Effectiveness of Immunotherapy in Non-Small Cell Lung Cancer Patients with a Diagnosis of COPD: Is This a Hidden Prognosticator for Survival and a Risk Factor for Immune-Related Adverse Events?
Abstract
Simple Summary
Abstract
1. Introduction
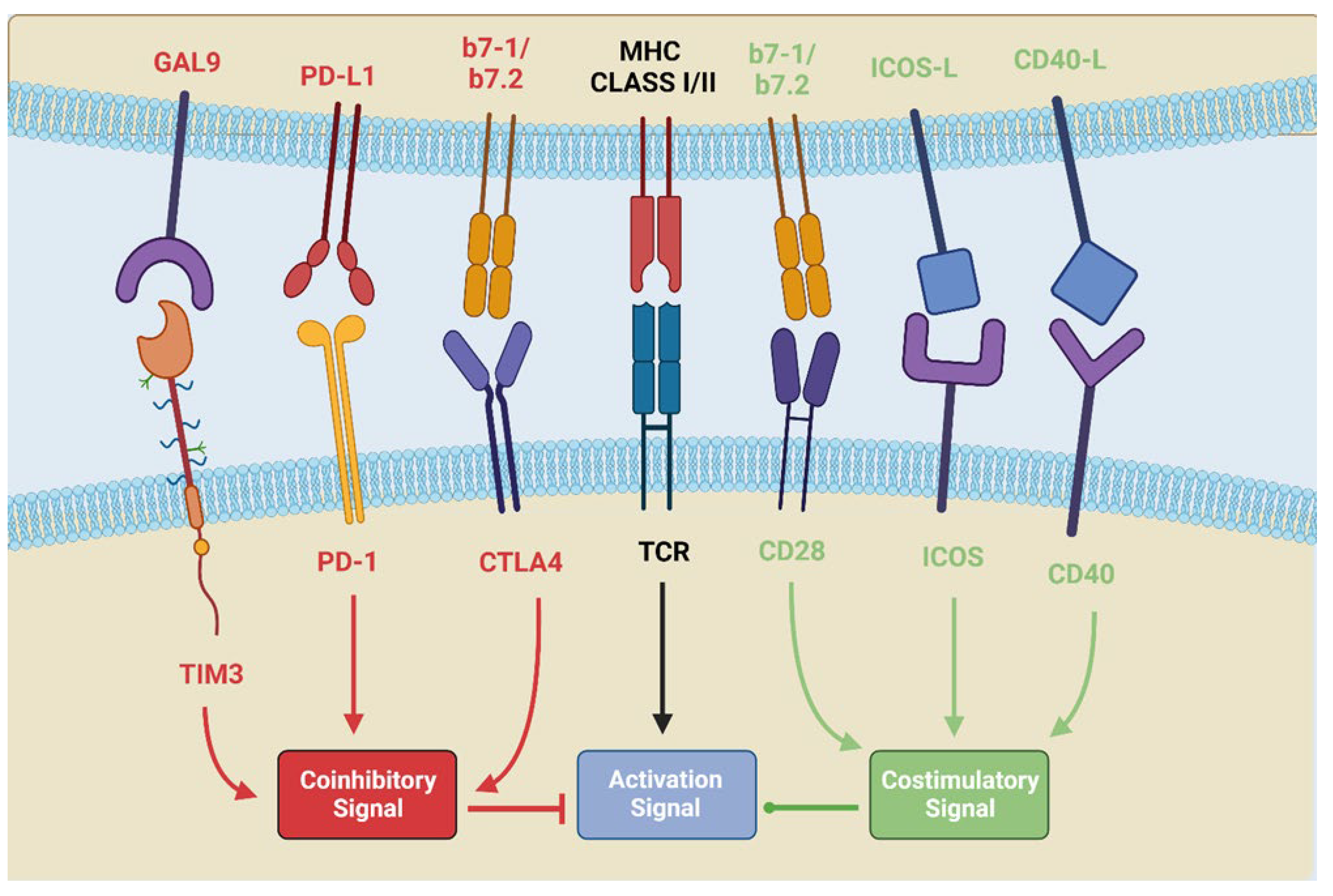
2. PD-L1/PD-1 Interaction and CTLA-4 Stimulation: Pathways of Immune Anergy
2.1. The Biological Pathway of CTLA4
2.2. The Biological Pathway of PD-1/PD-L1
2.3. Role of Tumour Microenvironment and Myeloid Cells
3. COPD Affects the TME by Altering the Response of NSCLC to ICIs
3.1. Common Pathways in NSCLC and COPD Affect PD-1/PD-L1 Expression and ICI Efficacy
3.2. COPD Comorbidity Enhances TILs and Th-1 Response in Lung Cancer Patients
3.3. Other Predictive Biomarkers Evaluated in NSCLC Patients with COPD
4. Observational Studies Investigating COPD’s Influence on NSCLC Patients Treated with ICIs
5. SAFETY and Interstitial Lung Disease (ILD)
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- The European Cancer Burden in 2020: Incidence and Mortality Estimates for 40 Countries and 25 Major Cancers-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/34560371/ (accessed on 8 December 2023).
