Assessing Trifecta and Pentafecta Success Rates between Robot-Assisted vs. Open Radical Cystectomy: A Propensity Score-Matched Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Surgical Intervention
2.3. Outcomes
- (1)
- Negative soft tissue surgical margins.
- (2)
- Lymphadenectomy of ≥16 LNs.
- (3)
- Absence of major complications at 90 days.
- (4)
- Treatment-free time between TURBt and RC shorter than 3 months.
- (5)
- Absence of local recurrence within 12 months after RC.
2.4. Statistical Analysis
Propensity Score Matching
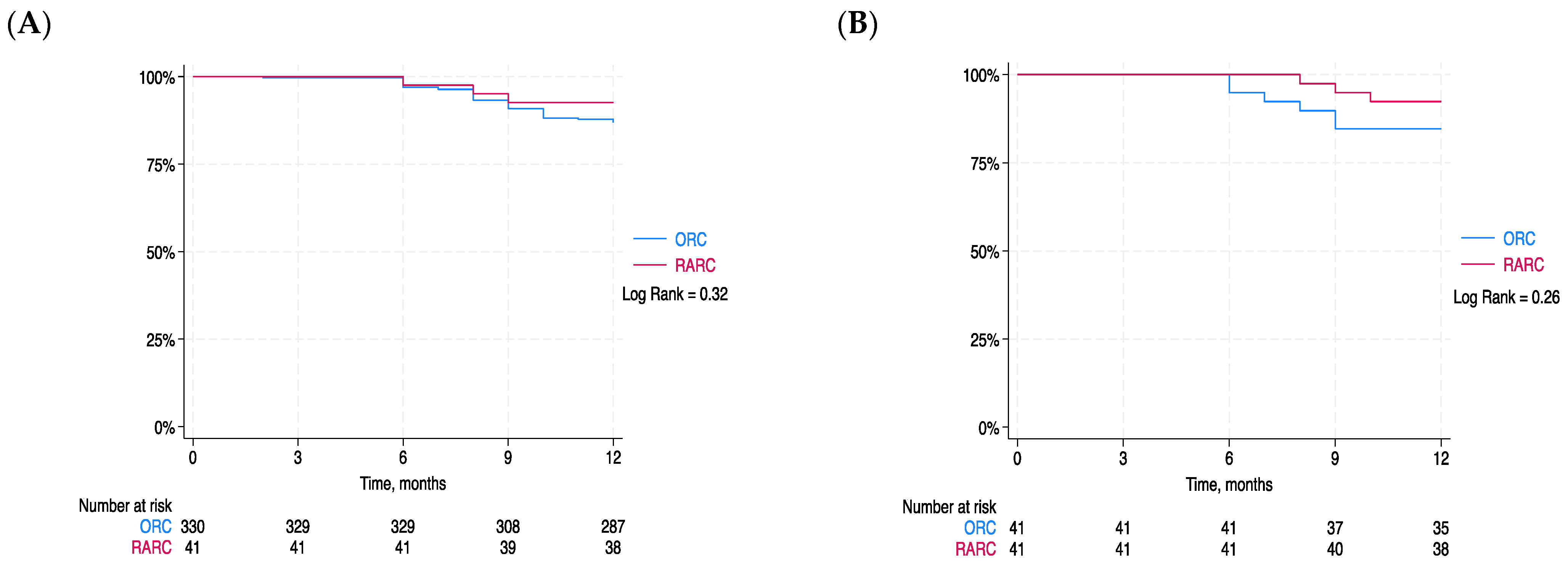
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cumberbatch, M.G.K.; Jubber, I.; Black, P.C.; Esperto, F.; Figueroa, J.D.; Kamat, A.M.; Kiemeney, L.; Lotan, Y.; Pang, K.; Silverman, D.T.; et al. Epidemiology of Bladder Cancer: A Systematic Review and Contemporary Update of Risk Factors in 2018. Eur. Urol. 2018, 74, 784–795. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.P.; Lieskovsky, G.; Cote, R.; Groshen, S.; Feng, A.-C.; Boyd, S.; Skinner, E.; Bochner, B.; Thangathurai, D.; Mikhail, M.; et al. Radical Cystectomy in the Treatment of Invasive Bladder Cancer: Long-Term Results in 1,054 Patients. J. Clin. Oncol. 2001, 19, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Postoperative Nomogram Predicting Risk of Recurrence after Radical Cystectomy for Bladder Cancer. J. Clin. Oncol. 2006, 24, 3967–3972. [CrossRef] [PubMed]
- Lowrance, W.T.; Rumohr, J.A.; Chang, S.S.; Clark, P.E.; Smith, J.A.; Cookson, M.S. Contemporary Open Radical Cystectomy: Analysis of Perioperative Outcomes. J. Urol. 2008, 179, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Moretto, S.; Gandi, C.; Bientinesi, R.; Totaro, A.; Marino, F.; Gavi, F.; Russo, A.; Aceto, P.; Pierconti, F.; Bassi, P.; et al. Robotic versus Open Pyeloplasty: Perioperative and Functional Outcomes. J. Clin. Med. 2023, 12, 2538. [Google Scholar] [CrossRef] [PubMed]
- Styn, N.R.; Montgomery, J.S.; Wood, D.P.; Hafez, K.S.; Lee, C.T.; Tallman, C.; He, C.; Crossley, H.; Hollenbeck, B.K.; Weizer, A.Z. Matched Comparison of Robotic-Assisted and Open Radical Cystectomy. Urology 2012, 79, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Challacombe, B.J.; Bochner, B.H.; Dasgupta, P.; Gill, I.; Guru, K.; Herr, H.; Mottrie, A.; Pruthi, R.; Redorta, J.P.; Wiklund, P. The Role of Laparoscopic and Robotic Cystectomy in the Management of Muscle-Invasive Bladder Cancer with Special Emphasis on Cancer Control and Complications. Eur. Urol. 2011, 60, 767–775. [Google Scholar] [CrossRef]
- Ng, C.K.; Kauffman, E.C.; Lee, M.-M.; Otto, B.J.; Portnoff, A.; Ehrlich, J.R.; Schwartz, M.J.; Wang, G.J.; Scherr, D.S. A Comparison of Postoperative Complications in Open versus Robotic Cystectomy. Eur. Urol. 2010, 57, 274–282. [Google Scholar] [CrossRef]
