years) | 0.5 | Rao et al. 2017 [39] | 0.5 | Rao et al. 2017 [39] |
| Mortality after local recurrence without salvage | ||||
| (5 years) | 0.7 | Rao et al. 2017 [39] | 0.7 | Rao et al. 2017 [39] |
| Mortality after distant recurrence | ||||
| (5 years) | 0.8 | Rao et al. 2017 [39] | 0.8 | Rao et al. 2017 [39] |
| Mortality after combined Local + distant recurrence | ||||
| (5 years) | 0.8 | Rao et al. 2017 [39] | 0.8 | Rao et al. 2017 [39] |
| Mortality of other causes | ||||
| (per year) | 0.02 | DESTATIS Zensus 2022 [17,40] | 0.02 | DESTATIS Zensus 2022 [17,40] |
| Utility Parameters: | NOM after TNT (I) | Resection after CRT (II) | ||
|---|---|---|---|---|
| Value | Citation | Value | Citation | |
| Initial state: NOM/surgery after CRT | 0.80 | Couwenberg et al. 2018 [41], Cui et al. 2022 [10] | 0.61 | Kosmala et al. 2021 [42,43] |
| Long term: NOM (no recurrence)/surgery after CRT (no recurrence) | 0.80 | Couwenberg et al. 2018 [41] | 0.70 | Couwenberg et al. 2018 [41], Kosmala et al. 2021 [42] |
| Salvage surgery (local recurrence) | 0.70 | Couwenberg et al. 2018 [41] | 0.70 | Couwenberg et al. 2018 [41] |
| Local recurrence | 0.67 | Van den Brink et al. 2004 [43] | 0.67 | Van den Brink et al. 2004 [43] |
| Distant recurrence | 0.70 | Van den Brink et al. 2004 [43] | 0.70 | Van den Brink et al. 2004 [43] |
| Local + distant recurrence | 0.48 | Van den Brink et al. 2004 [43] | 0.48 | Van den Brink et al. 2004 [43] |
| Death | 0 | - | 0 | - |
| Age Group | <25 | 25–<45 | 45–<65 | ≥65 | Total |
|---|---|---|---|---|---|
| Rectal cancer cases in 2019 [47] | 14 | 460 | 5807 | 11,614 | 17,895 |