- Rabe, K.F.; Watz, H. Chronic obstructive pulmonary disease. Lancet 2017, 389, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Forder, A.; Zhuang, R.; Souza, V.G.P.; Brockley, L.J.; Pewarchuk, M.E.; Telkar, N.; Stewart, G.L.; Benard, K.; Marshall, E.A.; Reis, P.P.; et al. Mechanisms Contributing to the Comorbidity of COPD and Lung Cancer. Int. J. Mol. Sci. 2023, 24, 2859. [Google Scholar] [CrossRef]
- Mark, N.M.; Kargl, J.; Busch, S.E.; Yang, G.H.Y.; Metz, H.E.; Zhang, H.; Hubbard, J.J.; Pipavath, S.N.J.; Madtes, D.K.; Houghton, A.M. Chronic Obstructive Pulmonary Disease Alters Immune Cell Composition and Immune Checkpoint Inhibitor Efficacy in Non–Small Cell Lung Cancer. Am. J. Respir. Crit. Care Med. 2018, 197, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Biton, J.; Ouakrim, H.; Dechartres, A.; Alifano, M.; Mansuet-Lupo, A.; Si, H.; Halpin, R.; Creasy, T.; Bantsimba-Malanda, C.; Arrondeau, J.; et al. Impaired Tumor-Infiltrating T Cells in Patients with Chronic Obstructive Pulmonary Disease Impact Lung Cancer Response to PD-1 Blockade. Am. J. Respir. Crit. Care Med. 2018, 198, 928–940. [Google Scholar] [CrossRef]
- Zhou, J.; Chao, Y.; Yao, D.; Ding, N.; Li, J.; Gao, L.; Zhang, Y.; Xu, X.; Zhou, J.; Halmos, B.; et al. Impact of chronic obstructive pulmonary disease on immune checkpoint inhibitor efficacy in advanced lung cancer and the potential prognostic factors. Transl. Lung Cancer Res. 2021, 10, 2148–2162. [Google Scholar] [CrossRef]
- GOLD Report. Global Initiative for Chronic Obstructive Lung Disease-GOLD. Available online: https://goldcopd.org/2023-gold-report-2/ (accessed on 23 August 2023).
- Garassino, M.C.; Gadgeel, S.; Speranza, G.; Felip, E.; Esteban, E.; Dómine, M.; Hochmair, M.J.; Powell, S.F.; Bischoff, H.G.; Peled, N.; et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non–Small-Cell Lung Cancer: 5-Year Outcomes from the Phase 3 KEYNOTE-189 Study. J. Clin. Oncol. 2023, 41, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.K.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef] [PubMed]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.-G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.; Kilickap, S.; Gümüş, M.; Bondarenko, I.; Özgüroğlu, M.; Gogishvili, M.; Turk, H.M.; Cicin, I.; Bentsion, D.; Gladkov, O.; et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 2021, 397, 592–604. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Menzies, A.M.; Johnson, D.B.; Ramanujam, S.; Atkinson, V.G.; Wong, A.N.M.; Park, J.J.; McQuade, J.L.; Shoushtari, A.N.; Tsai, K.K.; Eroglu, Z.; et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann. Oncol. 2017, 28, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Qureshi, O.S.; Zheng, Y.; Nakamura, K.; Attridge, K.; Manzotti, C.; Schmidt, E.M.; Baker, J.; Jeffery, L.E.; Kaur, S.; Briggs, Z.; et al. Trans-Endocytosis of CD80 and CD86: A Molecular Basis for the Cell-Extrinsic Function of CTLA-4. Science 2011, 332, 600–603. [Google Scholar] [CrossRef]
- Rudd, C.E.; Taylor, A.; Schneider, H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol. Rev. 2009, 229, 12–26. [Google Scholar] [CrossRef]
- Polverino, F.; Mirra, D.; Yang, C.X.; Esposito, R.; Spaziano, G.; Rojas-Quintero, J.; Sgambato, M.; Piegari, E.; Cozzolino, A.; Cione, E.; et al. Similar programmed death ligand 1 (PD-L1) expression profile in patients with mild COPD and lung cancer. Sci. Rep. 2022, 12, 22402. [Google Scholar] [CrossRef] [PubMed]
- Lenschow, D.J.; Walunas, T.L.; Bluestone, J.A. CD28/B7 System of T Cell Costimulation. Annu. Rev. Immunol. 1996, 14, 233–258. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.H. Costimulation of T lymphocytes: The role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell 1992, 71, 1065–1068. [Google Scholar] [CrossRef]
- Yi, M.; Niu, M.; Xu, L.; Luo, S.; Wu, K. Regulation of PD-L1 expression in the tumor microenvironment. J. Hematol. Oncol. 2021, 14, 10. [Google Scholar] [CrossRef]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Zerdes, I.; Matikas, A.; Bergh, J.; Rassidakis, G.Z.; Foukakis, T. Genetic, transcriptional and post-translational regulation of the programmed death protein ligand 1 in cancer: Biology and clinical correlations. Oncogene 2018, 37, 4639–4661. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Jiang, L.; Moser, E.K.; Jewett, L.B.; Wright, J.; Du, J.; Zhou, B.; Davis, S.D.; Krupp, N.L.; Braciale, T.J.; et al. Control of pathogenic effector T-cell activities in situ by PD-L1 expression on respiratory inflammatory dendritic cells during respiratory syncytial virus infection. Mucosal Immunol. 2015, 8, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, L.; Huang, F.; Zhang, Q.; Liu, S.; Ma, L.; You, Z. Inflammatory cytokines IL-17 and TNF-α up-regulate PD-L1 expression in human prostate and colon cancer cells. Immunol Lett. 2017, 184, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.J.; Xu, L.J.; Yang, L.; Tsai, Y.; Keng, P.C.; Chen, Y.; Lee, S.O.; Chen, Y. Radiation alters PD-L1/NKG2D ligand levels in lung cancer cells and leads to immune escape from NK cell cytotoxicity via IL-6-MEK/Erk signaling pathway. Oncotarget 2017, 8, 80506–80520. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and Its Ligands in Tolerance and Immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef]
