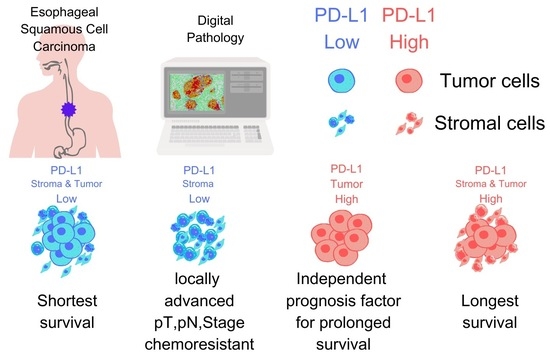
Contrasting Roles of Programmed Death-Ligand 1 Expression in Tumor and Stroma in Prognosis of Esophageal Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Immunohistochemistry
2.3. Image Acquisition and Analysis
2.4. Statistical Analysis
3. Results
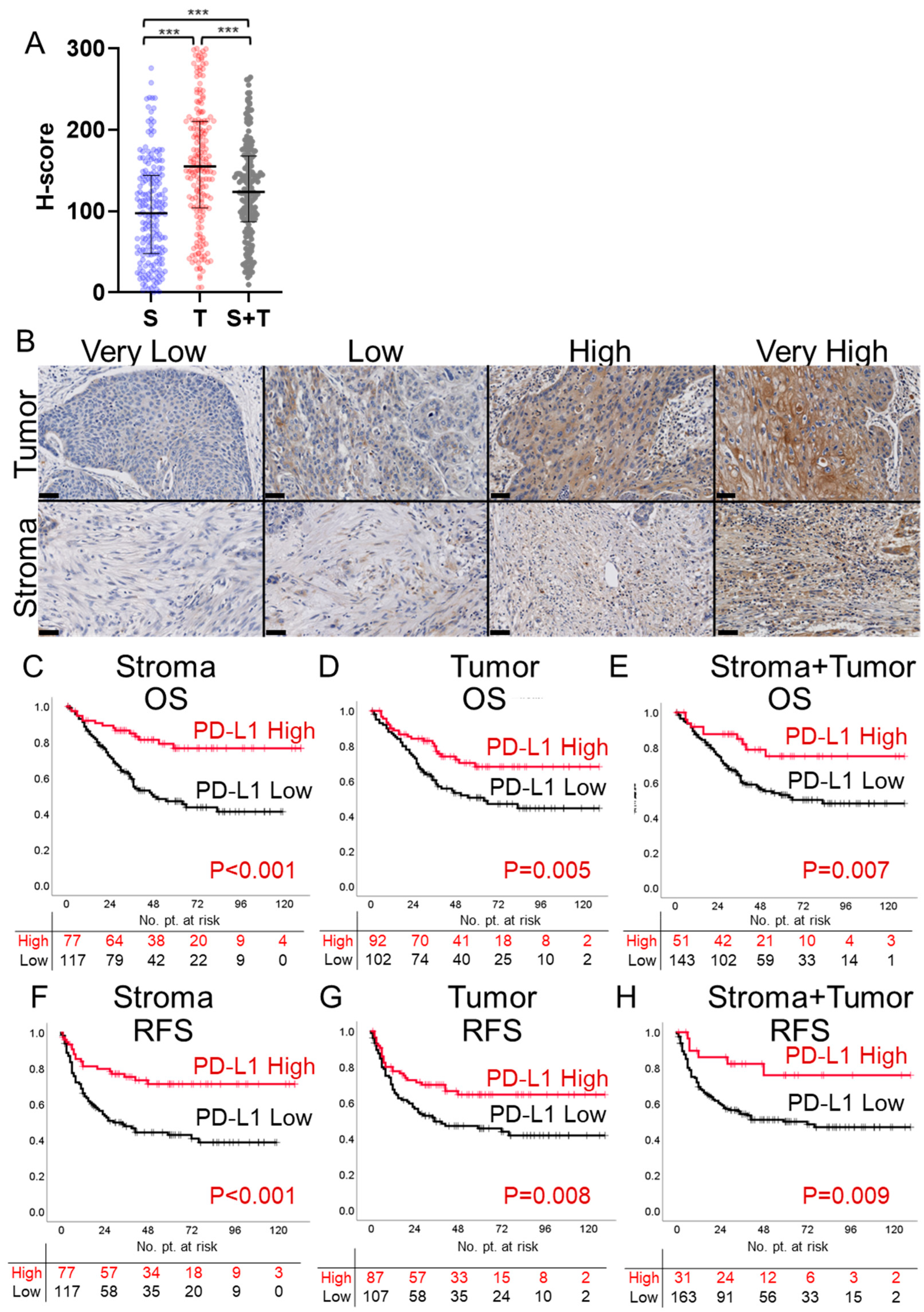
3.1. PD-L1 Expression in Stroma, Tumor, and Stroma + Tumor
3.2. Survival Analysis Based on PD-L1 H-Score
3.3. Multivariate Analysis of Clinicopathological Factors
3.4. Correlation Analysis of PD-L1 Expression and Clinicopathological Factors
3.5. PD-L1 Expression and Its Association with Recurrence Patterns and Metastatic Sites
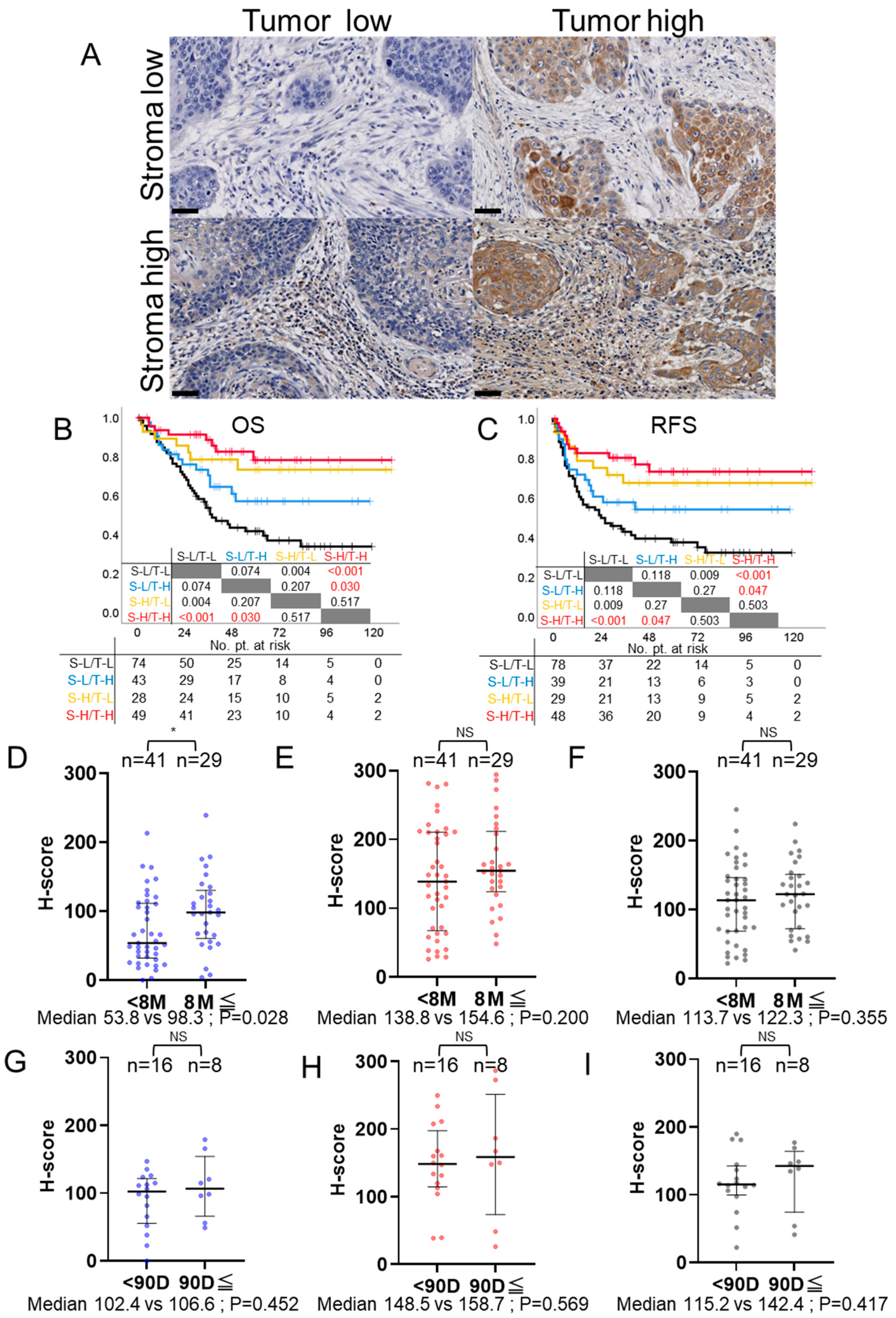
3.6. Comparison of Survival Times Based on PD-L1 Expression Patterns in Stroma and Tumor
3.7. PD-L1 Expression and Post-Recurrence Treatment Efficacy: CTx and Immune Checkpoint Inhibitors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Ishihara, R.; Ishikawa, H.; Ito, Y.; Oyama, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawachi, H.; et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: Part 1. Esophagus 2023, 20, 343–372. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Ishihara, R.; Ishikawa, H.; Ito, Y.; Oyama, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawachi, H.; et al. Esophageal cancer practice guidelines 2022 edited by the Japan Esophageal Society: Part 2. Esophagus 2023, 20, 373–389. [Google Scholar] [CrossRef]
- Obermannová, R.; Alsina, M.; Cervantes, A.; Leong, T.; Lordick, F.; Nilsson, M.; van Grieken, N.C.T.; Vogel, A.; Smyth, E.C. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 992–1004. [Google Scholar] [CrossRef]
- Kajiwara, Y.; Takahashi, A.; Ueno, H.; Kakeji, Y.; Hasegawa, H.; Eguchi, S.; Goi, T.; Saiura, A.; Sasaki, A.; Takiguchi, S.; et al. Annual report on National Clinical Database 2020 for gastroenterological surgery in Japan. Ann. Gastroenterol. Surg. 2023, 7, 367–406. [Google Scholar] [CrossRef]
- Booka, E.; Takeuchi, H.; Morita, Y.; Hiramatsu, Y.; Kikuchi, H. What is the best reconstruction procedure after esophagectomy? A meta-analysis comparing posterior mediastinal and retrosternal approaches. Ann. Gastroenterol. Surg. 2023, 7, 553–564. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, S.; Zhang, L.; Guo, S.; Shen, J.; Li, Q.; Yang, H.; Feng, Y.; Liu, M.; Lin, S.H.; et al. Recurrence Risk Based on Pathologic Stage after Neoadjuvant Chemoradiotherapy in Esophageal Squamous Cell Carcinoma: Implications for Risk-Based Postoperative Surveillance Strategies. Ann. Surg. Oncol. 2018, 25, 3639–3646. [Google Scholar] [CrossRef]
- Kato, K.; Cho, B.C.; Takahashi, M.; Okada, M.; Lin, C.Y.; Chin, K.; Kadowaki, S.; Ahn, M.J.; Hamamoto, Y.; Doki, Y.; et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 1506–1517. [Google Scholar] [CrossRef]
- Kojima, T.; Shah, M.A.; Muro, K.; Francois, E.; Adenis, A.; Hsu, C.H.; Doi, T.; Moriwaki, T.; Kim, S.B.; Lee, S.H.; et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J. Clin. Oncol. 2020, 38, 4138–4148. [Google Scholar] [CrossRef]
- PD-L1 IHC 22C3 pharmDx Interpretation Manual—Esophageal Cancer. Available online: https://www.agilent.com/cs/library/usermanuals/public/29439-d67239-pd-l1-ihc22c3-ec-kn590-int-man-en.pdf (accessed on 25 July 2023).
