Donor Lymphocyte Infusion in the Treatment of Post-Transplant Relapse of Acute Myeloid Leukemias and Myelodysplastic Syndromes Significantly Improves Overall Survival: A French–Italian Experience of 134 Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Definitions
2.3. Statistical Analysis
3. Results
3.1. Patient and Transplant Characteristics
3.2. Post-Relapse Therapy and Response
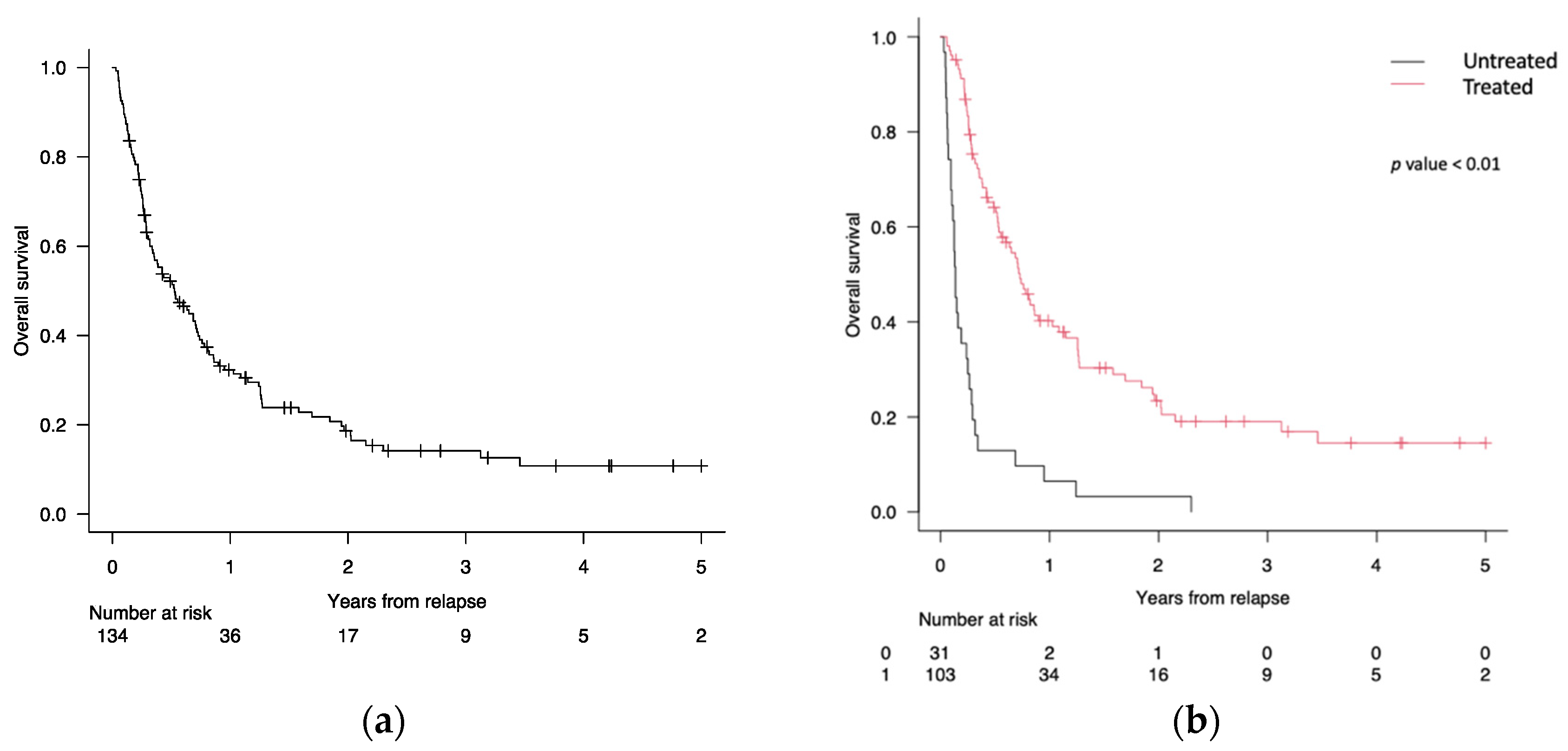
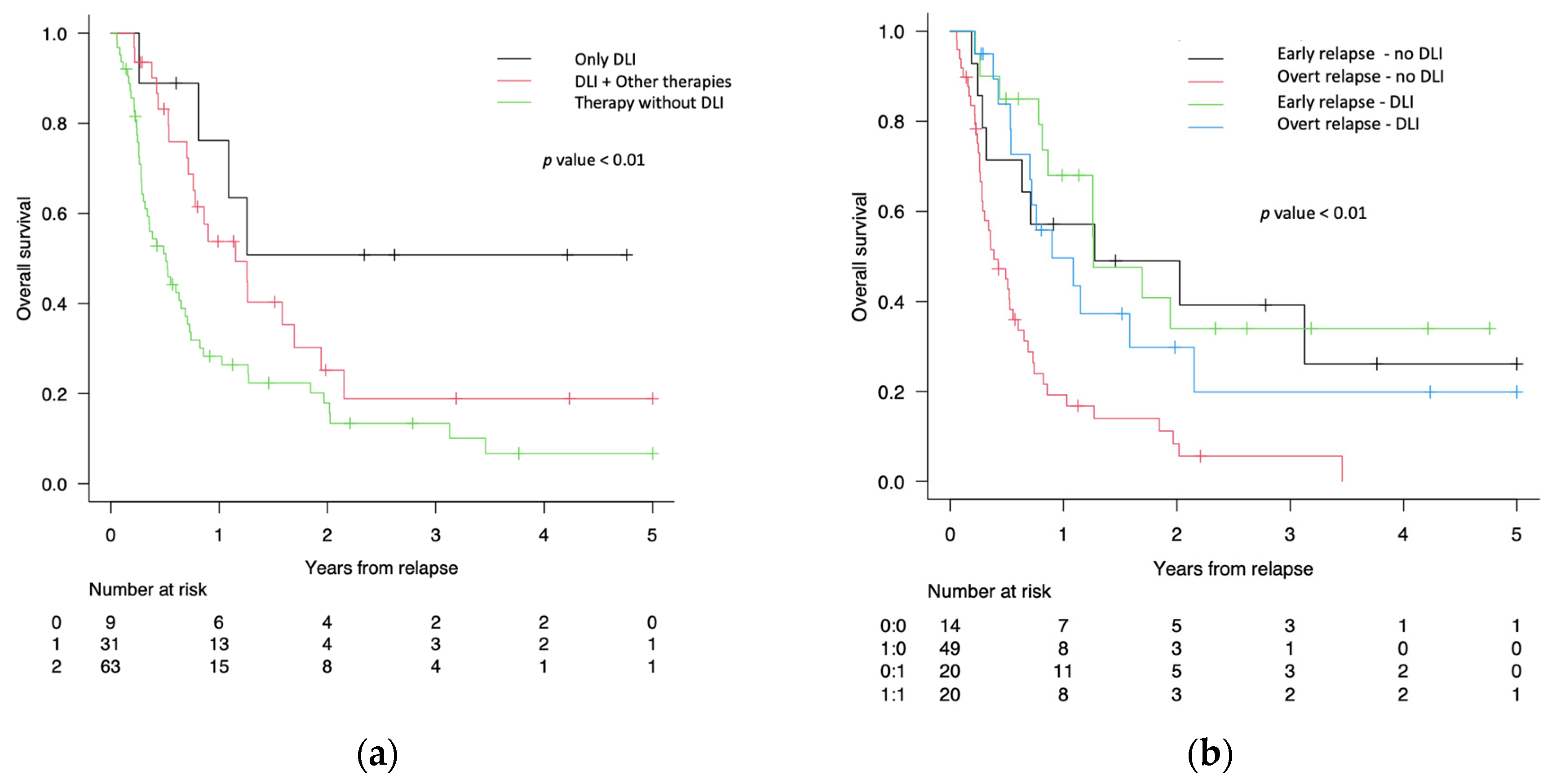
3.3. Survival Analysis after Post-Transplant Relapse
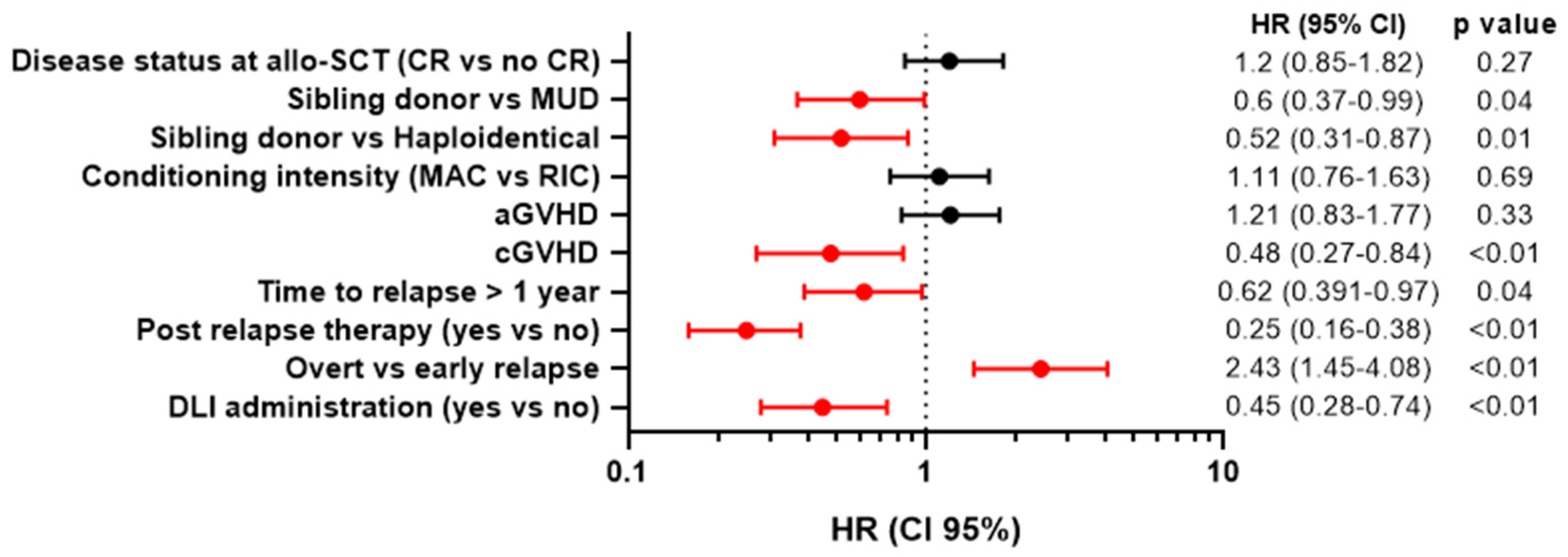
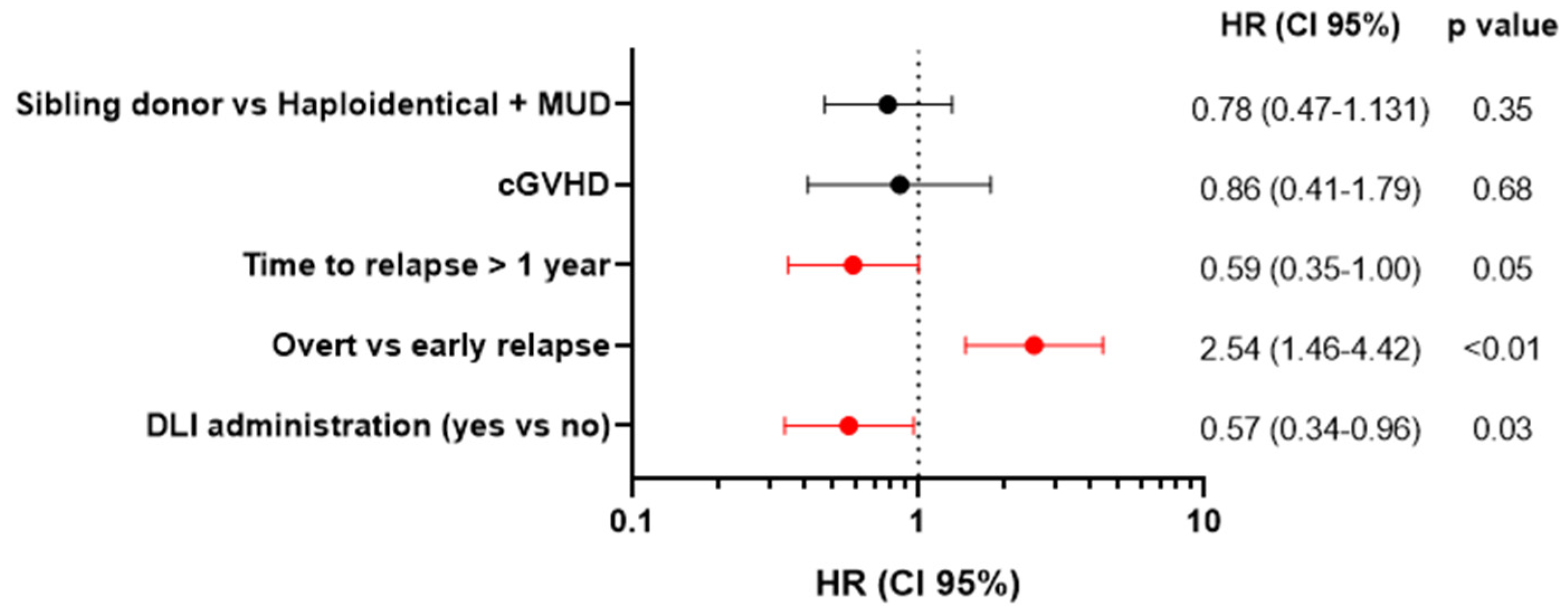
3.4. Univariate and Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and Management of AML in Adults: 2022 Recommendations from an International Expert Panel on Behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Tallman, M.S.; Weisdorf, D.J. Allogeneic Hematopoietic Cell Transplantation for Adults with Acute Myeloid Leukemia: Myths, Controversies, and Unknowns. Blood 2011, 117, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Loke, J.; Malladi, R.; Moss, P.; Craddock, C. The Role of Allogeneic Stem Cell Transplantation in the Management of Acute Myeloid Leukaemia: A Triumph of Hope and Experience. Br. J. Haematol. 2020, 188, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Morello, E.; Malagola, M.; Bernardi, S.; Pristipino, C.; Russo, D. The Role of Allogeneic Hematopoietic Stem Cell Transplantation in the Four P Medicine Era. Blood Res. 2018, 53, 3–6. [Google Scholar] [CrossRef]
- Malagola, M.; Polverelli, N.; Rubini, V.; Martino, M.; Patriarca, F.; Bruno, B.; Giaccone, L.; Grillo, G.; Bramanti, S.; Bernasconi, P.; et al. GITMO Registry Study on Allogeneic Transplantation in Patients Aged ≥60 Years from 2000 to 2017: Improvements and Criticisms. Transplant. Cell. Ther. 2022, 28, 96.e1–96.e11. [Google Scholar] [CrossRef] [PubMed]
- Zuanelli Brambilla, C.; Lobaugh, S.M.; Ruiz, J.D.; Dahi, P.B.; Goldberg, A.D.; Young, J.W.; Gyurkocza, B.; Shaffer, B.C.; Ponce, D.M.; Tamari, R. Relapse after Allogeneic Stem Cell Transplantation of Acute Myelogenous Leukemia and Myelodysplastic Syndrome and the Importance of Second Cellular Therapy. Transplant. Cell. Ther. 2021, 27, 771.e1–771.e10. [Google Scholar] [CrossRef]