- Fife, B.T.; Pauken, K.E.; Eagar, T.N.; Obu, T.; Wu, J.; Tang, Q.; Azuma, M.; Krummel, M.F.; Bluestone, J.A. Interactions between programmed death-1 and programmed death ligand-1 promote tolerance by blocking the T cell receptor-induced stop signal. Nat. Immunol. 2009, 10, 1185–1192. [Google Scholar] [CrossRef]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.P.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006, 439, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Wang, Y.-H.; Liu, Y.-M.; Ma, L.-X. Prognostic significance of the neutrophil to lymphocyte ratio in patients with non-small cell lung cancer: A systemic review and meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 3098–3106. [Google Scholar]
- Peña-Romero, A.C.; Orenes-Piñero, E. Dual Effect of Immune Cells within Tumour Microenvironment: Pro- and Anti-Tumour Effects and Their Triggers. Cancers 2022, 14, 1681. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, L.; Shan, B. Future of immune checkpoint inhibitors: Focus on tumor immune microenvironment. Ann. Transl. Med. 2020, 8, 1095. [Google Scholar] [CrossRef] [PubMed]
- El-Kenawi, A.; Hänggi, K.; Ruffell, B. The Immune Microenvironment and Cancer Metastasis. Cold Spring Harb. Perspect. Med. 2020, 10, a037424. [Google Scholar] [CrossRef]
- Hiemstra, P.S. Altered Macrophage Function in Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2013, 10, S180–S185. [Google Scholar] [CrossRef]
- Mateu-Jimenez, M.; Curull, V.; Pijuan, L.; Sánchez-Font, A.; Rivera-Ramos, H.; Rodríguez-Fuster, A.; Aguiló, R.; Gea, J.; Barreiro, E. Systemic and Tumor Th1 and Th2 Inflammatory Profile and Macrophages in Lung Cancer: Influence of Underlying Chronic Respiratory Disease. J. Thorac. Oncol. 2017, 12, 235–248. [Google Scholar] [CrossRef]
- Meng, H.; Long, Q.; Wang, R.; Zhou, X.; Su, H.; Wang, T.; Li, Y. Identification of the Key Immune-Related Genes in Chronic Obstructive Pulmonary Disease Based on Immune Infiltration Analysis. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 13–24. [Google Scholar] [CrossRef]
- Naessens, T.; Morias, Y.; Hamrud, E.; Gehrmann, U.; Budida, R.; Mattsson, J.; Baker, T.; Skogberg, G.; Israelsson, E.; Thörn, K.; et al. Human Lung Conventional Dendritic Cells Orchestrate Lymphoid Neogenesis during Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2020, 202, 535–548. [Google Scholar] [CrossRef]
- Mellor, A.L.; Keskin, D.B.; Johnson, T.; Chandler, P.; Munn, D.H. Cells Expressing Indoleamine 2,3-Dioxygenase Inhibit T Cell Responses. J. Immunol. 2002, 168, 3771–3776. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, P.C.; Ochoa, A.C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: Mechanisms and therapeutic perspectives. Immunol. Rev. 2008, 222, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Backman, M.; La Fleur, L.; Kurppa, P.; Djureinovic, D.; Elfving, H.; Brunnström, H.; Mattsson, J.S.M.; Lindberg, A.; Pontén, V.; Eltahir, M.; et al. Infiltration of NK and plasma cells is associated with a distinct immune subset in non-small cell lung cancer. J. Pathol. 2021, 255, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Yoshizawa, A.; Sumiyoshi, S.; Sonobe, M.; Kobayashi, M.; Koyanagi, I.; Aini, W.; Tsuruyama, T.; Date, H.; Haga, H. Stromal plasma cells expressing immunoglobulin G4 subclass in non–small cell lung cancer. Hum. Pathol. 2013, 44, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Lohr, M.; Edlund, K.; Botling, J.; Hammad, S.; Hellwig, B.; Othman, A.; Berglund, A.; Lambe, M.; Holmberg, L.; Ekman, S.; et al. The prognostic relevance of tumour-infiltrating plasma cells and immunoglobulin kappa C indicates an important role of the humoral immune response in non-small cell lung cancer. Cancer Lett. 2013, 333, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Tamminga, M.; Hiltermann, T.J.N.; Schuuring, E.; Timens, W.; Fehrmann, R.S.; Groen, H.J. Immune microenvironment composition in non-small cell lung cancer and its association with survival. Clin. Transl. Immunol. 2020, 9, e1142. [Google Scholar] [CrossRef]
- Teramoto, K.; Igarashi, T.; Kataoka, Y.; Ishida, M.; Hanaoka, J.; Sumimoto, H.; Daigo, Y.; Teramoto, K.; Igarashi, T.; Kataoka, Y.; et al. Prognostic impact of soluble PD-L1 derived from tumor-associated macrophages in non-small-cell lung cancer. Cancer Immunol. Immunother. 2023, 72, 3755–3764. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Lv, G.; Zhu, N.; Chen, X.; Shao, Y.; Liu, Y.; Zhao, W.; Shi, Y. Multidimensional profiling depicts infiltrating immune cell heterogeneity in the tumor microenvironment of stage IA non-small cell lung cancer. Thorac. Cancer 2022, 13, 947–955. [Google Scholar] [CrossRef]
