Treatment Outcomes of Adolescents Compared to Younger Pediatric Patients with Acute Myeloid Leukemia: Do They Need a Special Approach?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment
2.2. Statistical Analysis
3. Results
3.1. Patient’s Characteristics
3.2. Treatment Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czogała, M.; Balwierz, W.; Pawińska-Wąsikowska, K.; Książek, T.; Bukowska-Strakova, K.; Czogała, W.; Sikorska-Fic, B.; Matysiak, M.; Skalska-Sadowska, J.; Wachowiak, J.; et al. Advances in the First Line Treatment of Pediatric Acute Myeloid Leukemia in the Polish Pediatric Leukemia and Lymphoma Study Group from 1983 to 2019. Cancers 2021, 13, 4536. [Google Scholar] [CrossRef]
- Reinhardt, D.; Antoniou, E.; Waack, K. Pediatric Acute Myeloid Leukemia-Past, Present, and Future. J. Clin. Med. 2022, 11, 504. [Google Scholar] [CrossRef]
- Creutzig, U.; van den Heuvel-Eibrink, M.M.; Gibson, B.; Dworzak, M.N.; Adachi, S.; de Bont, E.; Harbott, J.; Hasle, H.; Johnston, D.; Kinoshita, A.; et al. AML Committee of the International BFM Study Group. Diagnosis and management of acute myeloid leukemia in children and adolescents: Recommendations from an international expert panel. Blood 2012, 120, 3187–3205. [Google Scholar] [CrossRef]
- Taga, T.; Tomizawa, D.; Takahashi, H.; Adachi, S. Acute myeloid leukemia in children: Current status and future directions. Pediatr. Int. 2016, 58, 71–80. [Google Scholar] [CrossRef]
- Pemmaraju, N.; Kantarjian, H.; Ravandi, F.; Nogueras-Gonzalez, G.M.; Huang, X.; O’Brien, S.; Wierda, W.; Garcia-Manero, G.; Thomas, D.; Pierce, S.; et al. Patient Characteristics and Outcomes in Adolescents and Young Adults (AYA) with Acute Myeloid Leukemia (AML). Clin. Lymphoma Myeloma Leuk. 2016, 16, 213–222.e2. [Google Scholar] [CrossRef]
- Larkin, K.T.; Nicolet, D.; Kelly, B.J.; Mrózek, K.; LaHaye, S.; Miller, K.E.; Wijeratne, S.; Wheeler, G.; Kohlschmidt, J.; Blachly, J.S.; et al. High early death rates, treatment resistance, and short survival of Black adolescents and young adults with AML. Blood Adv. 2022, 6, 5570–5581. [Google Scholar] [CrossRef]