- Sauerer, T.; Velázquez, G.F.; Schmid, C. Relapse of Acute Myeloid Leukemia after Allogeneic Stem Cell Transplantation: Immune Escape Mechanisms and Current Implications for Therapy. Mol. Cancer 2023, 22, 180. [Google Scholar] [CrossRef] [PubMed]
- Rautenberg, C.; Germing, U.; Haas, R.; Kobbe, G.; Schroeder, T. Relapse of Acute Myeloid Leukemia after Allogeneic Stem Cell Transplantation: Prevention, Detection, and Treatment. Int. J. Mol. Sci. 2019, 20, 228. [Google Scholar] [CrossRef]
- Bazarbachi, A.; Schmid, C.; Labopin, M.; Beelen, D.; Blau, I.W.; Potter, V.; Niittyvuopio, R.; Socie, G.; Blaise, D.; Sanz, J.; et al. Evaluation of Trends and Prognosis over Time in Patients with AML Relapsing after Allogeneic Hematopoietic Cell Transplant Reveals Improved Survival for Young Patients in Recent Years. Clin. Cancer Res. 2020, 26, 6475–6482. [Google Scholar] [CrossRef]
- Yanada, M.; Konuma, T.; Yamasaki, S.; Kondo, T.; Fukuda, T.; Shingai, N.; Sawa, M.; Ozawa, Y.; Tanaka, M.; Uchida, N.; et al. Relapse of Acute Myeloid Leukemia after Allogeneic Hematopoietic Cell Transplantation: Clinical Features and Outcomes. Bone Marrow Transplant. 2021, 56, 1126–1133. [Google Scholar] [CrossRef]
- Scott, B.L.; Pasquini, M.C.; Logan, B.R.; Wu, J.; Devine, S.M.; Porter, D.L.; Maziarz, R.T.; Warlick, E.D.; Fernandez, H.F.; Alyea, E.P.; et al. Myeloablative versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J. Clin. Oncol. 2017, 35, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Molina, B.; Gonzalez Vicent, M.; Herrero, B.; Deltoro, N.; Ruiz, J.; Perez Martinez, A.; Diaz, M.A. Kinetics and Risk Factors of Relapse after Allogeneic Stem Cell Transplantation in Children with Leukemia: A Long-Term Follow-Up Single-Center Study. Biol. Blood Marrow Transplant. 2019, 25, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, G.J.; Janssen, J.J.W.M.; van de Loosdrecht, A.A. Risk Factors for Relapse after Allogeneic Transplantation in Acute Myeloid Leukemia. Haematologica 2016, 101, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Sengsayadeth, S.; Savani, B.N.; Blaise, D.; Malard, F.; Nagler, A.; Mohty, M. Reduced Intensity Conditioning Allogeneic Hematopoietic Cell Transplantation for Adult Acute Myeloid Leukemia in Complete Remission—A Review from the Acute Leukemia Working Party of the EBMT. Haematologica 2015, 100, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Kreidieh, F.; Abou Dalle, I.; Moukalled, N.; El-Cheikh, J.; Brissot, E.; Mohty, M.; Bazarbachi, A. Relapse after Allogeneic Hematopoietic Stem Cell Transplantation in Acute Myeloid Leukemia: An Overview of Prevention and Treatment. Int. J. Hematol. 2022, 116, 330–340. [Google Scholar] [CrossRef] [PubMed]