- Mony, J.T.; Schuchert, M.J. Prognostic Implications of Heterogeneity in Intra-tumoral Immune Composition for Recurrence in Early Stage Lung Cancer. Front. Immunol. 2018, 9, 2298. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Y.; Zhao, Y.; Pei, L.; Yan, H. Immune Infiltration Profiling in Nonsmall Cell Lung Cancer and Their Clinical Significance: Study Based on Gene Expression Measurements. DNA Cell Biol. 2019, 38, 1387–1401. [Google Scholar] [CrossRef]
- Aloe, C.; Wang, H.; Vlahos, R.; Irving, L.; Steinfort, D.; Bozinovski, S. Emerging and multifaceted role of neutrophils in lung cancer. Transl. Lung Cancer Res. 2021, 10, 2806–2818. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Yin, Y.; Liu, B.; Wang, L.; Chen, M.; Zhu, Y.; Zhang, H.; Sun, D.; Qin, J. ZNF143 Expression is Associated with COPD and Tumor Microenvironment in Non-Small Cell Lung Cancer. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 685–700. [Google Scholar] [CrossRef]
- Eapen, M.S.; Hansbro, P.M.; Larsson-Callerfelt, A.-K.; Jolly, M.K.; Myers, S.; Sharma, P.; Jones, B.; Rahman, A.; Markos, J.; Chia, C.; et al. Chronic Obstructive Pulmonary Disease and Lung Cancer: Underlying Pathophysiology and New Therapeutic Modalities. Drugs 2018, 78, 1717–1740. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, G.; Zhou, J. Characteristics of elderly patients with COPD and newly diagnosed lung cancer, and factors associated with treatment decision. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Houghton, A.M. Common Mechanisms Linking Chronic Obstructive Pulmonary Disease and Lung Cancer. Ann. Am. Thorac. Soc. 2018, 15 (Suppl. S4), S273–S277. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Huang, Z.; Chen, Y.; Xiao, H.; Wang, T. Lung cancer patients with chronic obstructive pulmonary disease benefit from anti-PD-1/PD-L1 therapy. Front. Immunol. 2022, 13, 1038715. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.; He, X.; Hou, L.; Nguyen, N.P.; Zhu, G.; Cameron, R.B.; Lee, J.M. Classification of Non-Small Cell Lung Cancer’s Tumor Immune Micro-Environment and Strategies to Augment Its Response to Immune Checkpoint Blockade. Cancers 2021, 13, 2924. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-N.; Pan, X.; Qiu, D. Imbalances of Th17 and Treg cells and their respective cytokines in COPD patients by disease stage. Int. J. Clin. Exp. Med. 2014, 7, 5324–5329. [Google Scholar]
- Young, R.P.; Hopkins, R.J.; Christmas, T.; Black, P.N.; Metcalf, P.; Gamble, G.D. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur. Respir. J. 2009, 34, 380–386. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Yang, H.; Pan, C.; Li, H.; Luo, Y.; Cheng, T. Multiple microarray analyses identify key genes associated with the development of Non-Small Cell Lung Cancer from Chronic Obstructive Pulmonary Disease. J. Cancer 2021, 12, 996–1010. [Google Scholar] [CrossRef]
- Miao, T.-W.; Du, L.-Y.; Xiao, W.; Mao, B.; Wang, Y.; Fu, J.-J. Identification of Survival-Associated Gene Signature in Lung Cancer Coexisting With COPD. Front. Oncol. 2021, 11, 600243. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Cai, J.-H.; Tsai, J.J.P.; Wang, C.C.N. Identification of Hub Genes Associated with Development of Head and Neck Squamous Cell Carcinoma by Integrated Bioinformatics Analysis. Front. Oncol. 2020, 10, 681. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Zhang, Y.; Lv, J.-W.; Li, Y.-Q.; Wang, Y.-Q.; He, Q.-M.; Yang, X.-J.; Sun, Y.; Mao, Y.-P.; Yun, J.-P.; et al. Genomic Analysis of Tumor Microenvironment Immune Types across 14 Solid Cancer Types: Immunotherapeutic Implications. Theranostics 2017, 7, 3585–3594. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, J.A.; Sweis, R.F.; Bao, R.; Luke, J.J. T Cell–Inflamed versus Non-T Cell–Inflamed Tumors: A Conceptual Framework for Cancer Immunotherapy Drug Development and Combination Therapy Selection. Cancer Immunol. Res. 2018, 6, 990–1000. [Google Scholar] [CrossRef]
- Takayama, Y.; Nakamura, T.; Fukushiro, Y.; Mishima, S.; Masuda, K.; Shoda, H. Coexistence of Emphysema with Non-small-cell Lung Cancer Predicts the Therapeutic Efficacy of Immune Checkpoint Inhibitors. In Vivo 2021, 35, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Franklin, W.A.; Gazdar, A.F.; Haney, J.; I Wistuba, I.; La Rosa, F.G.; Kennedy, T.; Ritchey, D.M.; Miller, Y.E. Widely dispersed p53 mutation in respiratory epithelium. A novel mechanism for field carcinogenesis. J. Clin. Investig. 1997, 100, 2133–2137. [Google Scholar] [CrossRef] [PubMed]
- Spira, A.; E Beane, J.; Shah, V.; Steiling, K.; Liu, G.; Schembri, F.; Gilman, S.; Dumas, Y.-M.; Calner, P.; Sebastiani, P.; et al. Airway epithelial gene expression in the diagnostic evaluation of smokers with suspect lung cancer. Nat. Med. 2007, 13, 361–366. [Google Scholar] [CrossRef]
- Chen, K.; Pociask, D.A.; McAleer, J.P.; Chan, Y.R.; Alcorn, J.F.; Kreindler, J.L.; Keyser, M.R.; Shapiro, S.D.; Houghton, A.M.; Kolls, J.K.; et al. IL-17RA Is Required for CCL2 Expression, Macrophage Recruitment, and Emphysema in Response to Cigarette Smoke. PLoS ONE 2011, 6, e20333. [Google Scholar] [CrossRef]