- Smith, N.D.; Castle, E.P.; Gonzalgo, M.L.; Svatek, R.S.; Weizer, A.Z.; Montgomery, J.S.; Pruthi, R.S.; Woods, M.E.; Tollefson, M.K.; Konety, B.R.; et al. The RAZOR (Randomized Open vs Robotic Cystectomy) Trial: Study Design and Trial Update. BJU Int. 2015, 115, 198–205. [Google Scholar] [CrossRef]
- Yu, H.; Hevelone, N.D.; Lipsitz, S.R.; Kowalczyk, K.J.; Nguyen, P.L.; Choueiri, T.K.; Kibel, A.S.; Hu, J.C. Comparative Analysis of Outcomes and Costs Following Open Radical Cystectomy Versus Robot-Assisted Laparoscopic Radical Cystectomy: Results from the US Nationwide Inpatient Sample. Eur. Urol. 2012, 61, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Mastroianni, R.; Ferriero, M.; Tuderti, G.; Anceschi, U.; Bove, A.M.; Brassetti, A.; Misuraca, L.; Zampa, A.; Torregiani, G.; Ghiani, E.; et al. Open Radical Cystectomy versus Robot-Assisted Radical Cystectomy with Intracorporeal Urinary Diversion: Early Outcomes of a Single-Center Randomized Controlled Trial. J. Urol. 2022, 207, 982–992. [Google Scholar] [CrossRef]
- Baron, M.; Peyronnet, B.; Aublé, A.; Hascoet, J.; Castel-Lacanal, E.; Miget, G.; Le Doze, S.; Prudhomme, T.; Manunta, A.; Cornu, J.-N.; et al. Long-Term Discontinuation of Botulinum Toxin a Intradetrusor Injections for Neurogenic Detrusor Overactivity: A Multicenter Study. J. Urol. 2019, 201, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; Qi, M.; Shan, D.; Liu, S.; Na, T.; Chen, L. Trifecta Achievement in Patients Undergoing Partial Nephrectomy: A Systematic Review and Meta-Analysis of Predictive Factors. Int. Braz. J. Urol. 2022, 48, 625–635. [Google Scholar] [CrossRef]
- Bove, A.M.; Brassetti, A.; Ochoa, M.; Anceschi, U.; Ferriero, M.; Tuderti, G.; Misuraca, L.; Mastroianni, R.; Cartolano, S.; D’Annunzio, S.; et al. Robotic-Assisted Simple Prostatectomy: Long-Term, Trifecta- and Pentafecta-Based Analysis of Functional Outcomes. Ther. Adv. Urol. 2023, 15, 175628722211471. [Google Scholar] [CrossRef]
- Aziz, A.; Gierth, M.; Rink, M.; Schmid, M.; Chun, F.K.; Dahlem, R.; Roghmann, F.; Palisaar, R.-J.; Noldus, J.; Ellinger, J.; et al. Optimizing Outcome Reporting after Radical Cystectomy for Organ-Confined Urothelial Carcinoma of the Bladder Using Oncological Trifecta and Pentafecta. World J. Urol. 2015, 33, 1945–1950. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Rubin, D.B. The Use of Matched Sampling and Regression Adjustment to Remove Bias in Observational Studies. Biometrics 1973, 29, 185–203. [Google Scholar] [CrossRef]