| Percentage of incidence 2019 [%] | <0.5 | 2.6 | 32.5 | 64.9 | 100 |
| Employment rate 2022 [%] [17] | 38.2 | 78.0 | 73.7 | 3.4 | 28.2 |
| Gross income per month 2022 [mean, EUR] [17] | 2913 | 3881 | 4155 | 4557 | 4409 |
| Income and Co-Payment Values Derived from General Population Data | |
|---|---|
| Net household income per month 2021 [average, EUR] [17] | 3813 EUR |
| Gross pension payment per month 2022 [average, EUR] [17] | 1048 EUR |
| Reduced earning capacity pension 2022 [average, EUR] [17] | 925 EUR |
| Gross income (age-corrected) for employed patients in the rectal cancer cohort [47], 2022 [mean, EUR] | 4409 EUR |
| Net household income for rectal cancer cohort [47], 2022 [mean, EUR] | 2580 EUR |
| Co-payment exemption limit (mean household size: 2 persons [17]) | |
| 1% of gross income (for chronic diseases) | 411 EUR/month |
| 2% of gross income (general threshold) | 822 EUR/month |
| Patients with Rectal Cancer [N] | 44 | |
|---|---|---|
| Age [years] | ||
| Median | 67 | |
| Interquartile range (IQR), 25–75% | 58–75 | |
| Gender [N], (%) | ||
| Female | 19 (43%) | |
| Male | 25 (57%) | |
| Patients insured with a German statutory health insurance (SHI) [N], (%) | 38 (86%) | |
| Employment status of patients insured with SHI | Employed | 12 (32%) |
| Not employed | 4 (11%) | |
| Retired | 18 (47%) | |
| Not reported | 4 (11%) | |
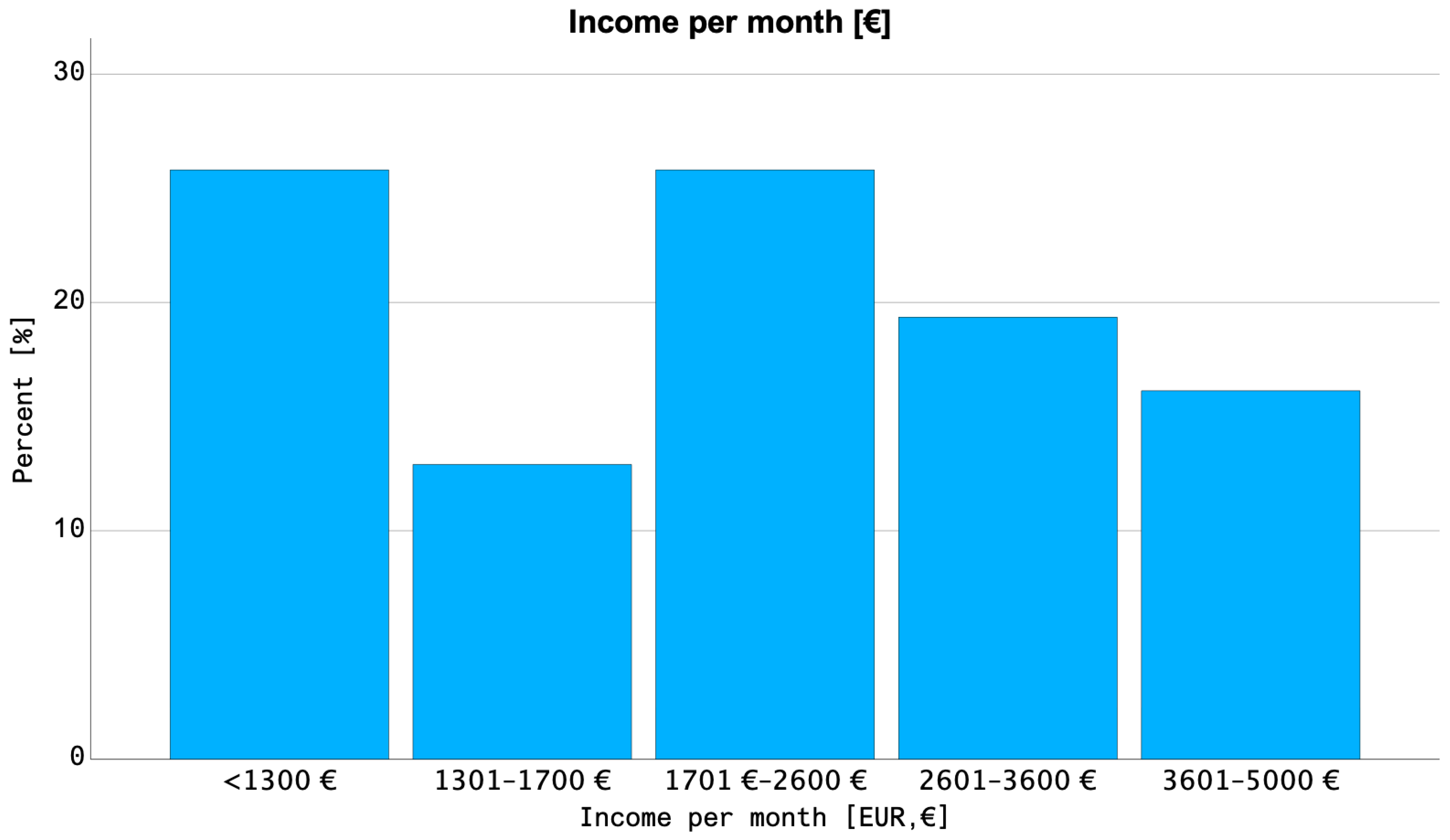
| Net household income of patients insured with SHI | <1300 EUR | 8 (21%) |
| 1300–1700 EUR | 4 (11%) | |
| 1701–2600 EUR | 8 (21%) | |
| 2601–3600 EUR | 6 (16%) | |
| 3601–5000 EUR | 5 (13%) | |
| >5000 EUR | 0 | |