- Appelbaum, F.R.; Gundacker, H.; Head, D.R.; Slovak, M.L.; Willman, C.L.; Godwin, J.E.; Anderson, J.E.; Petersdorf, S.H. Age and acute myeloid leukemia. Blood 2006, 107, 3481–3485. [Google Scholar] [CrossRef]
- Creutzig, U.; Kutny, M.A.; Barr, R.; Schlenk, R.F.; Ribeiro, R.C. Acute myelogenous leukemia in adolescents and young adults. Pediatr. Blood Cancer 2018, 65, e27089. [Google Scholar] [CrossRef]
- Hossain, M.J.; Xie, L.; Caywood, E.H. Prognostic factors of childhood and adolescent acute myeloid leukemia (AML) survival: Evidence from four decades of US population data. Cancer Epidemiol. 2015, 39, 720–726. [Google Scholar] [CrossRef]
- Gupta, S.; Baxter, N.N.; Sutradhar, R.; Pole, J.D.; Nagamuthu, C.; Lau, C.; Nathan, P.C. Adolescents and young adult acute myeloid leukemia outcomes at pediatric versus adult centers: A population-based study. Pediatr. Blood Cancer 2021, 68, e28939. [Google Scholar] [CrossRef]
- Wennström, L.; Edslev, P.W.; Abrahamsson, J.; Nørgaard, J.M.; Fløisand, Y.; Forestier, E.; Gustafsson, G.; Heldrup, J.; Hovi, L.; Jahnukainen, K.; et al. Acute Myeloid Leukemia in Adolescents and Young Adults Treated in Pediatric and Adult Departments in the Nordic Countries. Pediatr. Blood Cancer 2016, 63, 83–92. [Google Scholar] [CrossRef]
- Gramatges, M.M.; Rabin, K.R. The adolescent and young adult with cancer: State of the art—Acute leukemias. Curr. Oncol. Rep. 2013, 15, 317–324. [Google Scholar] [CrossRef]
- Advani, A.S.; Hunger, S.P.; Burnett, A.K. Acute leukemia in adolescents and young adults. Semin. Oncol. 2009, 36, 213–226. [Google Scholar] [CrossRef]
- Seval, G.C.; Ozcan, M. Treatment of Acute Myeloid Leukemia in Adolescent and Young Adult Patients. J. Clin. Med. 2015, 4, 441–459. [Google Scholar] [CrossRef]