- Messer, J.C.; Punnen, S.; Fitzgerald, J.; Svatek, R.; Parekh, D.J. Health-related Quality of Life from a Prospective Randomised Clinical Trial of Robot-assisted Laparoscopic vs Open Radical Cystectomy. BJU Int. 2014, 114, 896–902. [Google Scholar] [CrossRef]
- Khan, M.S.; Omar, K.; Ahmed, K.; Gan, C.; Van Hemelrijck, M.; Nair, R.; Thurairaja, R.; Rimington, P.; Dasgupta, P. Long-Term Oncological Outcomes from an Early Phase Randomised Controlled Three-Arm Trial of Open, Robotic, and Laparoscopic Radical Cystectomy (CORAL). Eur. Urol. 2020, 77, 110–118. [Google Scholar] [CrossRef]
- Maibom, S.L.; Røder, M.A.; Aasvang, E.K.; Rohrsted, M.; Thind, P.O.; Bagi, P.; Kistorp, T.; Poulsen, A.M.; Salling, L.N.; Kehlet, H.; et al. Open vs Robot-assisted Radical Cystectomy (BORARC): A Double-blinded, Randomised Feasibility Study. BJU Int. 2022, 130, 102–113. [Google Scholar] [CrossRef]
- Venkatramani, V.; Reis, I.M.; Castle, E.P.; Gonzalgo, M.L.; Woods, M.E.; Svatek, R.S.; Weizer, A.Z.; Konety, B.R.; Tollefson, M.; Krupski, T.L.; et al. Predictors of Recurrence, and Progression-Free and Overall Survival Following Open versus Robotic Radical Cystectomy: Analysis from the RAZOR Trial with a 3-Year Followup. J. Urol. 2020, 203, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Nix, J.; Smith, A.; Kurpad, R.; Nielsen, M.E.; Wallen, E.M.; Pruthi, R.S. Prospective Randomized Controlled Trial of Robotic versus Open Radical Cystectomy for Bladder Cancer: Perioperative and Pathologic Results. Eur. Urol. 2010, 57, 196–201. [Google Scholar] [CrossRef]
- Bochner, B.H.; Sjoberg, D.D.; Laudone, V.P. A Randomized Trial of Robot-Assisted Laparoscopic Radical Cystectomy. N. Engl. J. Med. 2014, 371, 389–390. [Google Scholar] [CrossRef]
- Mastroianni, R.; Tuderti, G.; Ferriero, M.; Anceschi, U.; Bove, A.M.; Brassetti, A.; Misuraca, L.; D’Annunzio, S.; Guaglianone, S.; Gallucci, M.; et al. Open versus Robot-assisted Radical Cystectomy: Pentafecta and Trifecta Achievement Comparison from a Randomised Controlled Trial. BJU Int. 2023, 132, 671–677. [Google Scholar] [CrossRef]
- Khetrapal, P.; Wong, J.K.L.; Tan, W.P.; Rupasinghe, T.; Tan, W.S.; Williams, S.B.; Boorjian, S.A.; Wijburg, C.; Parekh, D.J.; Wiklund, P.; et al. Robot-Assisted Radical Cystectomy Versus Open Radical Cystectomy: A Systematic Review and Meta-Analysis of Perioperative, Oncological, and Quality of Life Outcomes Using Randomized Controlled Trials. Eur. Urol. 2023, 84, 393–405. [Google Scholar] [CrossRef]
- Fallara, G.; Maida, F.D.; Bravi, C.A.; De Groote, R.; Piramide, F.; Turri, F.; Andras, I.; Moschovas, M.; Larcher, A.; Breda, A.; et al. A Systematic Review and Meta-Analysis of Robot-Assisted vs. Open Radical Cystectomy: Where Do We Stand and Future Perspective. Minerva Urol. Nephrol. 2023, 75, S2724–S6051. [Google Scholar] [CrossRef] [PubMed]