| Not reported | 7 (18%) | |
| Loss of income related to cancer treatment of patients insured with SHI | None | 25 (66%) |
| <100 EUR/month | 2 (5%) | |
| 100–500 EUR/month | 4 (11%) | |
| 500–1500 EUR/month | 4 (11%) | |
| Not reported | 3 (8%) | |
| Additional expenses related to cancer treatment of patients insured with SHI | None | 16 (42%) |
| <100 EUR/month | 3 (8%) | |
| 100–500 EUR/month | 12 (32%) | |
| 500–1500 EUR/month | 1 (3%) | |
| Not reported | 6 (16%) | |
| Of those: Reasons for additional expenses (multiple mentioning was possible) | Expenses for co-payments | 17 |
| Driving expenses | 10 | |
| Expenses for medication or health care products | 10 | |
| Other (not specified) | 1 | |
| Financial difficulties due to treatment in patients insured with SHI | None | 24 (63%) |
| Little | 5 (13%) | |
| Moderate | 4 (11%) | |
| Great | 2 (5%) | |
| Not reported | 3 (8%) | |
| Treatment Option | QALY | Costs | [EUR] | Δ Costs [EUR] | ICER | NMB [EUR] | iNMB [EUR] |
|---|---|---|---|---|---|---|---|
| TNT + NOM (I) | 3.87 | All patients | 4711 | −1540 | −2407 | 18,509 | 5380 |
| Employed | 15,519 | −1985 | −1590 | 7701 | 5825 | ||
| Retired | 813 | −1017 | −3101 | 22,407 | 4857 | ||
| CRT + Resection (II) | 3.23 | All patients | 6252 | 13,128 | |||
| Employed | 17,504 | 1876 | |||||
| Retired | 1830 | 17,550 |
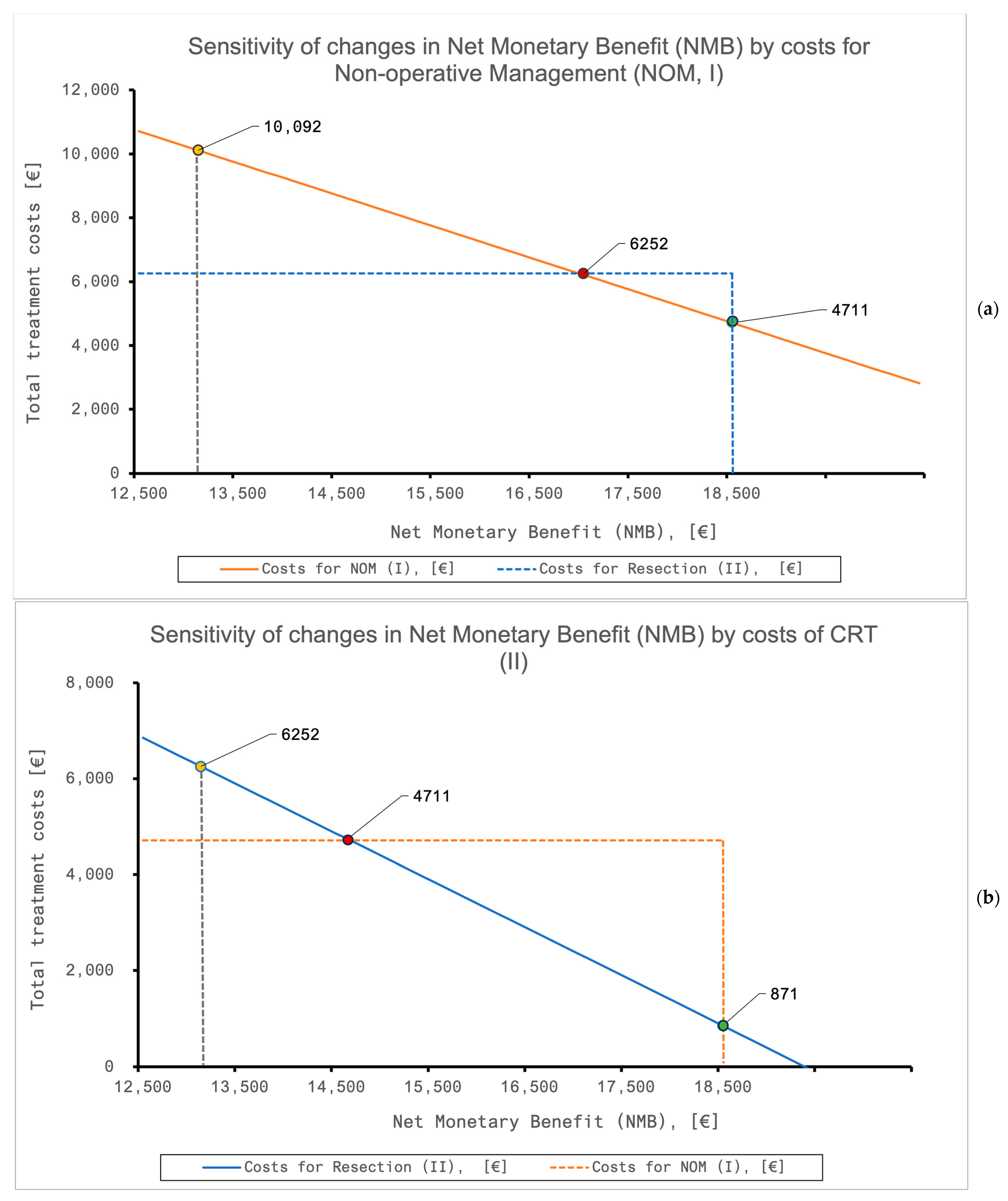
| QALY | QALY, Where NMB Becomes Equivalent | Costs | Costs, Where NMB Becomes Equivalent | ||
|---|---|---|---|---|---|
| NOM (I) | All patients | 3.87 | 2.97 | 4711 | 10,092 |
| Employed | 2.90 | 15,519 | 21,344 | ||