- Rubnitz, J.E.; Pounds, S.; Cao, X.; Jenkins, L.; Dahl, G.; Bowman, W.P.; Taub, J.W.; Pui, C.H.; Ribeiro, R.C.; Campana, D.; et al. Treatment outcome in older patients with childhood acute myeloid leukemia. Cancer 2012, 118, 6253–6259. [Google Scholar] [CrossRef]
- Woods, W.G.; Franklin, A.R.; Alonzo, T.A.; Gerbing, R.B.; Donohue, K.A.; Othus, M.; Horan, J.; Appelbaum, F.R.; Estey, E.H.; Bloomfield, C.D.; et al. Outcome of adolescents and young adults with acute myeloid leukemia treated on COG trials compared to CALGB and SWOG trials. Cancer 2013, 119, 4170–4179. [Google Scholar] [CrossRef]
- Canner, J.; Alonzo, T.A.; Franklin, J.; Freyer, D.R.; Gamis, A.; Gerbing, R.B.; Lange, B.J.; Meshinchi, S.; Woods, W.G.; Perentesis, J.; et al. Differences in outcomes of newly diagnosed acute myeloid leukemia for adolescent/young adult and younger patients: A report from the Children’s Oncology Group. Cancer 2013, 119, 4162–4169. [Google Scholar] [CrossRef]
- Creutzig, U.; Büchner, T.; Sauerland, M.C.; Zimmermann, M.; Reinhardt, D.; Döhner, H.; Schlenk, R.F. Significance of age in acute myeloid leukemia patients younger than 30 years: A common analysis of the pediatric trials AML-BFM 93/98 and the adult trials AMLCG 92/99 and AMLSG HD93/98A. Cancer 2008, 112, 562–571. [Google Scholar] [CrossRef]
- Schulpen, M.; Goemans, B.F.; Kaspers, G.J.L.; Raaijmakers, M.H.G.P.; Zwaan, C.M.; Karim-Kos, H.E. Increased survival disparities among children and adolescents & young adults with acute myeloid leukemia: A Dutch population-based study. Int. J. Cancer 2022, 150, 1101–1112. [Google Scholar] [CrossRef]
- Nasir, S.S.; Giri, S.; Nunnery, S.; Martin, M.G. Outcome of Adolescents and Young Adults Compared with Pediatric Patients with Acute Myeloid and Promyelocytic Leukemia. Clin. Lymphoma Myeloma Leuk. 2017, 17, 126–132.e1. [Google Scholar] [CrossRef]
- O’Dwyer, K.; Freyer, D.R.; Horan, J.T. Treatment strategies for adolescent and young adult patients with acute myeloid leukemia. Blood 2018, 132, 362–368. [Google Scholar] [CrossRef]
