Natural Killer Cell Dysfunction in Premenopausal BRCA1 Mutation Carriers: A Potential Mechanism for Ovarian Carcinogenesis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Sample Collection
2.3. 5β-Pregnanediol
2.4. Isolation of Peripheral Blood NK Cells
2.5. Cell Lines
2.6. Real-Time Cell Impedance-Based Cytotoxicity Assay
2.7. Flow Cytometry-Based Cytotoxicity Assay
2.8. Immunohistochemistry
2.9. Data Analysis
3. Results
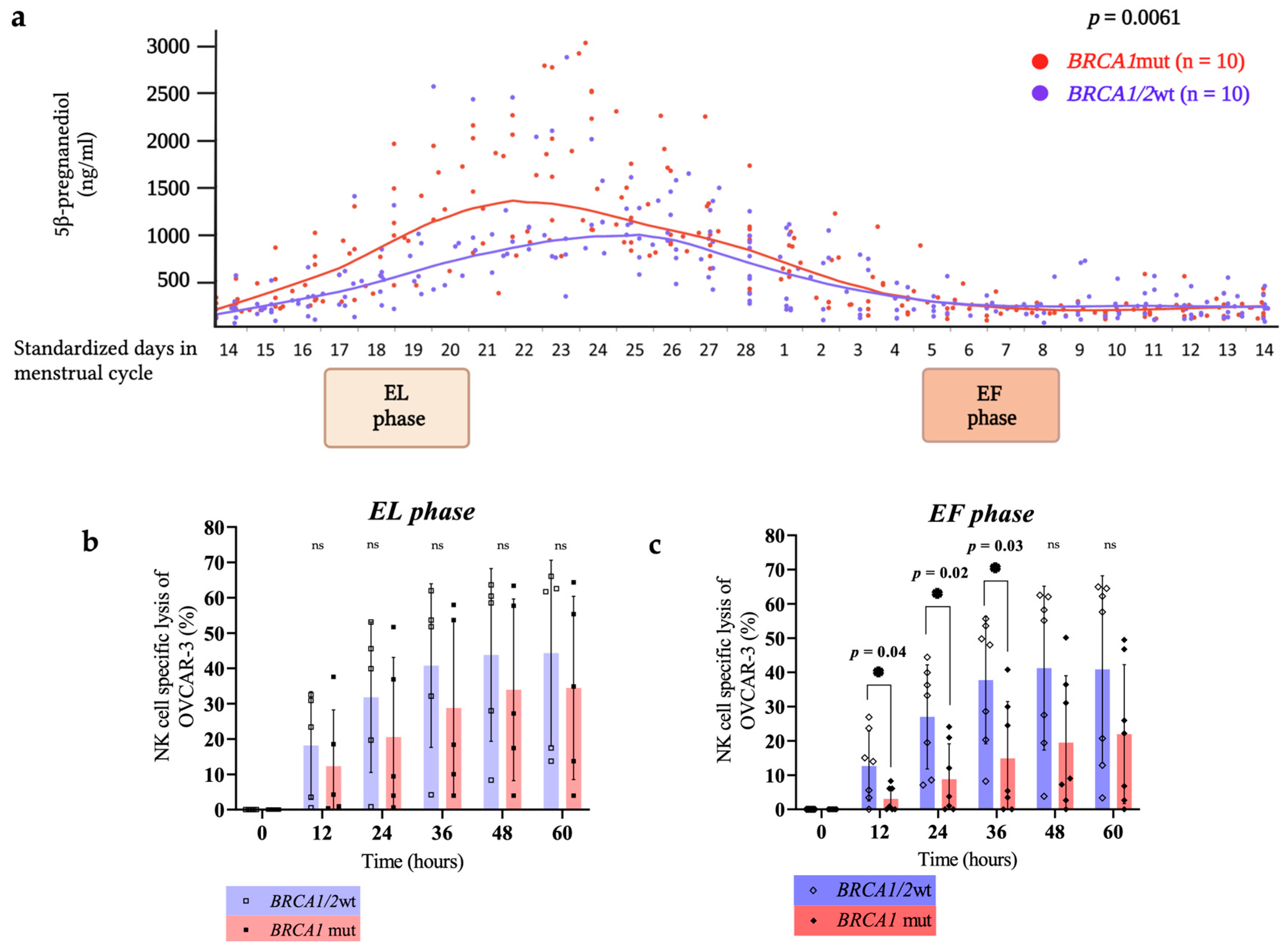
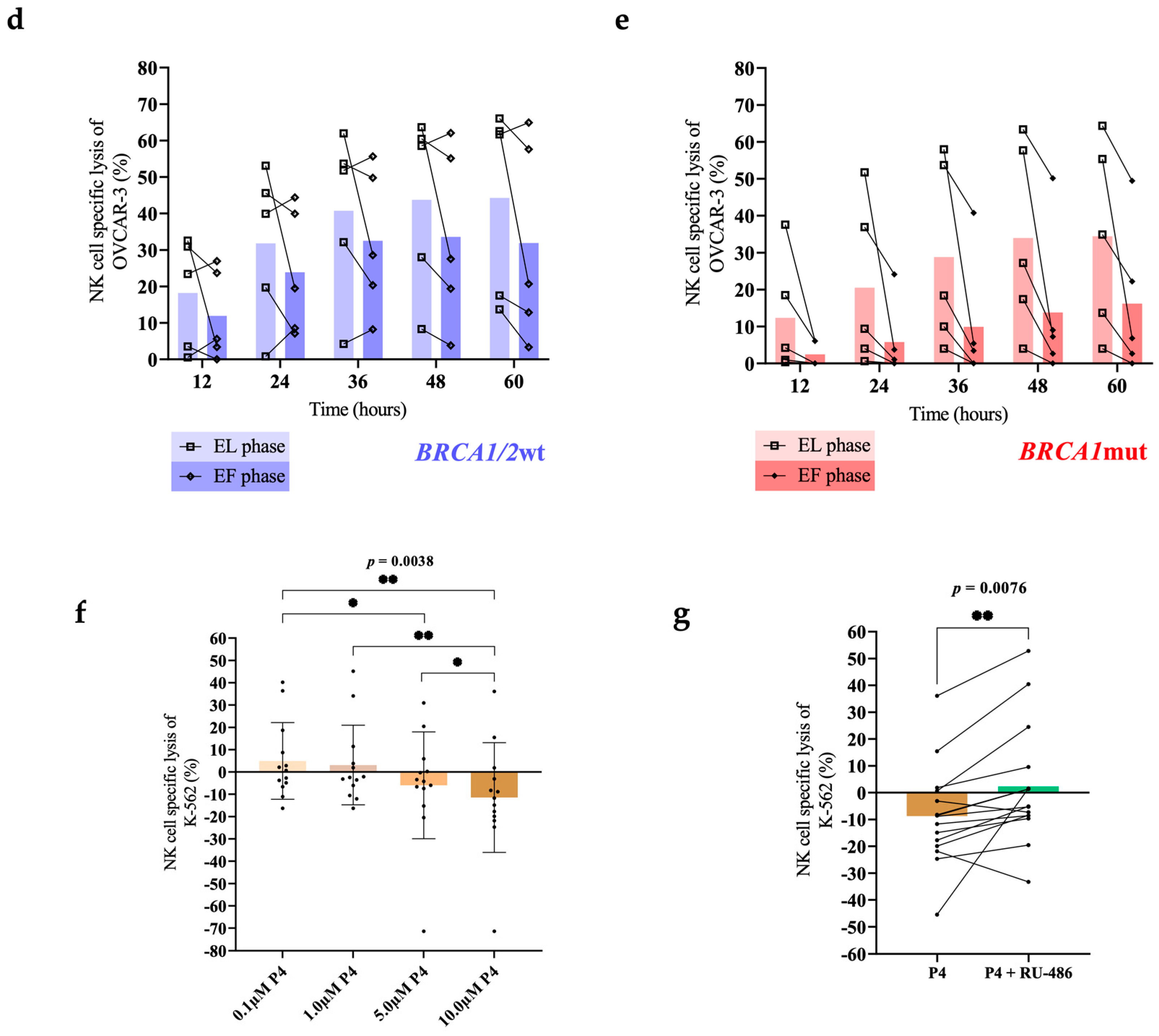
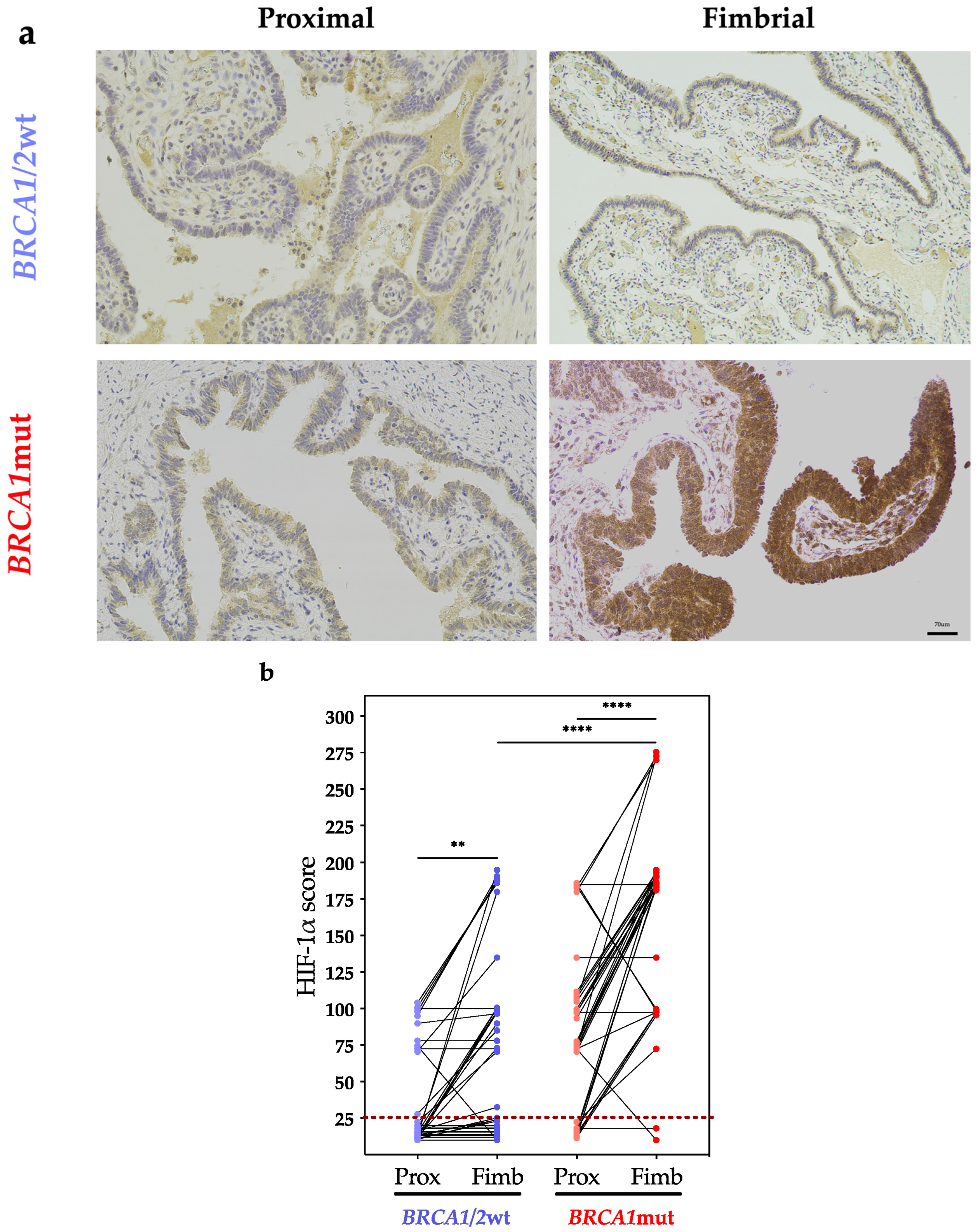
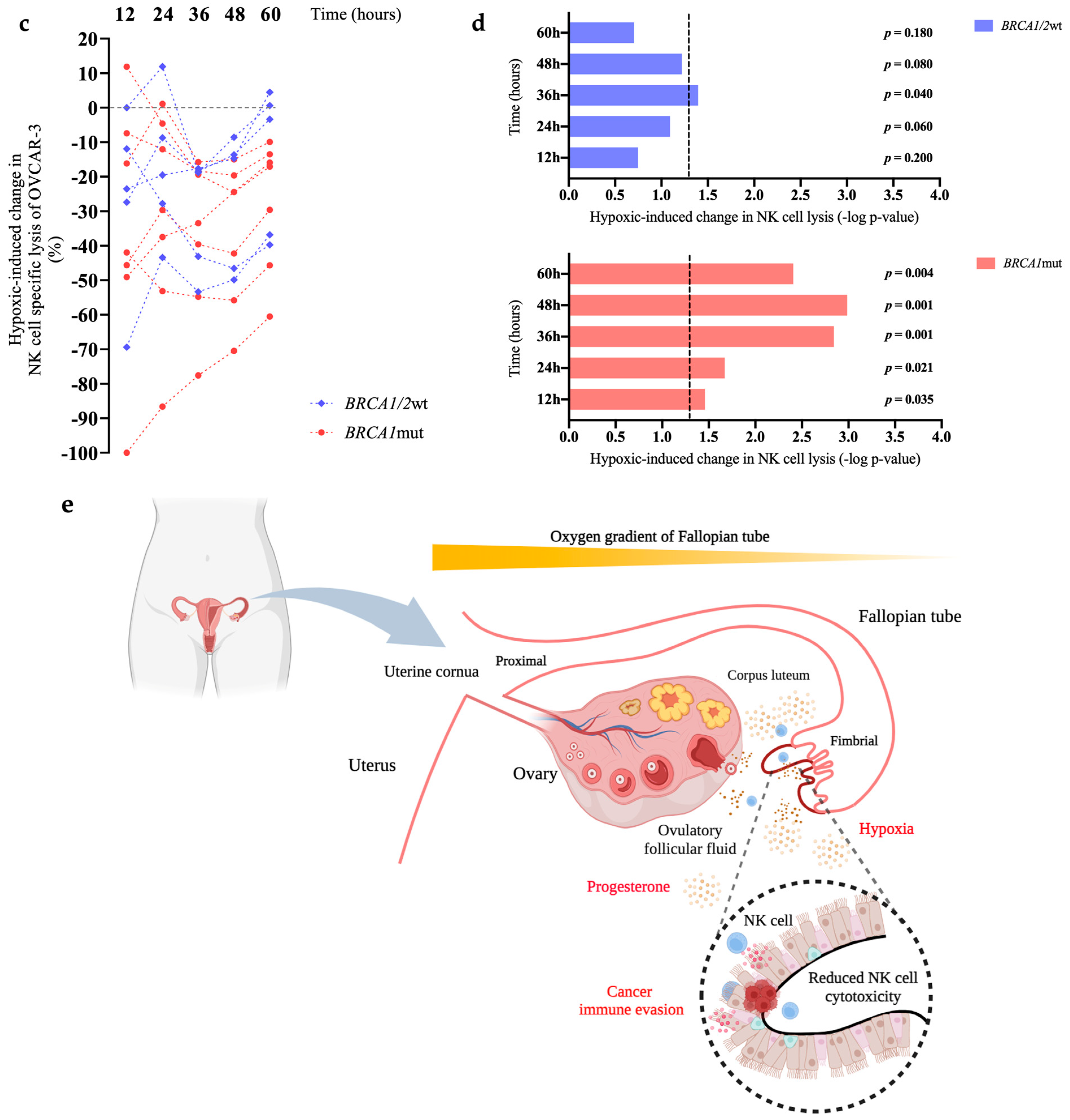
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Momozawa, Y.; Sasai, R.; Usui, Y.; Shiraishi, K.; Iwasaki, Y.; Taniyama, Y.; Parsons, M.T.; Mizukami, K.; Sekine, Y.; Hirata, M.; et al. Expansion of Cancer Risk Profile for BRCA1 and BRCA2 Pathogenic Variants. JAMA Oncol. 2022, 8, 871–878. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef] [PubMed]
- Tenuta, M.; Pandozzi, C.; Sciarra, F.; Campolo, F.; Gelibter, A.J.; Sirgiovanni, G.; Cortesi, E.; Lenzi, A.; Isidori, A.M.; Sbardella, E.; et al. Circulating Natural Killer Cells as Prognostic Value for Non-Small-Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors: Correlation with Sarcopenia. Cancers 2023, 15, 3592. [Google Scholar] [CrossRef]