| Retired | 3.06 | 813 | 5670 | ||
| Resection (II) | All patients | 3.23 | 4.13 | 6252 | 871 |
| Employed | 4.20 | 17,504 | 11,679 | ||
| Retired | 4.04 | 1830 | <0 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wurschi, G.W.; Rühle, A.; Domschikowski, J.; Trommer, M.; Ferdinandus, S.; Becker, J.-N.; Boeke, S.; Sonnhoff, M.; Fink, C.A.; Käsmann, L.; et al. Patient-Relevant Costs for Organ Preservation versus Radical Resection in Locally Advanced Rectal Cancer. Cancers 2024, 16, 1281. https://doi.org/10.3390/cancers16071281
Wurschi GW, Rühle A, Domschikowski J, Trommer M, Ferdinandus S, Becker J-N, Boeke S, Sonnhoff M, Fink CA, Käsmann L, et al. Patient-Relevant Costs for Organ Preservation versus Radical Resection in Locally Advanced Rectal Cancer. Cancers. 2024; 16(7):1281. https://doi.org/10.3390/cancers16071281
Chicago/Turabian StyleWurschi, Georg W., Alexander Rühle, Justus Domschikowski, Maike Trommer, Simone Ferdinandus, Jan-Niklas Becker, Simon Boeke, Mathias Sonnhoff, Christoph A. Fink, Lukas Käsmann, and et al. 2024. "Patient-Relevant Costs for Organ Preservation versus Radical Resection in Locally Advanced Rectal Cancer" Cancers 16, no. 7: 1281. https://doi.org/10.3390/cancers16071281
APA StyleWurschi, G. W., Rühle, A., Domschikowski, J., Trommer, M., Ferdinandus, S., Becker, J.-N., Boeke, S., Sonnhoff, M., Fink, C. A., Käsmann, L., Schneider, M., Bockelmann, E., Krug, D., Nicolay, N. H., Fabian, A., & Pietschmann, K. (2024). Patient-Relevant Costs for Organ Preservation versus Radical Resection in Locally Advanced Rectal Cancer. Cancers, 16(7), 1281. https://doi.org/10.3390/cancers16071281