- Chang, S.H.; Mirabolfathinejad, S.G.; Katta, H.; Cumpian, A.M.; Gong, L.; Caetano, M.S.; Moghaddam, S.J.; Dong, C. T helper 17 cells play a critical pathogenic role in lung cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 5664–5669. [Google Scholar] [CrossRef]
- Zhou, Q.; Munger, M.E.; Veenstra, R.G.; Weigel, B.J.; Hirashima, M.; Munn, D.H.; Murphy, W.J.; Azuma, M.; Anderson, A.C.; Kuchroo, V.K.; et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood 2011, 117, 4501–4510. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Zhu, C.; Kuchroo, V.K. Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 2017, 276, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Sen, D.; Kaminski, J.; Barnitz, R.; Kurachi, M.; Gerdemann, U.; Yates, K.; Tsao, H.; Godec, J.; LaFleur, M.; Brown, F.; et al. The epigenetic landscape of T cell exhaustion. Science 2016, 354, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Kargl, J.; Busch, S.E.; Yang, G.H.; Kim, K.H.; Hanke, M.L.; Metz, H.E.; Hubbard, J.J.; Lee, S.M.; Madtes, D.K.; McIntosh, M.W.; et al. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat. Commun. 2017, 8, 14381. [Google Scholar] [CrossRef]
- Lizotte, P.H.; Ivanova, E.V.; Awad, M.M.; Jones, R.E.; Keogh, L.; Liu, H.; Dries, R.; Almonte, C.; Herter-Sprie, G.S.; Santos, A.; et al. Multiparametric profiling of non–small-cell lung cancers reveals distinct immunophenotypes. J. Clin. Investig. 2016, 1, e89014. [Google Scholar] [CrossRef]
- Chen, B.; Khodadoust, M.S.; Liu, C.L.; Newman, A.M.; Alizadeh, A.A. Profiling Tumor Infiltrating Immune Cells with CIBERSORT. Methods Protoc. 2018, 1711, 243–259. [Google Scholar] [CrossRef]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ–related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef]
- Aldarouish, M.; Su, X.; Qiao, J.; Gao, C.; Chen, Y.; Dai, A.; Zhang, T.; Shu, Y.; Wang, C. Immunomodulatory effects of chemotherapy on blood lymphocytes and survival of patients with advanced non-small cell lung cancer. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419839592. [Google Scholar] [CrossRef] [PubMed]
- Capone, A.; Volpe, E. Transcriptional Regulators of T Helper 17 Cell Differentiation in Health and Autoimmune Diseases. Front. Immunol. 2020, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; She, X.; Gao, W.; Liu, X.; Shi, B. Correlation between the Treg/Thl7 Index and the Efficacy of PD-1 Monoclonal Antibody in Patients with Advanced Non-Small-Cell Lung Cancer Complicated with Chronic Obstructive Pulmonary Disease. Comput. Math. Methods Med. 2022, 2022, 2923998. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-C.; Narva, S.; Zhou, K.; Zhang, W. A Review on the Antitumor Activity of Various Nitrogenous-based Heterocyclic Compounds as NSCLC Inhibitors. Mini-Rev. Med. Chem. 2019, 19, 1517–1530. [Google Scholar] [CrossRef] [PubMed]
- Nambirajan, A.; Singh, V.; Bhardwaj, N.; Mittal, S.; Kumar, S.; Jain, D. SMARCA4/BRG1–Deficient Non–Small Cell Lung Carcinomas: A Case Series and Review of the Literature. Arch. Pathol. Lab. Med. 2021, 145, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Szentkereszty, M.; Komlósi, Z.I.; Szűcs, G.; Barna, G.; Tamási, L.; Losonczy, G.; Gálffy, G. Effect of COPD on Inflammation, Lymphoid Functions and Progression-Free Survival during First-Line Chemotherapy in Advanced Non-small Cell Lung Cancer. Pathol. Oncol. Res. 2019, 26, 1117–1128. [Google Scholar] [CrossRef]
- Mok, T.; Gorbunova, V.; Juhasz, E.; Szima, B.; Burdaeva, O.; Orlov, S.; Yu, C.-J.; Archer, V.; Hilton, M.; Delmar, P.; et al. A Correlative Biomarker Analysis of the Combination of Bevacizumab and Carboplatin-Based Chemotherapy for Advanced Nonsquamous Non–Small-Cell Lung Cancer: Results of the Phase II Randomized ABIGAIL Study (BO21015). J. Thorac. Oncol. 2014, 9, 848–855. [Google Scholar] [CrossRef][Green Version]
- Briukhovetska, D.; Dörr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in cancer: From biology to therapy. Nat. Rev. Cancer 2021, 21, 481–499. [Google Scholar] [CrossRef]
- Yuen, K.C.; Liu, L.-F.; Gupta, V.; Madireddi, S.; Keerthivasan, S.; Li, C.; Rishipathak, D.; Williams, P.; Kadel, E.E., 3rd; Koeppen, H.; et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat. Med. 2020, 26, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Baggiolini, M.; Walz, A.; Kunkel, S.L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J. Clin. Investig. 1989, 84, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Fernando, R.I.; Castillo, M.D.; Litzinger, M.; Hamilton, D.H.; Palena, C. IL-8 Signaling Plays a Critical Role in the Epithelial–Mesenchymal Transition of Human Carcinoma Cells. Cancer Res 2011, 71, 5296–5306. [Google Scholar] [CrossRef]
- David, J.M.; Dominguez, C.; Hamilton, D.H.; Palena, C. The IL-8/IL-8R Axis: A Double Agent in Tumor Immune Resistance. Vaccines 2016, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka-Chrebelska, K.H.; Mukherjee, D.; Maryanchik, S.V.; Rudzinska-Radecka, M. Biological and Genetic Mechanisms of COPD, Its Diagnosis, Treatment, and Relationship with Lung Cancer. Biomedicines 2023, 11, 448. [Google Scholar] [CrossRef]