- Tomizawa, D.; Watanabe, T.; Hanada, R.; Horibe, K.; Horikoshi, Y.; Iwamoto, S.; Kinoshita, A.; Moritake, H.; Nakayama, H.; Shimada, A.; et al. Outcome of adolescent patients with acute myeloid leukemia treated with pediatric protocols. Int. J. Hematol. 2015, 102, 318–326. [Google Scholar] [CrossRef]
- Bolouri, H.; Farrar, J.E.; Triche, T., Jr.; Ries, R.E.; Lim, E.L.; Alonzo, T.A.; Ma, Y.; Moore, R.; Mungall, A.J.; Marra, M.A.; et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat. Med. 2018, 24, 103–112, Erratum in Nat. Med. 2018, 24, 526; Erratum in Nat. Med. 2019, 25, 530. [Google Scholar] [CrossRef]
- Desai, P.; Hassane, D.; Roboz, G.J. Clonal Hematopoiesis and risk of Acute Myeloid Leukemia. Best. Pract. Res. Clin. Haematol. 2019, 32, 177–185. [Google Scholar] [CrossRef]
- Jones, L.M.; Tarlock, K.; Cooper, T. Targeted Therapy in Pediatric AML: An Evolving Landscape. Paediatr. Drugs. 2021, 23, 485–497. [Google Scholar] [CrossRef]
- Ramanujachar, R.; Richards, S.; Hann, I.; Webb, D. Adolescents with acute lymphoblastic leukaemia: Emerging from the shadow of paediatric and adult treatment protocols. Pediatr. Blood Cancer 2006, 47, 748–756. [Google Scholar] [CrossRef]
- Usvasalo, A.; Räty, R.; Knuutila, S.; Vettenranta, K.; Harila-Saari, A.; Jantunen, E.; Kauppila, M.; Koistinen, P.; Parto, K.; Riikonen, P.; et al. Acute lymphoblastic leukemia in adolescents and young adults in Finland. Haematologica 2008, 93, 1161–1168. [Google Scholar] [CrossRef]

| Feature | 0–0.9 Years n = 31 | 1–9.9 Years n = 91 | 10–14.9 Years n = 59 | Above 15 Years n = 39 | p |
|---|---|---|---|---|---|
| Median age (range) | 0.48 (0.09–0.92) | 3.88 (1.0–9.5) | 12.9 (9.5–14.9) | 16.4 (15.1–17.8) | |
| Sex | NS | ||||
| Female | 11 (35.5%) | 50 (55%) | 32 (54%) | 25 (64%) | |
| Male | 20 (64.5%) | 41 (45%) | 27 (46%) | 14 (36%) | |
| Treatment protocol | NS | ||||
| AML-BFM 2012 Registry | 23 (74%) | 53 (58%) | 32 (54%) | 23 (59%) | |