- De Lima, M.; Giralt, S.; Thall, P.F.; De Padua Silva, L.; Jones, R.B.; Komanduri, K.; Braun, T.M.; Nguyen, H.Q.; Champlin, R.; Garcia-Manero, G. Maintenance Therapy with Low-Dose Azacitidine after Allogeneic Hematopoietic Stem Cell Transplantation for Recurrent Acute Myelogenous Leukemia or Myelodysplastic Syndrome: A Dose and Schedule Finding Study. Cancer 2010, 116, 5420–5431. [Google Scholar] [CrossRef]
- Oran, B.; de Lima, M.; Garcia-Manero, G.; Thall, P.F.; Lin, R.; Popat, U.; Alousi, A.M.; Hosing, C.; Giralt, S.; Rondon, G.; et al. A Phase 3 Randomized Study of 5-Azacitidine Maintenance vs Observation after Transplant in High-Risk AML and MDS Patients. Blood Adv. 2020, 4, 5580–5588. [Google Scholar] [CrossRef] [PubMed]
- Leotta, S.; Condorelli, A.; Sciortino, R.; Milone, G.A.; Bellofiore, C.; Garibaldi, B.; Schininà, G.; Spadaro, A.; Cupri, A.; Milone, G. Prevention and Treatment of Acute Myeloid Leukemia Relapse after Hematopoietic Stem Cell Transplantation: The State of the Art and Future Perspectives. J. Clin. Med. 2022, 11, 253. [Google Scholar] [CrossRef]
- Christopeit, M.; Kuss, O.; Finke, J.; Bacher, U.; Beelen, D.W.; Bornhäuser, M.; Schwerdtfeger, R.; Bethge, W.A.; Basara, N.; Gramatzki, M.; et al. Second Allograft for Hematologic Relapse of Acute Leukemia after First Allogeneic Stem-Cell Transplantation from Related and Unrelated Donors: The Role of Donor Change. J. Clin. Oncol. 2013, 31, 3259–3271. [Google Scholar] [CrossRef]
- Platzbecker, U.; Wermke, M.; Radke, J.; Oelschlaegel, U.; Seltmann, F.; Kiani, A.; Klut, I.M.; Knoth, H.; Röllig, C.; Schetelig, J.; et al. Azacitidine for Treatment of Imminent Relapse in MDS or AML Patients after Allogeneic HSCT: Results of the RELAZA Trial. Leukemia 2012, 26, 381–389. [Google Scholar] [CrossRef]
- Najima, Y. Overcoming Relapse: Prophylactic or Pre-Emptive Use of Azacitidine or FLT3 Inhibitors after Allogeneic Transplantation for AML or MDS. Int. J. Hematol. 2023, 118, 169–182. [Google Scholar] [CrossRef]