- Jung, H.; Kim, H.S.; Kim, J.Y.; Sun, J.-M.; Ahn, J.S.; Ahn, M.-J.; Park, K.; Esteller, M.; Lee, S.-H.; Choi, J.K. DNA methylation loss promotes immune evasion of tumours with high mutation and copy number load. Nat. Commun. 2019, 10, 4278. [Google Scholar] [CrossRef]
- Duruisseaux, M.; Martínez-Cardús, A.; Calleja-Cervantes, M.E.; Moran, S.; de Moura, M.C.; Davalos, V.; Piñeyro, D.; Sanchez-Cespedes, M.; Girard, N.; Brevet, M.; et al. Epigenetic prediction of response to anti-PD-1 treatment in non-small-cell lung cancer: A multicentre, retrospective analysis. Lancet Respir. Med. 2018, 6, 771–781. [Google Scholar] [CrossRef]
- Huo, X.; Jin, S.; Wang, Y.; Ma, L. DNA methylation in chronic obstructive pulmonary disease. Epigenomics 2021, 13, 1145–1155. [Google Scholar] [CrossRef]
- Suzuki, M.; Wada, H.M.; Tian, L.; Shigematsu, H.; Suzuki, H.; Alaa, M.; Tamura, H.; Fujiwara, T.; Nagato, K.; Motohashi, S.; et al. Molecular characterization of chronic obstructive pulmonary disease-related nonsmall cell lung cancer through aberrant methylation and alterations of EGFR signaling. Ann. Surg. Oncol. 2010, 17, 878–888. [Google Scholar] [CrossRef]
- Tessema, M.; Yingling, C.M.; Picchi, M.A.; Wu, G.; Liu, Y.; Weissfeld, J.L.; Siegfried, J.M.; Tesfaigzi, Y.; Belinsky, S.A. Epigenetic Repression of CCDC37 and MAP1B Links Chronic Obstructive Pulmonary Disease to Lung Cancer. J. Thorac. Oncol. 2015, 10, 1181–1188. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Arora, S.; Prakasam, G.; Calin, G.A.; Syed, M.A. MicroRNA in lung cancer: Role, mechanisms, pathways and therapeutic relevance. Mol. Asp. Med. 2019, 70, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Thai, P.; Statt, S.; Chen, C.H.; Liang, E.; Campbell, C.; Wu, R. Characterization of a Novel Long Noncoding RNA, SCAL1, Induced by Cigarette Smoke and Elevated in Lung Cancer Cell Lines. Am. J. Respir. Cell Mol. Biol. 2013, 49, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Groot, M.; Zhang, D.; Jin, Y. Long Non-Coding RNA Review and Implications in Lung Diseases. JSM Bioinform. Genom. Preteomics 2018, 3, 1033. [Google Scholar]
- Ji, P.; Diederichs, S.; Wang, W.; Böing, S.; Metzger, R.; Schneider, P.M.; Tidow, N.; Brandt, B.; Buerger, H.; Bulk, E.; et al. MALAT-1, a novel noncoding RNA, and thymosin β4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 2003, 22, 8031–8041. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Lu, J.H.; Chen, W.Y.; Gu, A.Q. Upregulated lncRNA-UCA1 contributes to progression of lung cancer and is closely related to clinical diagnosis as a predictive biomarker in plasma. Int. J. Clin. Exp. Med. 2015, 8, 11824–11830. [Google Scholar] [PubMed]
- Liu, X.-H.; Liu, Z.-L.; Sun, M.; Liu, J.; Wang, Z.-X.; De, W. The long non-coding RNA HOTAIR indicates a poor prognosis and promotes metastasis in non-small cell lung cancer. BMC Cancer 2013, 13, 464. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Endo, H.; Yokoyama, M.; Abe, J.; Tamai, K.; Tanaka, N.; Sato, I.; Takahashi, S.; Kondo, T.; Satoh, K. Large noncoding RNA HOTAIR enhances aggressive biological behavior and is associated with short disease-free survival in human non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2013, 436, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wu, D.; Fan, S.; Zhang, Z.; Chen, G.; Lu, J. Upregulation of miR-675-5p induced by lncRNA H19 was associated with tumor progression and development by targeting tumor suppressor p53 in non–small cell lung cancer. J. Cell. Biochem. 2019, 120, 18724–18735. [Google Scholar] [CrossRef]
- Mirra, D.; Esposito, R.; Spaziano, G.; La Torre, C.; Vocca, C.; Tallarico, M.; Cione, E.; Gallelli, L.; D’Agostino, B. Lung microRNAs Expression in Lung Cancer and COPD: A Preliminary Study. Biomedicines 2023, 11, 736. [Google Scholar] [CrossRef]
- Xian, J.; Su, W.; Liu, L.; Rao, B.; Lin, M.; Feng, Y.; Qiu, F.; Chen, J.; Zhou, Q.; Zhao, Z.; et al. Identification of Three Circular RNA Cargoes in Serum Exosomes as Diagnostic Biomarkers of Non–Small-Cell Lung Cancer in the Chinese Population. J. Mol. Diagn. 2020, 22, 1096–1108. [Google Scholar] [CrossRef]
- Shi, K.; Li, Q.-Y.; Zhang, Y.-Q.; Huang, H.; Ding, D.-X.; Luo, W.-M.; Zhang, J.; Guo, Q. HLA-DPA1 overexpression inhibits cancer progression, reduces resistance to cisplatin, and correlates with increased immune infiltration in lung adenocarcinoma. Aging 2023, 15, 11067–11091. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Jiang, G.; Chen, Y.; Xu, Y.; Wan, Y.; Chen, R.; Liu, F.; Mao, W.; Zheng, M.; Xu, J. HLA class II molecule HLA-DRA identifies immuno-hot tumors and predicts the therapeutic response to anti-PD-1 immunotherapy in NSCLC. BMC Cancer 2022, 22, 738. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, I.M.; Giatromanolaki, A.; Mitrakas, A.; Koukourakis, M.I. Loss of HLA-class-I expression in non-small-cell lung cancer: Association with prognosis and anaerobic metabolism. Cell. Immunol. 2022, 373, 104495. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, Y.; Guo, X.; Zhu, Z.; Kong, X.; Yu, F.; Wang, Q. Identification of differentially expressed genes and signaling pathways in chronic obstructive pulmonary disease via bioinformatic analysis. FEBS Open Bio 2019, 9, 1880–1899. [Google Scholar] [CrossRef] [PubMed]