- McCarty, K.S., Jr.; Szabo, E.; Flowers, J.L.; Cox, E.B.; Leight, G.S.; Miller, L.; Konrath, J.; Soper, J.T.; Budwit, D.A.; Creasman, W.T.; et al. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Res. 1986, 46, 4244s–4248s. [Google Scholar] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Q.; Xu, J.; Zhou, Z.G.; Jin, L.L.; Yu, X.J.; Xiao, G.; Lin, J.; Zhuang, S.M.; Zhang, Y.J.; Zheng, L. Expression patterns of programmed death ligand 1 correlate with different microenvironments and patient prognosis in hepatocellular carcinoma. Br. J. Cancer 2018, 119, 80–88. [Google Scholar] [CrossRef]
- Thompson, E.D.; Zahurak, M.; Murphy, A.; Cornish, T.; Cuka, N.; Abdelfatah, E.; Yang, S.; Duncan, M.; Ahuja, N.; Taube, J.M.; et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut 2017, 66, 794–801. [Google Scholar] [CrossRef]
- Zhai, Q.; Fan, J.; Lin, Q.; Liu, X.; Li, J.; Hong, R.; Wang, S. Tumor stromal type is associated with stromal PD-L1 expression and predicts outcomes in breast cancer. PLoS ONE 2019, 14, e0223325. [Google Scholar] [CrossRef]
- Sumitomo, R.; Hirai, T.; Fujita, M.; Murakami, H.; Otake, Y.; Huang, C.L. PD-L1 expression on tumor-infiltrating immune cells is highly associated with M2 TAM and aggressive malignant potential in patients with resected non-small cell lung cancer. Lung Cancer 2019, 136, 136–144. [Google Scholar] [CrossRef]
- Teramoto, K.; Igarashi, T.; Kataoka, Y.; Ishida, M.; Hanaoka, J.; Sumimoto, H.; Daigo, Y. Clinical significance of PD-L1-positive cancer-associated fibroblasts in pN0M0 non-small cell lung cancer. Lung Cancer 2019, 137, 56–63. [Google Scholar] [CrossRef]
- Wu, X.; Ke, X.; Ni, Y.; Kuang, L.; Zhang, F.; Lin, Y.; Lin, W.; Xiong, X.; Huang, H.; Lin, X.; et al. Tumor-Infiltrating Immune Cells and PD-L1 as Prognostic Biomarkers in Primary Esophageal Small Cell Carcinoma. J. Immunol. Res. 2020, 2020, 8884683. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Y.; Long, Q.; Li, Q.; Tian, J.; Liu, T.; Wu, Y.; Ding, Z. Increased coexpression of PD-L1 and TIM3/TIGIT is associated with poor overall survival of patients with esophageal squamous cell carcinoma. J. Immunother. Cancer 2021, 9, PMC8504357. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, S.; Morita, Y.; Matsumoto, A.; Ida, S.; Muraki, R.; Kitajima, R.; Takeda, M.; Kikuchi, H.; Hiramatsu, Y.; Takeuchi, H. Tenascin C in pancreatic cancer-associated fibroblasts enhances epithelial mesenchymal transition and is associated with resistance to immune checkpoint inhibitor. Am. J. Cancer Res. 2023, 13, 5641–5655. [Google Scholar] [PubMed]
- Okadome, K.; Baba, Y.; Nomoto, D.; Yagi, T.; Kalikawe, R.; Harada, K.; Hiyoshi, Y.; Nagai, Y.; Ishimoto, T.; Iwatsuki, M.; et al. Prognostic and clinical impact of PD-L2 and PD-L1 expression in a cohort of 437 oesophageal cancers. Br. J. Cancer 2020, 122, 1535–1543. [Google Scholar] [CrossRef]