- Craddock, C.; Jilani, N.; Siddique, S.; Yap, C.; Khan, J.; Nagra, S.; Ward, J.; Ferguson, P.; Hazlewood, P.; Buka, R.; et al. Tolerability and Clinical Activity of Post-Transplantation Azacitidine in Patients Allografted for Acute Myeloid Leukemia Treated on the RICAZA Trial. Biol. Blood Marrow Transplant. 2016, 22, 385–390. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Potter, V.T.; Barber, L.D.; Kulasekararaj, A.G.; Lim, Z.Y.; Pearce, R.M.; de Lavallade, H.; Kenyon, M.; Ireland, R.M.; Marsh, J.C.W.; et al. Outcome of Donor Lymphocyte Infusion after T Cell-Depleted Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myelogenous Leukemia and Myelodysplastic Syndromes. Biol. Blood Marrow Transplant. 2013, 19, 562–568. [Google Scholar] [CrossRef]
- Guillaume, T.; Malard, F.; Magro, L.; Labopin, M.; Tabrizi, R.; Borel, C.; Chevallier, P.; Vigouroux, S.; Peterlin, P.; Garnier, A.; et al. Prospective Phase II Study of Prophylactic Low-Dose Azacitidine and Donor Lymphocyte Infusions Following Allogeneic Hematopoietic Stem Cell Transplantation for High-Risk Acute Myeloid Leukemia and Myelodysplastic Syndrome. Bone Marrow Transplant. 2019, 54, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Dominietto, A.; Pozzi, S.; Miglino, M.; Albarracin, F.; Piaggio, G.; Bertolotti, F.; Grasso, R.; Zupo, S.; Raiola, A.M.; Gobbi, M.; et al. Donor lymphocyte infusions for the treatment of minimal residual disease in acute leukemia. Blood. 2007, 109, 5063–5064. [Google Scholar] [CrossRef]
- Biederstädt, A.; Rezvani, K. How I Treat High-Risk Acute Myeloid Leukemia Using Preemptive Adoptive Cellular Immunotherapy. Blood 2023, 141, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Blouin, A.G.; Ye, F.; Williams, J.; Askar, M. A Practical Guide to Chimerism Analysis: Review of the Literature and Testing Practices Worldwide. Hum. Immunol. 2021, 82, 838–849. [Google Scholar] [CrossRef]
- Valero-Garcia, J.; del Carmen González-Espinosa, M.; Barrios, M.; Carmona-Antoñanzas, G.; García-Planells, J.; Ruiz-Lafora, C.; Fuentes-Gálvez, A.; Jiménez-Velasco, A. Earlier Relapse Detection after Allogeneic Haematopoietic Stem Cell Transplantation by Chimerism Assays: Digital PCR versus Quantitative Real-Time PCR of Insertion/Deletion Polymorphisms. PLoS ONE 2019, 14, e0212708. [Google Scholar] [CrossRef]