- Mkorombindo, T.; Tran-Nguyen, T.K.; Yuan, K.; Zhang, Y.; Xue, J.; Criner, G.J.; Kim, Y.-I.; Pilewski, J.M.; Gaggar, A.; Cho, M.H.; et al. HLA-C and KIR permutations influence chronic obstructive pulmonary disease risk. J. Clin. Investig. 2021, 6, e150187. [Google Scholar] [CrossRef] [PubMed]
- Baßler, K.; Fujii, W.; Kapellos, T.S.; Dudkin, E.; Reusch, N.; Horne, A.; Reiz, B.; Luecken, M.D.; Osei-Sarpong, C.; Warnat-Herresthal, S.; et al. Alveolar macrophages in early stage COPD show functional deviations with properties of impaired immune activation. Front. Immunol. 2022, 13, 917232. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.-S.; Ban, W.H.; Sohng, K.-Y. Effect of COPD on symptoms, quality of life and prognosis in patients with advanced non-small cell lung cancer. BMC Cancer 2018, 18, 1053. [Google Scholar] [CrossRef]
- Ytterstad, E.; Moe, P.C.; Hjalmarsen, A. COPD in primary lung cancer patients: Prevalence and mortality. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Shiroyama, T.; Masuhiro, K.; Amiya, S.; Enomoto, T.; Adachi, Y.; Hara, R.; Niitsu, T.; Naito, Y.; Miyake, K.; et al. Quantitative evaluation of emphysema for predicting immunotherapy response in patients with advanced non-small-cell lung cancer. Sci. Rep. 2022, 12, 8881. [Google Scholar] [CrossRef]
- Dong, W.; Yin, Y.; Yang, S.; Liu, B.; Chen, X.; Wang, L.; Su, Y.; Jiang, Y.; Shi, D.; Sun, D.; et al. Impact of chronic obstructive pulmonary disease on the efficacy and safety of neoadjuvant immune checkpoint inhibitors combined with chemotherapy for resectable non-small cell lung cancer: A retrospective cohort study. BMC Cancer 2024, 24, 153. [Google Scholar] [CrossRef]
- Takamori, S.; Takada, K.; Shimokawa, M.; Jinnnouchi, M.; Matsubara, T.; Haratake, N.; Miura, N.; Toyozawa, R.; Yamaguchi, M.; Takenoyama, M.; et al. Prognostic impact of primary cancer adjoining emphysematous bullae in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Cancer Immunol. Immunother. 2021, 70, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Park, H.Y.; Im, Y.; Jung, H.A.; Sun, J.; Ahn, J.S.; Ahn, M.; Park, K.; Lee, H.Y.; Lee, S. Improved treatment outcome of pembrolizumab in patients with nonsmall cell lung cancer and chronic obstructive pulmonary disease. Int. J. Cancer 2019, 145, 2433–2439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhou, C.; Gao, J.; Yu, P.; Lin, X.; Xie, X.; Liu, M.; Zhang, J.; Xie, Z.; Cui, F.; et al. Treatment response and safety of immunotherapy for advanced non-small cell lung cancer with comorbid chronic obstructive pulmonary disease: A retrospective cohort study. Transl. Lung Cancer Res. 2022, 11, 2306–2317. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Zhou, J.; Hsu, S.; Ding, N.; Li, J.; Zhang, Y.; Xu, X.; Tang, X.; Wei, T.; Zhu, Z.; et al. Risk factors for immune checkpoint inhibitor-related pneumonitis in non-small cell lung cancer. Transl. Lung Cancer Res. 2022, 11, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Z.; Hou, X.; Zhang, Y.; Zhou, T.; Liu, J.; Lin, Z.; Fang, W.; Yang, Y.; Ma, Y.; et al. Immune-related pneumonitis associated with immune checkpoint inhibitors in lung cancer: A network meta-analysis. J. Immunother. Cancer 2020, 8, e001170. [Google Scholar] [CrossRef]
- Kim, S.; Lim, J.U. Immune checkpoint inhibitor-related interstitial lung disease in patients with advanced non-small cell lung cancer: Systematic review of characteristics, incidence, risk factors, and management. J. Thorac. Dis. 2022, 14, 1684–1695. [Google Scholar] [CrossRef]
- Deng, H.; Deng, J.; Lin, X.; Guan, W.; Lin, Z.; Qiu, Y.; Yang, Y.; Wu, J.; Qiu, G.; Sun, N.; et al. A Risk-Scoring Model for Severe Checkpoint Inhibitor-Related Pneumonitis: A Case–Control Study. Clin. Drug Investig. 2023, 43, 347–357. [Google Scholar] [CrossRef]
- Galant-Swafford, J.; Troesch, A.; Tran, L.; Weaver, A.; Doherty, T.A.; Patel, S.P. Landscape of Immune-Related Pneumonitis in Cancer Patients with Asthma Being Treated with Immune Checkpoint Blockade. Oncology 2020, 98, 123–130. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Reck, M.; Ciuleanu, T.-E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open 2021, 6, 100273. [Google Scholar] [CrossRef]
- Duan, M.-C.; Zhong, X.-N.; Liu, G.-N.; Wei, J.-R. The Treg/Th17 Paradigm in Lung Cancer. J. Immunol. Res. 2014, 2014, 730380. [Google Scholar] [CrossRef] [PubMed]
- Kanai, O.; Kim, Y.H.; Demura, Y.; Kanai, M.; Ito, T.; Fujita, K.; Yoshida, H.; Akai, M.; Mio, T.; Hirai, T. Efficacy and safety of nivolumab in non-small cell lung cancer with preexisting interstitial lung disease. Thorac. Cancer 2018, 9, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Shimizu, J.; Hasegawa, T.; Horio, Y.; Inaba, Y.; Yatabe, Y.; Hida, T. Pre-existing pulmonary fibrosis is a risk factor for anti-PD-1-related pneumonitis in patients with non-small cell lung cancer: A retrospective analysis. Lung Cancer 2018, 125, 212–217. [Google Scholar] [CrossRef] [PubMed]