- Jiang, C.; Zhu, Y.; Tang, S.; Zhang, G.; Lin, Q.; Xu, Y.; Shang, J. High PD-L1 expression is associated with a favorable prognosis in patients with esophageal squamous cell carcinoma undergoing postoperative adjuvant radiotherapy. Oncol. Lett. 2019, 17, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, F.; Shao, F.; Wang, P.; Li, Z.; Yang, X.; He, Z.; Shi, S.; Gao, Y.; He, J. PD-L1 expression on tumor cells associated with favorable prognosis in surgically resected esophageal squamous cell carcinoma. Hum. Pathol. 2019, 84, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Watanabe, H.; Hashimura, M.; Matsumoto, T.; Yokoi, A.; Nakagawa, M.; Ishibashi, Y.; Ito, T.; Ohhigata, K.; Saegusa, M. A combination of stromal PD-L1 and tumoral nuclear β-catenin expression as an indicator of colorectal carcinoma progression and resistance to chemoradiotherapy in locally advanced rectal carcinoma. J. Pathol. Clin. Res. 2022, 8, 458–469. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, Z.; Gu, J.; Li, Z.; Xu, X.; Xue, C.; Li, X.; Zhao, L.; Zhou, J.; Bai, C.; et al. Cancer-Associated Fibroblasts Promote the Upregulation of PD-L1 Expression Through Akt Phosphorylation in Colorectal Cancer. Front. Oncol. 2021, 11, 748465. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Ishida, M.; Yanai, H.; Tsuta, K.; Sekimoto, M.; Sugie, T. Prognostic significance of PD-L1-positive cancer-associated fibroblasts in patients with triple-negative breast cancer. BMC Cancer 2021, 21, 239. [Google Scholar] [CrossRef]
- Farlow, J.L.; Brenner, J.C.; Lei, Y.L.; Chinn, S.B. Immune deserts in head and neck squamous cell carcinoma: A review of challenges and opportunities for modulating the tumor immune microenvironment. Oral. Oncol. 2021, 120, 105420. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer–immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef] [PubMed]
| Variables | N | (%) |
|---|---|---|
| Age | ||
| under 70 y.o | 114 | (59%) |
| over and 70 y.o | 80 | (41%) |
| Sex | ||
| male | 169 | (88%) |
| female | 25 | (13%) |
| Tumor Location | ||
| Ce | 4 | (2%) |
| Ut | 22 | (11%) |
| Mt | 95 | (49%) |
| Lt | 72 | (37%) |
| Ae | 1 | (1%) |
| Differentiation | ||
| differentiated | 140 | (73%) |
| include poorly differentiated | 41 | (21%) |
| NA | 13 | (7%) |
| Preoperative treatment | ||
| None | 97 | (50%) |
| Chemotherapy | 87 | (45%) |
| Chemotherapy+Radiation | 10 | (5%) |
| pT | ||
| T1 | 94 | (49%) |
| T2 | 26 | (13%) |
| T3 | 74 | (38%) |
| pN | ||
| N0 | 71 | (37%) |
| N1 | 65 | (34%) |
| N2 | 34 | (18%) |
| N3 | 24 | (12%) |
| Lymphatic invasion | ||
| negative | 70 | (36%) |
| positive | 124 | (64%) |
| Venous invasion | ||
| negative | 55 | (28%) |
| positive | 139 | (72%) |
| Intramural metastasis | ||
| negative | 176 | (91%) |
| positive | 18 | (9%) |
| pStage * | ||
| I | 55 | (28%) |
| II | 43 | (22%) |
| III | 59 | (31%) |
| IVA | 37 | (19%) |
| Total recurrence cases | 85 | (44%) |
| Lymphogenous metastasis | ||