| AML-BFM 2019 | 8 (26%) | 38 (42%) | 27 (46%) | 16 (41%) | |
| FAB classification | <0.001 | ||||
| M0 | 1 (3.2%) | 5 (5.5%) | 1 (1.7%) | 1 (2.6%) | |
| M1 | 1 (3.2%) | 11 (12.1%) | 8 (13.6%) | 3 (7.7%) | |
| M2 | 0 | 24 (26.4%) | 23 (39%) | 12 (30.8%) | |
| M4 | 3 (9.7%) | 5 (5.5%) | 9 (15.3%) | 12 (30.8%) | |
| M5 | 16 (51.6%) | 29 (31.9%) | 18 (30.5%) | 11 (28.2%) | |
| M6 | 1 (3.2%) | 0 | 0 | 0 | |
| M7 | 6 (19.4%) | 16 (17.6%) | 0 | 0 | |
| SG | 3 (9.7%) | 1 (1.1%) | 0 | 0 | |
| Risk group | 0.012 | ||||
| SRG | 0 | 21 (23%) | 17 (29%) | 15 (39%) | |
| IRG | 15 (48%) | 35 (38.5%) | 18 (30.5%) | 11 (28%) | |
| HRG | 16 (52%) | 35 (38.5%) | 24 (40.5%) | 13 (33%) | |
| WBC at diagnosis (/μL) | NS | ||||
| <10,000 | 10 (32%) | 27 (30%) | 16 (27%) | 9 (23%) | |
| 10,000–50,000 | 9 (29%) | 34 (37%) | 21 (36%) | 10 (26%) | |
| 50,000–100,000 | 3 (10%) | 10 (11%) | 4 (7%) | 6 (15%) | |
| >100,000 | 7 (22.5%) | 13 (14%) | 16 (27%) | 10 (26%) | |
| ND | 2 (6.5%) | 7 (8%) | 2 (3%) | 4 (10%) | |
| Genetic abnormalities | |||||
| Low-risk genetics | 0 | 19 (21%) | 12 (20%) | 15 (38%) | 0.010 |
| inv(16)(p13;q22)/CBFB::MYH11 | 0 | 3 (3.5%) | 5 (9%) | 10 (26%) | |
| t(8;21)(q22;q22)/RUNX1::RUNX1T1 | 0 | 16 (18%) | 7 (13%) | 5 (13%) | |
| High-risk genetics | 13 (42%) | 31 (34%) | 14 (26%) | 12 (31%) | 0.005 |
| FLT3-ITD and WT1 | 0 | 2 (2.5%) | 4 (7%) | 3 (7.5%) | |
| Monosomy-7 | 1 (3.2%) | 0 | 2 (4%) | 1 (2.5%) | |
| (9;22)(q24;q11.2)/BCR::ABL1 | 1 (3.2%) | 1 (1%) | 0 | 0 | |
| (6;11)(q27;q23)/KMT2A::AF6 | 0 | 1 (1%) | 0 | 2 (5%) | |
| (10;11)(p12;q23)/KMT2A::MLLT10 | 7 (22.5%) | 8 (9%) | 3 (5%) | 3 (7.5%) | |
| (6;9)(p23;q34)/DEK::NUP214 | 0 | 1 (1%) | 3 (5%) | 0 | |
| Complex karyotype | 4 (13%) | 18 (20%) | 2 (4%) | 3 (7.5%) | |
| Intermediate-risk genetics | 18 (58%) | 41 (45%) | 29 (54%) | 12 (31%) | NS |
| Normal karyotype | 6 (19%) | 16 (18%) | 13 (24%) | 7 (18%) | |
| Other | 12 (39%) | 25 (28%) | 16 (29%) | 5 (13%) | |
| ND of genetic analysis | 0 | 1 | 4 | 0 | |