- Bernson, E.; Huhn, O.; Karlsson, V.; Hawkes, D.; Lycke, M.; Cazzetta, V.; Mikulak, J.; Hall, J.; Piskorz, A.M.; Portuesi, R.; et al. Identification of Tissue-Resident Natural Killer and T Lymphocytes with Anti-Tumor Properties in Ascites of Ovarian Cancer Patients. Cancers 2023, 15, 3362. [Google Scholar] [CrossRef]
- Pawlowska, A.; Rekowska, A.; Kurylo, W.; Panczyszyn, A.; Kotarski, J.; Wertel, I. Current Understanding on Why Ovarian Cancer Is Resistant to Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2023, 24, 10859. [Google Scholar] [CrossRef]
- Vazquez-Garcia, I.; Uhlitz, F.; Ceglia, N.; Lim, J.L.P.; Wu, M.; Mohibullah, N.; Niyazov, J.; Ruiz, A.E.B.; Boehm, K.M.; Bojilova, V.; et al. Ovarian cancer mutational processes drive site-specific immune evasion. Nature 2022, 612, 778–786. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Jang, B.; Hur, S.; Jung, U.; Kil, K.; Na, B.; Lee, M.; Choi, Y.; Fukui, A.; et al. Fluctuation of peripheral blood T, B, and NK cells during a menstrual cycle of normal healthy women. J. Immunol. 2010, 185, 756–762. [Google Scholar] [CrossRef]
- Donnez, J.; Langerock, S.; Thomas, K. Peritoneal fluid volume and 17 beta-estradiol and progesterone concentrations in ovulatory, anovulatory, and postmenopausal women. Obstet. Gynecol. 1982, 59, 687–692. [Google Scholar]
- Widschwendter, M.; Dubeau, L. Non-Surgical Cancer Risk Reduction in BRCA1 Mutation Carriers: Disabling the Remote Control. Cancers 2020, 12, 547. [Google Scholar] [CrossRef] [PubMed]