- Delgado, J.; Pereira, A.; Villamor, N.; López-Guillermo, A.; Rozman, C. Survival Analysis in Hematologic Malignancies: Recommendations for Clinicians. Haematologica 2014, 99, 1410–1420. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software “EZR” for Medical Statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Schmid, C.; Labopin, M.; Nagler, A.; Bornhäuser, M.; Finke, J.; Fassas, A.; Volin, L.; Gürman, G.; Maertens, J.; Bordigoni, P.; et al. Donor Lymphocyte Infusion in the Treatment of First Hematological Relapse after Allogeneic Stem-Cell Transplantation in Adults with Acute Myeloid Leukemia: A Retrospective Risk Factors Analysis and Comparison with Other Strategies by the EBMT Acute Leukemia Working Party. J. Clin. Oncol. 2007, 25, 4938–4945. [Google Scholar] [CrossRef] [PubMed]
- Loke, J.; Vyas, H.; Craddock, C. Optimizing Transplant Approaches and Post-Transplant Strategies for Patients with Acute Myeloid Leukemia. Front. Oncol. 2021, 11, 666091. [Google Scholar] [CrossRef] [PubMed]
- Malagola, M.; Polverelli, N.; Beghin, A.; Bolda, F.; Comini, M.; Farina, M.; Morello, E.; Radici, V.; Accorsi Buttini, E.; Bernardi, S.; et al. Bone Marrow CD34+ Molecular Chimerism as an Early Predictor of Relapse after Allogeneic Stem Cell Transplantation in Patients with Acute Myeloid Leukemia. Front. Oncol. 2023, 13, 1133418. [Google Scholar] [CrossRef]
- Schmid, C.; Labopin, M.; Schaap, N.; Veelken, H.; Brecht, A.; Stadler, M.; Finke, J.; Baron, F.; Collin, M.; Bug, G.; et al. Long-Term Results and GvHD after Prophylactic and Preemptive Donor Lymphocyte Infusion after Allogeneic Stem Cell Transplantation for Acute Leukemia. Bone Marrow Transplant. 2022, 57, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.; De Wreede, L.C.; Van Biezen, A.; Finke, J.; Ehninger, G.; Ganser, A.; Volin, L.; Niederwieser, D.; Beelen, D.; Alessandrino, P.; et al. Outcome after Relapse of Myelodysplastic Syndrome and Secondary Acute Myeloid Leukemia Following Allogeneic Stem Cell Transplantation: A Retrospective Registry Analysis on 698 Patients by the Chronic Malignancies Working Party of the European Society of Blood and Marrow Transplantation. Haematologica 2018, 103, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Rettinger, E.; Willasch, A.M.; Kreyenberg, H.; Borkhardt, A.; Holter, W.; Kremens, B.; Strahm, B.; Woessmann, W.; Mauz-Koerholz, C.; Gruhn, B.; et al. Preemptive Immunotherapy in Childhood Acute Myeloid Leukemia for Patients Showing Evidence of Mixed Chimerism after Allogeneic Stem Cell Transplantation. Blood J. Am. Soc. Hematol. 2011, 118, 5681–5688. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, L.; Yuan, X.; Huang, H.; Luo, Y. Optimization of Donor Lymphocyte Infusion for AML Relapse After Allo-HCT in the Era of New Drugs and Cell Engineering. Front. Oncol. 2022, 11, 790299. [Google Scholar] [CrossRef]