| HSCT in I CR | |||||
| Yes | 10 (32%) | 31 (34%) | 16 (27%) | 11 (28%) | NS |
| Feature | 0–0.9 Years n = 31 | 1–9.9 Years n = 91 | 10–14.9 Years n = 59 | Above 15 Years n = 39 | p |
|---|---|---|---|---|---|
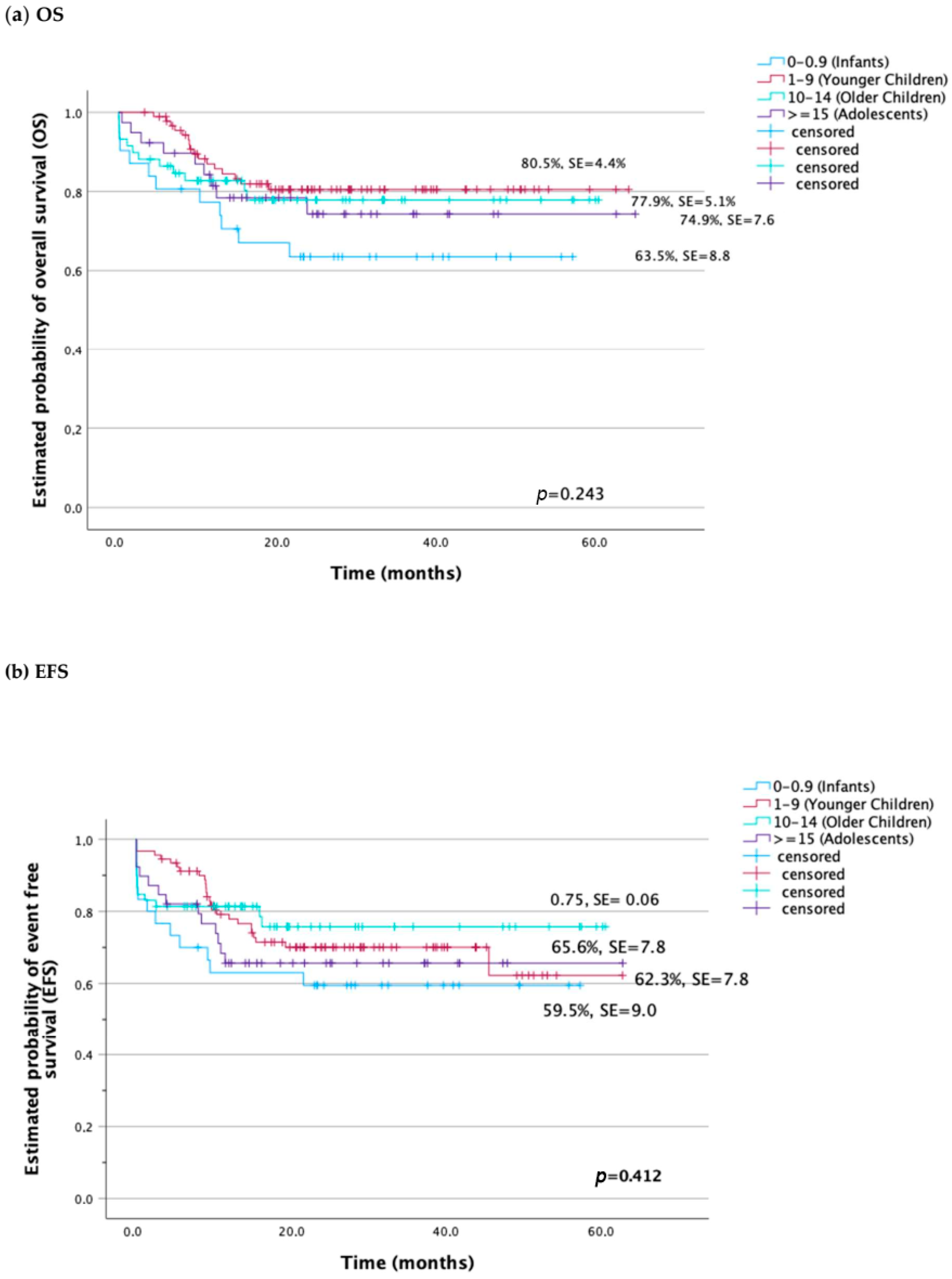
| Probability of 5-year OS ± SD | 63.5 ± 8.8 | 80.5 ± 4.4 | 77.9 ± 5.1 | 74.3 ± 7.6 | NS |
| Probability of 5-year EFS ± SD | 59.5 ± 9.0 | 62.3 ± 8.6 | 75.7 ± 6.1 | 65.6 ± 7.8 | NS |
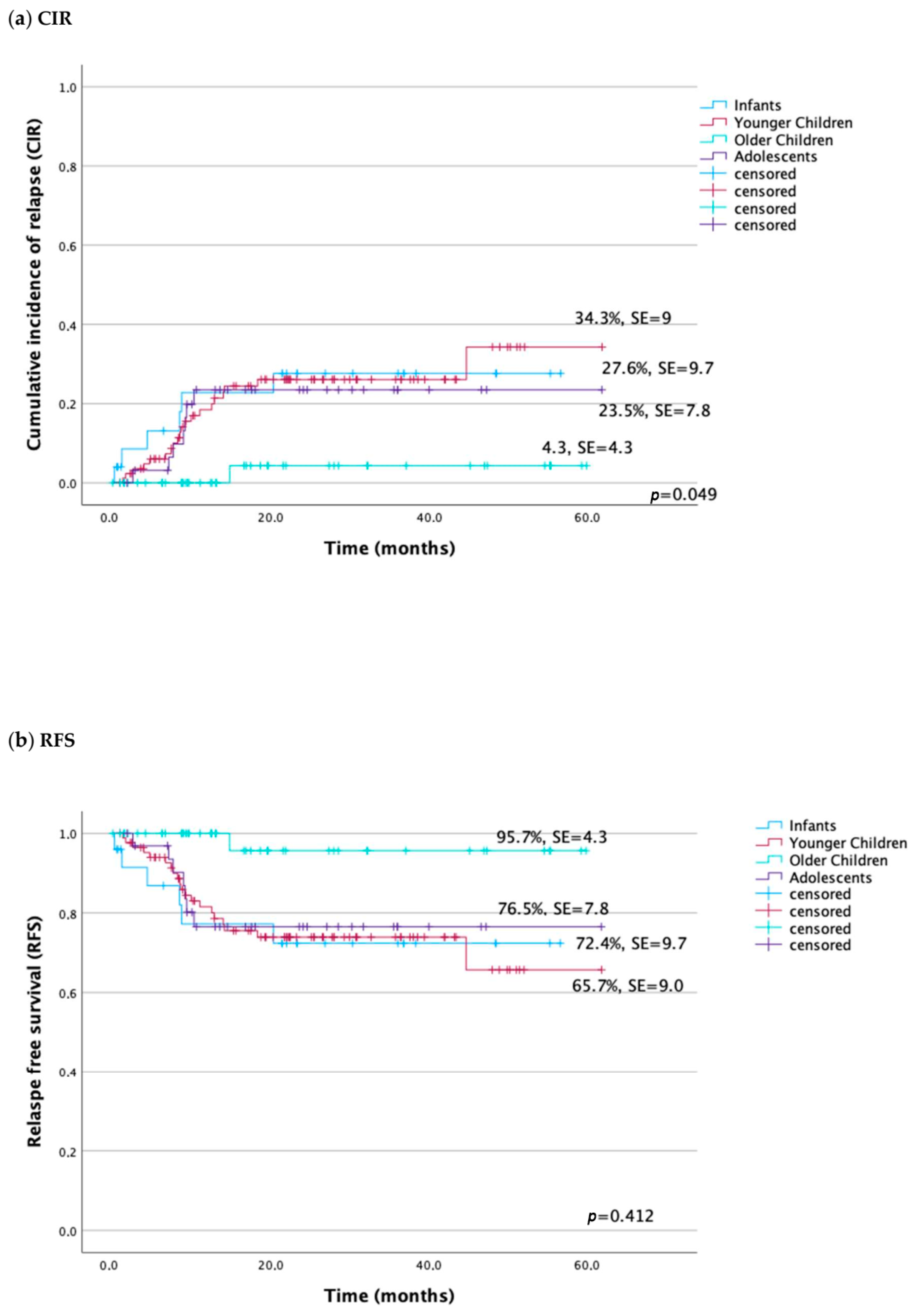
| Probability of 5-year RFS ± SD | 72.4 ± 9.7 | 65.7 ± 9.0 | 95.7 ± 4.3 | 76.5 ± 7.8 | 0.049 |
| Probability of 5-year CIR ± SD | 27.6 ± 9.7 | 34.3 ± 9.0 | 4.3 ± 4.3 | 23.5 ± 7.8 | 0.049 |
| CR (%) | 27 (87) | 87 (95) | 53 (89.8) | 35 (89.7) | 0.015 |
| NR (%) | 0 | 4 (4.4) | 1 (1.6) | 3 (7.6) | |
| Relapse (%) | 6 (19.4) | 19 (20.9) | 1 (1.6) | 7 (17.9) | |
| Deaths (in total) (%) | 11 (35.5) | 16 (17.6) | 12 (20.3) | 9 (23.1) | NS |
| Early deaths (%) | 4 (12.9) | 0 | 5 (8.4) | 1 (2.6) | 0.039 |
| <15 days | 3 (9.7) | 0 | 3 (5.1) | 1 | |
| 15–42 days | 1 (3.2) | 0 | 2 (3.4) | 0 | |
| TRM (%) | 2 (6.5) | 4 (4.4) | 3 (5.1) | 4 (10.3) | NS |
| Death in progression (%) | 7 (22.5) | 12 (13) | 4 (6.7) | 4 (10.3) | NS |
| Features | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| OS | |||||
| Age | <15 years | 1 | 1 | ||
| >15 years | 1 (0.5–2.2) | NS | 1.5 (0.7–3.2) | NS | |
| Risk group | SRG | 1 | 1 | ||
| IRG | 3.7 (1.0–12.6) | 0.038 | 3.8 (1.1–13.3) | 0.03 | |
| HRG | 6.3 (1.9–21.0) | 0.002 | 6.3 (1.6–23.7) | 0.006 | |
| Genotype | Non-high risk | 1 | 1 | ||
| High risk | 2.0 (1.1–3.6) | 0.014 | 1.8 (1.0–3.3) | 0.046 | |
| WBC at diagnosis (/μL) | <100,000 | 1 | 1 | ||
| ≥100,000 | 2.4 (1.3–4.6) | 0.004 | 2.4 (1.3–4.6) | 0.004 | |
| EFS | |||||
| Age | <15 years | 1 | 1 | ||
| >15 years | 1.2 (0.6–2.2) | 0.490 | 1.8 (0.9–3.4) | NS | |
| Risk group | SRG | 1 | 1 | ||
| IRG | 2.8 (1.1–7.0) | 0.021 | 3.3 (1.3–8.2) | 0.01 | |
| HRG | 4.0 (1.6–9.5) | 0.002 | 5.6 (2–15.5) | <0.001 | |