- Widschwendter, M.; Rosenthal, A.N.; Philpott, S.; Rizzuto, I.; Fraser, L.; Hayward, J.; Intermaggio, M.P.; Edlund, C.K.; Ramus, S.J.; Gayther, S.A.; et al. The sex hormone system in carriers of BRCA1/2 mutations: A case-control study. Lancet Oncol. 2013, 14, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, T.E.; Evans, I.; Jones, A.; Barrett, J.E.; Haran, S.; Reisel, D.; Papaikonomou, K.; Jones, L.; Herzog, C.; Pashayan, N.; et al. Antiprogestins reduce epigenetic field cancerization in breast tissue of young healthy women. Genome Med. 2022, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.; Park, E.Y.; Kwon, S.Y.; Shin, S.; Emerson, R.E.; Shin, Y.H.; DeMayo, F.J.; Lydon, J.P.; Coffey, D.M.; Hawkins, S.M.; et al. Targeting progesterone signaling prevents metastatic ovarian cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 31993–32004. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.N. The potential of mifepristone (RU486) as a female contraceptive drug. Int. J. Clin. Pract. 2002, 56, 140–144. [Google Scholar] [CrossRef]
- Sulke, A.N.; Jones, D.B.; Wood, P.J. Hormonal modulation of human natural killer cell activity in vitro. J. Reprod. Immunol. 1985, 7, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Uksila, J. Human NK cell activity is not inhibited by pregnancy and cord serum factors and female steroid hormones in vitro. J. Reprod. Immunol. 