| negative | 16 | (19%) |
| positive | 69 | (81%) |
| Hematogenous metastasis | ||
| negative | 33 | (39%) |
| positive | 52 | (61%) |
| Locoregional recurrence | ||
| negative | 76 | (89%) |
| positive | 9 | (11%) |
| Pleural/peritoneal dissemination | ||
| negative | 66 | (78%) |
| positive | 19 | (22%) |
| Variables | N | Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | ||||
| Age | |||||||||
| under 70 y.o | 114 | 1.00 | 0.48 | 1.30 | 0.355 | ||||
| over and 70 y.o | 80 | 0.79 | |||||||
| Sex | |||||||||
| male | 169 | 1.00 | 0.31 | 1.47 | 0.321 | ||||
| female | 25 | 0.67 | |||||||
| Differentiation | |||||||||
| differentiated | 140 | 1.00 | 0.95 | 2.79 | 0.077 | ||||
| include poorly diff. | 41 | 1.63 | |||||||
| Preoperative treatment | |||||||||
| none | 97 | 1.00 | 1.77 | 4.97 | <0.001 | 1.00 | 0.95 | 3.08 | 0.075 |
| chemotherapy(+/-Radiation) | 97 | 2.97 | 1.71 | ||||||
| pT * | |||||||||
| <T2 | 94 | 1.00 | 2.54 | 7.82 | <0.001 | 1.00 | 0.95 | 3.65 | 0.069 |
| T2 and over | 100 | 4.46 | 1.86 | ||||||
| pN * | |||||||||
| negative | 71 | 1.00 | 2.80 | 12.28 | <0.001 | 1.00 | 1.12 | 6.52 | 0.027 |
| positive | 123 | 5.86 | 2.70 | ||||||
| Lymphatic invasion | |||||||||
| negative | 70 | 1.00 | 1.77 | 6.11 | <0.001 | 1.00 | 0.47 | 2.27 | 0.943 |
| positive | 124 | 3.28 | 1.03 | ||||||
| Venous invasion | |||||||||
| negative | 55 | 1.00 | 1.96 | 9.35 | <0.001 | 1.00 | 0.61 | 3.89 | 0.357 |
| positive | 139 | 4.23 | 1.55 | ||||||
| Intramural metastasis | |||||||||
| negative | 176 | 1.00 | 1.58 | 5.28 | <0.001 | 1.00 | 0.80 | 2.93 | 0.201 |
| positive | 18 | 2.88 | 1.53 | ||||||
| PD-L1 Stroma | |||||||||
| low | 117 | 1.00 | 0.17 | 0.55 | <0.001 | 1.00 | 0.21 | 1.17 | 0.108 |
| high | 77 | 0.30 | 0.49 | ||||||
| PD-L1 Tumor | |||||||||
| low | 102 | 1.00 | 0.30 | 0.82 | 0.006 | 1.00 | 0.27 | 0.83 | 0.010 |
| high | 92 | 0.49 | 0.47 | ||||||
| PD-L1 Stroma+Tumor | |||||||||
| low | 143 | 1.00 | 0.20 | 0.80 | 0.010 | 1.00 | 0.45 | 3.84 | 0.616 |
| high | 51 | 0.40 | 1.32 | ||||||
| Variables | N | Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | ||||
| Age | |||||||||
| under 70 y.o | 114 | 1.00 | 0.52 | 1.26 | 0.344 | ||||
| over and 70 y.o | 80 | 0.81 | |||||||
| Sex | |||||||||
| male | 169 | 1.00 | 0.25 | 1.18 | 0.121 | ||||
| female | 25 | 0.54 | |||||||
| Differentiation | |||||||||
| differentiated | 140 | 1.00 | 1.05 | 2.74 | 0.031 | 1.00 | 0.71 | 1.92 | 0.534 |
| include poorly diff. | 41 | 1.70 | 1.17 | ||||||
| Preoperative treatment | |||||||||
| none | 97 | 1.00 | 1.91 | 4.79 | <0.001 | 1.00 | 0.99 | 2.98 | 0.051 |
| chemotherapy(+/−Radiation) | 97 | 3.03 | 1.72 | ||||||
| pT * | |||||||||
| <T2 | 94 | 1.00 | 2.36 | 6.13 | <0.001 | 1.00 | 0.89 | 2.91 | 0.114 |
| T2 and over | 100 | 3.80 | 1.61 | ||||||
| pN * | |||||||||
| negative | 71 | 1.00 | 2.68 | 9.15 | <0.001 | 1.00 | 1.17 | 5.58 | 0.018 |
| positive | 123 | 4.96 | 2.56 | ||||||