- Yan, C.H.; Wang, J.Z.; Liu, D.H.; Xu, L.P.; Chen, H.; Liu, K.Y.; Huang, X.J. Chemotherapy Followed by Modified Donor Lymphocyte Infusion as a Treatment for Relapsed Acute Leukemia after Haploidentical Hematopoietic Stem Cell Transplantation without in Vitro T-Cell Depletion: Superior Outcomes Compared with Chemotherapy Alone and an Analysis of Prognostic Factors. Eur. J. Haematol. 2013, 91, 304–314. [Google Scholar] [CrossRef]
- Zhao, P.; Ni, M.; Ma, D.; Fang, Q.; Zhang, Y.; Li, Y.; Huang, Y.; Chen, Y.; Chai, X.; Zhan, Y.; et al. Venetoclax plus Azacitidine and Donor Lymphocyte Infusion in Treating Acute Myeloid Leukemia Patients Who Relapse after Allogeneic Hematopoietic Stem Cell Transplantation. Ann. Hematol. 2022, 101, 119–130. [Google Scholar] [CrossRef]
- Patriarca, F.; Sperotto, A.; Lorentino, F.; Oldani, E.; Mammoliti, S.; Isola, M.; Picardi, A.; Arcese, W.; Saporiti, G.; Sorasio, R.; et al. Donor Lymphocyte Infusions After Allogeneic Stem Cell Transplantation in Acute Leukemia: A Survey from the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Front. Oncol. 2020, 10, 572918. [Google Scholar] [CrossRef]
- Sairafi, D.; Remberger, M.; Uhlin, M.; Ljungman, P.; Ringdén, O.; Mattsson, J. Leukemia Lineage-Specific Chimerism Analysis and Molecular Monitoring Improve Outcome of Donor Lymphocyte Infusions. Biol. Blood Marrow Transplant. 2010, 16, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Soverini, S.; Bernardi, S.; Galimberti, S. Molecular Testing in CML between Old and New Methods: Are We at a Turning Point? J. Clin. Med. 2020, 9, 3865. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Malagola, M.; Farina, M.; Polverelli, N.; Re, F.; Russo, D. Digital PCR as a New Method for Minimal Residual Disease Monitoring and Treatment Free Remission Management in Chronic Myeloid Leukemia Patients: Is It Reliable? Hemato 2022, 4, 1–11. [Google Scholar] [CrossRef]
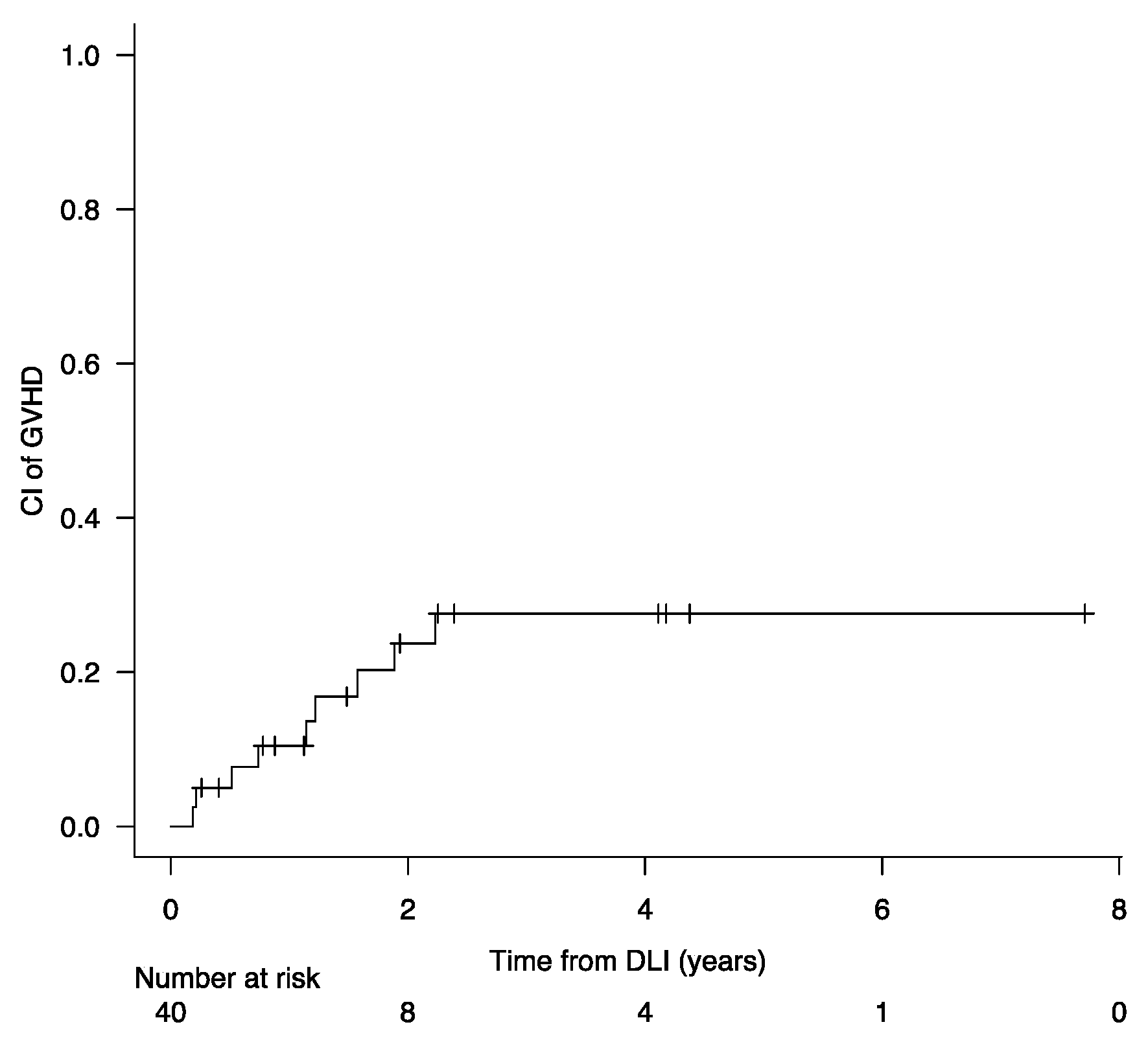

| All Patients (n = 103) | Overt Relapse (n = 69) | Early Relapse (n = 34) | p Value | |
|---|---|---|---|---|