1985, 7, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Szekeres-Bartho, J.; Hadnagy, J.; Pacsa, A.S. The suppressive effect of progesterone on lymphocyte cytotoxicity: Unique progesterone sensitivity of pregnancy lymphocytes. J. Reprod. Immunol. 1985, 7, 121–128. [Google Scholar] [CrossRef]
- Arruvito, L.; Giulianelli, S.; Flores, A.C.; Paladino, N.; Barboza, M.; Lanari, C.; Fainboim, L. NK cells expressing a progesterone receptor are susceptible to progesterone-induced apoptosis. J. Immunol. 2008, 180, 5746–5753. [Google Scholar] [CrossRef]
- Schrijver, L.H.; Antoniou, A.C.; Olsson, H.; Mooij, T.M.; Roos-Blom, M.J.; Azarang, L.; Adlard, J.; Ahmed, M.; Barrowdale, D.; Davidson, R.; et al. Oral contraceptive use and ovarian cancer risk for BRCA1/2 mutation carriers: An international cohort study. Am. J. Obstet. Gynecol. 2021, 225, e1–e17. [Google Scholar] [CrossRef]
- Pressley, M.; Gallaher, J.A.; Brown, J.S.; Tomaszewski, M.R.; Borad, P.; Damaghi, M.; Gillies, R.J.; Whelan, C.J. Cycling hypoxia selects for constitutive HIF stabilization. Sci. Rep. 2021, 11, 5777. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Rayoo, M.; Takano, E.A.; Investigators, K.C.; Fox, S.B. BRCA1 tumours correlate with a HIF-1alpha phenotype and have a poor prognosis through modulation of hydroxylase enzyme profile expression. Br. J. Cancer 2009, 101, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Sabry, M.; Zubiak, A.; Hood, S.P.; Simmonds, P.; Arellano-Ballestero, H.; Cournoyer, E.; Mashar, M.; Pockley, A.G.; Lowdell, M.W. Tumor- and cytokine-primed human natural killer cells exhibit distinct phenotypic and transcriptional signatures. PLoS ONE 2019, 14, e0218674. [Google Scholar] [CrossRef]
- Fitzpatrick, D.; Pirie, K.; Reeves, G.; Green, J.; Beral, V. Combined and progestagen-only hormonal contraceptives and breast cancer risk: A UK nested case-control study and meta-analysis. PLoS Med. 2023, 20, e1004188. [Google Scholar] [CrossRef]
- Burn, J.; Sheth, H.; Elliott, F.; Reed, L.; Macrae, F.; Mecklin, J.P.; Moslein, G.; McRonald, F.E.; Bertario, L.; Evans, D.G.; et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: A double-blind, randomised, placebo-controlled trial. Lancet 2020, 395, 1855–1863. [Google Scholar] [CrossRef]
- Manchanda, R.; Gaba, F.; Talaulikar, V.; Pundir, J.; Gessler, S.; Davies, M.; Menon, U.; Royal College of Obstetricians and Gynaecologists. Risk-Reducing Salpingo-Oophorectomy and the Use of Hormone Replacement Therapy Below the Age of Natural Menopause: Scientific Impact Paper No. 66 October 2021: Scientific Impact Paper No. 66. BJOG 2022, 129, e16–e34. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haran, S.; Chindera, K.; Sabry, M.; Wilkinson, N.; Arora, R.; Zubiak, A.; Bartlett, T.E.; Evans, I.; Jones, A.; Reisel, D.; et al. Natural Killer Cell Dysfunction in Premenopausal BRCA1 Mutation Carriers: A Potential Mechanism for Ovarian Carcinogenesis. Cancers 2024, 16, 1186. https://doi.org/10.3390/cancers16061186
Haran S, Chindera K, Sabry M, Wilkinson N, Arora R, Zubiak A, Bartlett TE, Evans I, Jones A, Reisel D, et al. Natural Killer Cell Dysfunction in Premenopausal BRCA1 Mutation Carriers: A Potential Mechanism for Ovarian Carcinogenesis. Cancers. 2024; 16(6):1186. https://doi.org/10.3390/cancers16061186
Chicago/Turabian StyleHaran, Shaun, Kantaraja Chindera, May Sabry, Nafisa Wilkinson, Rupali Arora, Agnieszka Zubiak, Thomas E. Bartlett, Iona Evans, Allison Jones, Daniel Reisel, and et al. 2024. "Natural Killer Cell Dysfunction in Premenopausal BRCA1 Mutation Carriers: A Potential Mechanism for Ovarian Carcinogenesis" Cancers 16, no. 6: 1186. https://doi.org/10.3390/cancers16061186
APA StyleHaran, S., Chindera, K., Sabry, M., Wilkinson, N., Arora, R., Zubiak, A., Bartlett, T. E., Evans, I., Jones, A., Reisel, D., Herzog, C., Alkasalias, T., Newman, M., Kim, J., Rådestad, A. F., Gemzell-Danielsson, K., Rosenthal, A. N., Dubeau, L., Lowdell, M. W., & Widschwendter, M. (2024). Natural Killer Cell Dysfunction in Premenopausal BRCA1 Mutation Carriers: A Potential Mechanism for Ovarian Carcinogenesis. Cancers, 16(6), 1186. https://doi.org/10.3390/cancers16061186