- Linder, B.J.; Frank, I.; Cheville, J.C.; Tollefson, M.K.; Thompson, R.H.; Tarrell, R.F.; Thapa, P.; Boorjian, S.A. The Impact of Perioperative Blood Transfusion on Cancer Recurrence and Survival Following Radical Cystectomy. Eur. Urol. 2013, 63, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Catto, J.W.F.; Khetrapal, P.; Ricciardi, F.; Ambler, G.; Williams, N.R.; Al-Hammouri, T.; Khan, M.S.; Thurairaja, R.; Nair, R.; Feber, A.; et al. Effect of Robot-Assisted Radical Cystectomy with Intracorporeal Urinary Diversion vs Open Radical Cystectomy on 90-Day Morbidity and Mortality among Patients with Bladder Cancer: A Randomized Clinical Trial. JAMA 2022, 327, 2092. [Google Scholar] [CrossRef]
- Salomon, L.; Saint, F.; Anastasiadis, A.G.; Sebe, P.; Chopin, D.; Abbou, C.-C. Combined Reporting of Cancer Control and Functional Results of Radical Prostatectomy. Eur. Urol. 2003, 44, 656–660. [Google Scholar] [CrossRef]
- Cacciamani, G.E.; Winter, M.; Medina, L.G.; Ashrafi, A.N.; Miranda, G.; Tafuri, A.; Landsberger, H.; Lin-Brande, M.; Rajarubendra, N.; De Castro Abreu, A.; et al. Radical Cystectomy Pentafecta: A Proposal for Standardisation of Outcomes Reporting Following Robot-assisted Radical Cystectomy. BJU Int. 2020, 125, 64–72. [Google Scholar] [CrossRef]
- Dotan, Z.A.; Kavanagh, K.; Yossepowitch, O.; Kaag, M.; Olgac, S.; Donat, M.; Herr, H.W. Positive Surgical Margins in Soft Tissue Following Radical Cystectomy for Bladder Cancer and Cancer Specific Survival. J. Urol. 2007, 178, 2308–2313. [Google Scholar] [CrossRef]
- Herr, H.W.; Smith, J.A.; Montie, J.E. Standardization of Radical Cystectomy: Time to Count and Be Counted. BJU Int. 2004, 94, 481–482. [Google Scholar] [CrossRef]
- Foschi, N.; Santoro, P.; Borrelli, I.; Gavi, F.; Amantea, C.; Russo, P.; Moscato, U. Urological Safety and COVID-19 Vaccinations. Vaccines 2022, 10, 1887. [Google Scholar] [CrossRef] [PubMed]
- Gavi, F.; Santoro, P.E.; Amantea, C.; Russo, P.; Marino, F.; Borrelli, I.; Moscato, U.; Foschi, N. Impact of COVID-19 on Uro-Oncological Patients: A Comprehensive Review of the Literature. Microorganisms 2023, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Shabsigh, A.; Korets, R.; Vora, K.C.; Brooks, C.M.; Cronin, A.M.; Savage, C.; Raj, G.; Bochner, B.H.; Dalbagni, G.; Herr, H.W.; et al. Defining Early Morbidity of Radical Cystectomy for Patients with Bladder Cancer Using a Standardized Reporting Methodology. Eur. Urol. 2009, 55, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Brassetti, A.; Cacciamani, G.; Anceschi, U.; Ferriero, M.; Tuderti, G.; Miranda, G.; Mastroianni, R.; Desai, M.; Aron, M.; Gill, I.; et al. Long-Term Oncologic Outcomes of Robot-Assisted Radical Cystectomy (RARC) with Totally Intracorporeal Urinary Diversion (ICUD): A Multi-Center Study. World J. Urol. 2020, 38, 837–843. [Google Scholar] [CrossRef]