| Genotype | Non-high risk | 1 | 1 | ||
| High risk | 1.1 (0.8–2.4) | NS | 1.3 (0.7–2.2) | NS | |
| WBC at diagnosis (/μL) | <100,000 | 1 | 1 | ||
| ≥100,000 | 2.1 (1.2–3.6) | 0.007 | 2 (1.2–3.5) | 0.009 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawińska-Wąsikowska, K.; Czogała, M.; Bukowska-Strakova, K.; Surman, M.; Rygielska, M.; Książek, T.; Sadowska, B.; Pac, A.; Skalska-Sadowska, J.; Samborska, M.; et al. Treatment Outcomes of Adolescents Compared to Younger Pediatric Patients with Acute Myeloid Leukemia: Do They Need a Special Approach? Cancers 2024, 16, 1145. https://doi.org/10.3390/cancers16061145
Pawińska-Wąsikowska K, Czogała M, Bukowska-Strakova K, Surman M, Rygielska M, Książek T, Sadowska B, Pac A, Skalska-Sadowska J, Samborska M, et al. Treatment Outcomes of Adolescents Compared to Younger Pediatric Patients with Acute Myeloid Leukemia: Do They Need a Special Approach? Cancers. 2024; 16(6):1145. https://doi.org/10.3390/cancers16061145
Chicago/Turabian StylePawińska-Wąsikowska, Katarzyna, Małgorzata Czogała, Karolina Bukowska-Strakova, Marta Surman, Monika Rygielska, Teofila Książek, Beata Sadowska, Agnieszka Pac, Jolanta Skalska-Sadowska, Magdalena Samborska, and et al. 2024. "Treatment Outcomes of Adolescents Compared to Younger Pediatric Patients with Acute Myeloid Leukemia: Do They Need a Special Approach?" Cancers 16, no. 6: 1145. https://doi.org/10.3390/cancers16061145
APA StylePawińska-Wąsikowska, K., Czogała, M., Bukowska-Strakova, K., Surman, M., Rygielska, M., Książek, T., Sadowska, B., Pac, A., Skalska-Sadowska, J., Samborska, M., Wachowiak, J., Ciebiera, M., Chaber, R., Tomaszewska, R., Szczepański, T., Zielezińska, K., Urasiński, T., Moj-Hackemer, M., Kałwak, K., ... Skoczeń, S. (2024). Treatment Outcomes of Adolescents Compared to Younger Pediatric Patients with Acute Myeloid Leukemia: Do They Need a Special Approach? Cancers, 16(6), 1145. https://doi.org/10.3390/cancers16061145