| Age at allo-SCT, years, median (range) | 60.1 (20–74) | 60.1 (20–74) | 60.0 (28.9–67.5) | 1.00 |
| Male, n (%) | 60 (58.2) | 39 (56.5) | 21 (61.8) | 0.67 |
| Diagnosis, n (%) | 0.38 | |||
| AML | 89 (86.4) | 58 (84.1) | 31 (91.2) | |
| MDS | 14 (13.6) | 11 (15.9) | 3 (8.8) | |
| Disease status, n (%) | 1.00 | |||
| CR | 52 (50.5) | 35 (50.7) | 17 (50) | |
| No-CR | 51 (49.5) | 34 (49.3) | 17 (50) | |
| Lines of therapy, n (%) | 0.53 | |||
| 1 | 59 (56.3) | 38 (55.1) | 21 (61.8) | |
| >1 | 44 (42.7) | 31 (44.9) | 13 (38.2) | |
| Donor type, n (%) | 0.18 | |||
| Sibling | 32 (31) | 19 (27.5) | 13 (38.2) | |
| MUD/MMUD | 39 (37.9) | 24 (34.8) | 15 (44.1) | |
| Haploidentical | 31 (30) | 25 (36.2) | 6 (17.6) | |
| UCB | 1 (1) | 1 (1.4) | 0 (0) | |
| Stem cell source, n (%) | 0.60 | |||
| PBSC | 96 (93.2) | 65 (94.2) | 31 (91.2) | |
| BM | 6 (5.8) | 3 (4.3) | 3 (8.8) | |
| UCB | 1 (1) | 1 (1.4) | 0 (0) | |
| Conditioning regimen, n (%) | 1.00 | |||
| MAC | 56 (54.4) | 37 (53.6) | 19 (55.9) | |
| RIC | 47 (45.6) | 32 (46.4) | 15 (44.1) | |
| GVHD prophylaxis, n (%) | <0.01 | |||
| CsA + MTX | 6 (5.8) | 3 (4.3) | 3 (8.8) | |
| CsA + MTX + ATG | 29 (28.2) | 15 (21.7) | 14 (41.2) | |
| CsA + MMF + ATG | 24 (23.3) | 16 (23.2) | 8 (23.5) | |
| CsA/Sir + MMF + PTCy | 7 (6.8) | 3 (4.3) | 4 (11.8) | |
| CsA + MMF + ATG + PTCy | 23 (22.3) | 22 (31.9) | 1 (2.9) | |
| Other | 14 (13.6) | 10 (14.5) | 4 (11.8) | |
| aGVHD | 47 (45.6) | 34 (49.3) | 13 (38.2) | 0.30 |
| cGVHD | 21 (20.4) | 10 (14.5) | 11 (32.4) | 0.04 |
| Time to relapse, months, median (range) | 12 (0.8–60.5) | 12 (0.8–60.5) | 12 (1.0–56.3) | 1.00 |
| Follow-up, months, median (range) | 1.65 (0.2–8.1) | 1.65 (0.2–8.1) | 1.61 (0.5–7.8) | 1.00 |
| Overt Relapse, n (%) | Early Relapse, n (%) | |
|---|---|---|
| Post-relapse therapy including DLI, n = 40 | 20 (50) | 20 (50) |
| HMA | 2 (10) | 2 (10) |
| HMA + venetoclax | 7 (35) | 5 (25) |
| FLT3-inhibitors | 2 (10) | 1 (5) |
| Intensive chemotherapy | 4 (20) | 1 (5) |
| 2nd allo-SCT | 3 (15) | 2 (10) |
| Other | 1 (5) | 1 (5) |
| Only DLI | 1 (5) | 8 (40) |
| Post-relapse therapy without DLI, n =63 | 49 (78) | 14 (22) |
| HMA | 6 (12) | 4 (29) |
| HMA + venetoclax | 24 (49) | 3 (21) |
| FLT3-inhibitors | 2 (4) | 2 (14) |
| Intensive chemotherapy | 5 (10) | 1 (7) |
| 2nd allo-SCT | 7 (14) | 2 (14) |
| Other | 5 (10) | 2 (14) |
| Overt Relapse, n (%) | Early Relapse, n (%) | p Value | |