- Ahmadi, H.; Daneshmand, S. Association between Use of ERAS Protocols and Complications after Radical Cystectomy. World J. Urol. 2022, 40, 1311–1316. [Google Scholar] [CrossRef]
- Peerbocus, M.; Wang, Z.-J. Enhanced Recovery after Surgery and Radical Cystectomy: A Systematic Review and Meta-Analysis. Res. Rep. Urol. 2021, 13, 535–547. [Google Scholar] [CrossRef]
- Baack Kukreja, J.; Kamat, A.M. Strategies to Minimize Readmission Rates Following Major Urologic Surgery. Ther. Adv. Urol. 2017, 9, 111–119. [Google Scholar] [CrossRef]
- Mastroianni, R.; Tuderti, G.; Ferriero, M.; Anceschi, U.; Bove, A.M.; Brassetti, A.; D’Annunzio, S.; Misuraca, L.; Torregiani, G.; Covotta, M.; et al. Robot-Assisted Radical Cystectomy with Totally Intracorporeal Urinary Diversion Versus Open Radical Cystectomy: 3-Year Outcomes from a Randomised Controlled Trial. Eur. Urol. 2024, S0302283824000538. [Google Scholar] [CrossRef] [PubMed]
| Variables | RARC (n = 41) | ORC (n = 330) | p-Value |
|---|---|---|---|
| Male sex, n (%) | 33 (80) | 262 (80) | 0.89 |
| Median BMI, kg/m2 (IQR) | 25 (18–30) | 25 (17–40) | 0.96 |
| Median age, yr (IQR) | 62 (45–76) | 70 (43–93) | 0.001 |
| Smokers, n (%) | 32 (78) | 259 (79) | 0.78 |
| Charlson comorbidity index, n (%) | 0.35 | ||
| 0–1 | 2 (5) | 25 (7) | |
| 2–3 | 16 (39) | 125 (38) | |
| ≥4 | 23 (56) | 180 (55) | |
| Neadjuvant chemotherapy, n (%) | 22 (53) | 123 (38) | 0.12 |
| Urinary diversion type, n (%) | 0.01 | ||
| Ileal conduit, n (%) | 23 (56) | 246 (75) | |
| Orthotopic neobladder, n (%) | 18 (44) | 57 (17) | |
| Ureterocutaneostomy, n (%) | 0 | 26 (8) | |
| Total operating time, min | 0.001 | ||
| Median (IQR) | 442 (360–522) | 351 (304–404) | |
| Mean ± sd | 447 ± 90 | 356 ± 80 | |
| Pathologic stage, n (%) | 0.44 | ||
| T0 | 0 | 12 (4) | |
| Tis | 4 (10) | 36 (11) | |
| T1 | 6 (15) | 31 (9) | |
| T2 | 25 (61) | 169 (51) | |
| T3 | 4 (10) | 54 (17) | |
| T4 | 2 (5) | 28 (8) | |
| Histology, n (%) | 0.44 | ||
| Urothelial cell carcinoma | 34 (84) | 289 (88) | |
| Squamous cell carcinoma | 2 (5) | 17 (5) | |
| Adenocarcinoma | 0 | 2 (1) | |
| Other | 5 (14) | 22 (7) | |
| Lymph node-positive patients, n (%) | 7 (17) | 40 (16) | 0.53 |
| No. 90-day complications, n (%): | 0.01 | ||
| Clavien–Dindo I–II | 21 (51) | 285 (86) | |
| Clavien–Dindo III–IV | 6 (15) | 45 (14) |
| Before PS Matching | After 1:1 PS Matching | ||||
|---|---|---|---|---|---|
| RARC (n = 41) | ORC (n = 330) | p-Value | ORC (n = 41) | p-Value | |