| Immune Cell | NSCLC [45,46,47,48,49,50,51,52,53] | COPD [42,48,51] | NLCSC and COPD [48,53] |
|---|---|---|---|
| Plasma cells | x | ||
| M2 macrophages | x | ||
| M0/M1 macrophages | x | x | x |
| CD8 T cells | x | x | x |
| Resting CD4 T cells | x | ||
| CD4 T cells | x | ||
| CD4 Treg cells | x | x | |
| Mast cells | x | ||
| Memory B cells | x | ||
| Dendritic cells | x | ||
| Neutrophils | x | x | x |
| NK | x |
| Post-Transcriptional Regulations | Involved Molecule | NSCLC | COPD | NSCLC and COPD |
|---|---|---|---|---|
| lnc-RNA | SCAL1 [104,105] | • | • | |
| MALAT1 [106] | • | |||
| UCA1 [107] | • | |||
| HOTAIR [108,109] | • | |||
| H19 [110] | • | • | ||
| miRNA |  miR-675 [110] miR-675 [110] | • | • | |
 miR-33a-5p [111] miR-33a-5p [111] | • | • | • | |
 miR 149-3p [111] miR 149-3p [111] | • | |||
 miR 197-3p [111] miR 197-3p [111] | • | |||
 miR 199a-5p [111] miR 199a-5p [111] | • | • | ||
 miR 320a-3p [111] miR 320a-3p [111] | • | • | ||
 miR-34a-5p [111] miR-34a-5p [111] | • | • | ||
| circ-RNA |  circ_0047921 [112] circ_0047921 [112] | • | ||
 circ_0056285 [112] circ_0056285 [112] | • | |||
 circ_0007761 [112] circ_0007761 [112] | • |
| Authors | Year | Type of Study | Sample Size | Type of Drug | Name of Drug | Mean Age (±SD) | COPD | No COPD | HR OS | HR PFS | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | PFS | ORR | OS | PFS | ORR | |||||||||
| Mark et al. [5] | 2018 | Retrospective | 72 | Anti-PD-1 Anti-PD-L1 | Nivolumab Pembrolizumab Atezolizumab | 66.4 ± 9.1 | 359 d * | 153 d | - | 145 d * | 54 d | - | Higher survival in COPD and smoker patients (p = 0.0350) | 0.58 (p = 0.033) |
| Biton et al. [6] | 2018 | Retrospective | 39 | Anti-PD-1 | Nivolumab | 64 ± 9 | 250 d ** | 100 d ** | - | 450 d ** | 150 d ** | - | Higher survival in COPD and smoker patients | Higher survival and disease response in COPD and smoker patients |
| Suzuki et al. [101] | 2019 | Retrospective | 229 | NR | NR | NR | 30 ms | - | - | 36 ms | - | - | Higher survival when genetic factors were stratified | - |
| Shin et al. [124] | 2019 | Retrospective | 133 | Anti-PD-1 | Pembrolizumab | 63 | 5 ms ** | 2 ms ** | 38.2% | 8 ms ** | 6 ms ** | 20.5% | 0.51 # (p < 0.001) | 0.61 # (p < 0.001) |
| Takamori et al. [123] § | 2020 | Retrospective | 257 | Anti-PD-1 Anti-PD-L1 | Nivolumab Pembrolizumab Atezolizumab | 66 | 14 ms | 6 ms | - | 28 ms | 3 ms | - | 0.526 (p < 0.0001) | 0.672 (p = 0.0006) |
| Takayama et al. [68] § | 2021 | Retrospective | 153 | Anti-PD-1 Anti-PD-L1 | Nivolumab Pembrolizumab Atezolizumab | 68 ± 9.5 | 19.5 ms | 6.6 ms | 32.4% | 11.6 ms | 2.7 | 15.9% | 0.58 (p = 0.03) | 0.47 (p < 0.001) |
| Zhou et al. [7] | 2021 | Retrospective | 156 | Anti-PD-1 Anti-PD-L1 | NR | NR | 510 d | 316 d | - | Not reached | 186 d | - | 0.56 # (p = 0.018) | 0.56 # (p = 0.034) |
| Noda et al. [121] § | 2022 | Retrospective | 56 | Anti-PD-1 Anti-PD-L1 | Nivolumab Pembrolizumab Atezolizumab | 70 | 20.6 ms | 6.5 ms | 34.1% | 10.8 ms | 2.3 ms | 6.7% | 0.36 (p = 0.004) | 0.30 (p = 0.005) |
| Dong et al. [122] | 2024 | Retrospective | 74 | Anti-PD-1 Anti-PD-L1 | Nivolumab Pembrolizumab Atezolizumab Durvalumab | 63.87 ± 5.87 | - | Not reached | 70% | - | 17 ms | 63.6% | - | χ2 = 6.247 (p = 0.012) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riondino, S.; Rosenfeld, R.; Formica, V.; Morelli, C.; Parisi, G.; Torino, F.; Mariotti, S.; Roselli, M. Effectiveness of Immunotherapy in Non-Small Cell Lung Cancer Patients with a Diagnosis of COPD: Is This a Hidden Prognosticator for Survival and a Risk Factor for Immune-Related Adverse Events? Cancers 2024, 16, 1251. https://doi.org/10.3390/cancers16071251
Riondino S, Rosenfeld R, Formica V, Morelli C, Parisi G, Torino F, Mariotti S, Roselli M. Effectiveness of Immunotherapy in Non-Small Cell Lung Cancer Patients with a Diagnosis of COPD: Is This a Hidden Prognosticator for Survival and a Risk Factor for Immune-Related Adverse Events? Cancers. 2024; 16(7):1251. https://doi.org/10.3390/cancers16071251
Chicago/Turabian StyleRiondino, Silvia, Roberto Rosenfeld, Vincenzo Formica, Cristina Morelli, Giusy Parisi, Francesco Torino, Sabrina Mariotti, and Mario Roselli. 2024. "Effectiveness of Immunotherapy in Non-Small Cell Lung Cancer Patients with a Diagnosis of COPD: Is This a Hidden Prognosticator for Survival and a Risk Factor for Immune-Related Adverse Events?" Cancers 16, no. 7: 1251. https://doi.org/10.3390/cancers16071251
APA StyleRiondino, S., Rosenfeld, R., Formica, V., Morelli, C., Parisi, G., Torino, F., Mariotti, S., & Roselli, M. (2024). Effectiveness of Immunotherapy in Non-Small Cell Lung Cancer Patients with a Diagnosis of COPD: Is This a Hidden Prognosticator for Survival and a Risk Factor for Immune-Related Adverse Events? Cancers, 16(7), 1251. https://doi.org/10.3390/cancers16071251