|---|---|---|---|
| Pts treated with DLI-based regimen, n = 40 | 20 (50) | 20 (50) | |
| Response | |||
| CRmol | 14 (70) | 11 (55) | |
| NR | 6 (30) | 9 (45) | 0.51 |
| GVHD post DLI | 1 (5) | 8 (40) | 0.02 |
| Deaths | 13 (65) | 11 (55) | 0.75 |
| Deaths due to disease progression | 11 (55) | 7 (35) | 0.36 |
| Pts treated without DLI, n = 63 | 49 (%) | 14 (%) | |
| Response | |||
| RCmol | 8 (16) | 8 (57) | |
| NR | 41 (84) | 6 (43) | <0.01 |
| Deaths | 43 (88) | 9 (64) | 0.06 |
| Deaths due to disease progression | 41 (84) | 7 (50) | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Accorsi Buttini, E.; Doran, C.; Malagola, M.; Radici, V.; Galli, M.; Rubini, V.; Leoni, A.; Farina, M.; Polverelli, N.; Re, F.; et al. Donor Lymphocyte Infusion in the Treatment of Post-Transplant Relapse of Acute Myeloid Leukemias and Myelodysplastic Syndromes Significantly Improves Overall Survival: A French–Italian Experience of 134 Patients. Cancers 2024, 16, 1278. https://doi.org/10.3390/cancers16071278
Accorsi Buttini E, Doran C, Malagola M, Radici V, Galli M, Rubini V, Leoni A, Farina M, Polverelli N, Re F, et al. Donor Lymphocyte Infusion in the Treatment of Post-Transplant Relapse of Acute Myeloid Leukemias and Myelodysplastic Syndromes Significantly Improves Overall Survival: A French–Italian Experience of 134 Patients. Cancers. 2024; 16(7):1278. https://doi.org/10.3390/cancers16071278
Chicago/Turabian StyleAccorsi Buttini, Eugenia, Cristina Doran, Michele Malagola, Vera Radici, Marco Galli, Vicky Rubini, Alessandro Leoni, Mirko Farina, Nicola Polverelli, Federica Re, and et al. 2024. "Donor Lymphocyte Infusion in the Treatment of Post-Transplant Relapse of Acute Myeloid Leukemias and Myelodysplastic Syndromes Significantly Improves Overall Survival: A French–Italian Experience of 134 Patients" Cancers 16, no. 7: 1278. https://doi.org/10.3390/cancers16071278
APA StyleAccorsi Buttini, E., Doran, C., Malagola, M., Radici, V., Galli, M., Rubini, V., Leoni, A., Farina, M., Polverelli, N., Re, F., Bernardi, S., Mohty, M., Russo, D., & Brissot, E. (2024). Donor Lymphocyte Infusion in the Treatment of Post-Transplant Relapse of Acute Myeloid Leukemias and Myelodysplastic Syndromes Significantly Improves Overall Survival: A French–Italian Experience of 134 Patients. Cancers, 16(7), 1278. https://doi.org/10.3390/cancers16071278