| Trifecta, n (%) | 26 (63) | 188 (57) | 0.43 | 27 (65) | 0.46 |
| Pentafecta, n (%) | 13 (32) | 128 (39) | 0.38 | 13 (32) | 0.86 |
| Negative STSMs, n (%) | 40 (98) | 317 (96) | 0.62 | 37 (96) | 0.88 |
| Lymph node count ≥ 16, n (%) | 35 (85) | 265 (80) | 0.43 | 33 (82) | 0.32 |
| Absence of Clavien–Dindo grade ≥ III complications at 90 days, n (%) | 35 (85) | 284 (86) | 0.36 | 36 (88) | 0.22 |
| ≤3 months between TURBT and RC, n (%) | 25 (61) | 258 (78) | 0.01 | 30 (72) | 0.02 |
| Absence of local recurrence within 12 months after RC, n (%) | 35 (85) | 237 (71) | 0.01 | 34 (83) | 0.55 |
| Before PS Matching | After 1:1 PS Matching | ||||
|---|---|---|---|---|---|
| RARC (n = 41) | ORC (n = 330) | p Value | ORC (n = 41) | p Value | |
| Lenght of hospital stay, mean ± sd | 16 ± 11 | 15 ± 12 | 0.53 | 15 ± 11 | 0.60 |
| Mean estimated blood loss, mL ± sd | 317 ± 26 | 622 ± 22 | 0.01 | 525 ± 65 | 0.01 |
| Perioperative transfusion, n (%) | 9 (21) | 125 (38) | 0.01 | 18 (42) | 0.01 |
| 90-day hospital readmissions, n (%) | 8 (20) | 70 (21) | 0.90 | 10 (24) | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavi, F.; Foschi, N.; Fettucciari, D.; Russo, P.; Giannarelli, D.; Ragonese, M.; Gandi, C.; Balocchi, G.; Francocci, A.; Bizzarri, F.P.; et al. Assessing Trifecta and Pentafecta Success Rates between Robot-Assisted vs. Open Radical Cystectomy: A Propensity Score-Matched Analysis. Cancers 2024, 16, 1270. https://doi.org/10.3390/cancers16071270
Gavi F, Foschi N, Fettucciari D, Russo P, Giannarelli D, Ragonese M, Gandi C, Balocchi G, Francocci A, Bizzarri FP, et al. Assessing Trifecta and Pentafecta Success Rates between Robot-Assisted vs. Open Radical Cystectomy: A Propensity Score-Matched Analysis. Cancers. 2024; 16(7):1270. https://doi.org/10.3390/cancers16071270
Chicago/Turabian StyleGavi, Filippo, Nazario Foschi, Daniele Fettucciari, Pierluigi Russo, Diana Giannarelli, Mauro Ragonese, Carlo Gandi, Giovanni Balocchi, Alessandra Francocci, Francesco Pio Bizzarri, and et al. 2024. "Assessing Trifecta and Pentafecta Success Rates between Robot-Assisted vs. Open Radical Cystectomy: A Propensity Score-Matched Analysis" Cancers 16, no. 7: 1270. https://doi.org/10.3390/cancers16071270
APA StyleGavi, F., Foschi, N., Fettucciari, D., Russo, P., Giannarelli, D., Ragonese, M., Gandi, C., Balocchi, G., Francocci, A., Bizzarri, F. P., Marino, F., Filomena, G. B., Palermo, G., Totaro, A., Racioppi, M., Bientinesi, R., & Sacco, E. (2024). Assessing Trifecta and Pentafecta Success Rates between Robot-Assisted vs. Open Radical Cystectomy: A Propensity Score-Matched Analysis. Cancers, 16(7), 1270. https://doi.org/10.3390/cancers16071270