| Lymphatic invasion | |||||||||
| negative | 70 | 1.00 | 1.66 | 4.82 | <0.001 | 1.00 | 0.53 | 2.15 | 0.850 |
| positive | 124 | 2.83 | 1.07 | ||||||
| Venous invasion | |||||||||
| negative | 55 | 1.00 | 1.71 | 5.80 | <0.001 | 1.00 | 0.62 | 3.03 | 0.436 |
| positive | 139 | 3.14 | 1.37 | ||||||
| Intramural metastasis | |||||||||
| negative | 176 | 1.00 | 1.44 | 4.56 | 0.001 | 1.00 | 0.70 | 2.44 | 0.397 |
| positive | 18 | 2.56 | 1.31 | ||||||
| PD-L1 Stroma | |||||||||
| low | 117 | 1.00 | 0.24 | 0.65 | <0.001 | 1.00 | 0.43 | 1.48 | 0.465 |
| high | 77 | 0.39 | 0.79 | ||||||
| PD-L1 Tumor | |||||||||
| low | 108 | 1.00 | 0.35 | 0.87 | 0.010 | 1.00 | 0.32 | 0.91 | 0.022 |
| high | 87 | 0.55 | 0.54 | ||||||
| PD-L1 Stroma+Tumor | |||||||||
| low | 163 | 1.00 | 0.15 | 0.80 | 0.013 | 1.00 | 0.33 | 2.77 | 0.930 |
| high | 31 | 0.35 | 0.95 | ||||||
| Variables | PD-L1 Stroma | PD-L1 Tumor | PD-L1 Stroma + Tumor | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low N = 117 | High N = 77 | p-Value | Low N = 102 | High N = 92 | p-Value | Low N = 143 | High N = 51 | p-Value | |
| Age | |||||||||
| under 70 y.o | 37% | 22% | 0.333 | 31% | 27% | 0.756 | 44% | 14% | 0.514 |
| over and 70 y.o | 23% | 18% | 21% | 20% | 29% | 12% | |||
| Sex | |||||||||
| male | 55% | 32% | 0.074 | 47% | 40% | 0.357 | 65% | 22% | 0.237 |
| female | 6% | 7% | 6% | 7% | 8% | 5% | |||
| Tumor Location | |||||||||
| Ce | 2% | 1% | 0.736 | 2% | 1% | 0.298 | 2% | 1% | 0.498 |
| Ut | 7% | 4% | 7% | 5% | 8% | 4% | |||
| Mt | 29% | 20% | 28% | 21% | 37% | 12% | |||
| Lt | 22% | 15% | 16% | 21% | 27% | 10% | |||
| Ae | 0% | 1% | 0% | 1% | 0% | 1% | |||
| Differentiation | |||||||||
| differentiated | 42% | 30% | 0.701 | 36% | 37% | 0.267 | 53% | 19% | 0.962 |
| include poorly differentiated | 14% | 7% | 12% | 9% | 15% | 6% | |||
| NA | 4% | 3% | 5% | 2% | 5% | 2% | |||
| Preoperative treatment | |||||||||
| None | 28% | 22% | 0.394 | 28% | 22% | 0.538 | 35% | 15% | 0.510 |
| Chemotherapy | 29% | 16% | 22% | 23% | 35% | 10% | |||
| Chemotherapy+Radiation | 4% | 2% | 3% | 2% | 4% | 1% | |||
| pT * | |||||||||
| T1 | 22% | 27% | <0.001 | 25% | 23% | 0.988 | 32% | 16% | 0.051 |
| T2 | 10% | 4% | 7% | 6% | 10% | 3% | |||
| T3 | 29% | 9% | 20% | 18% | 31% | 7% | |||
| pN * | |||||||||
| N0 | 15% | 21% | <0.001 | 18% | 19% | 0.362 | 23% | 14% | 0.041 |
| N1 | 22% | 11% | 21% | 13% | 26% | 7% | |||
| N2 | 13% | 5% | 8% | 9% | 14% | 3% | |||
| N3 | 10% | 3% | 6% | 6% | 10% | 2% | |||
| Lymphatic invasion | |||||||||
| negative | 16% | 20% | <0.001 | 19% | 18% | 0.810 | 24% | 12% | 0.057 |
| positive | 44% | 20% | 34% | 30% | 50% | 14% | |||
| Venous invasion | |||||||||
| negative | 10% | 19% | <0.001 | 13% | 15% | 0.352 | 16% | 12% | 0.002 |
| positive | 51% | 21% | 39% | 32% | 57% | 14% | |||
| Intramural metastasis | |||||||||
| negative | 54% | 37% | 0.278 | 47% | 43% | 0.790 | 67% | 24% | 0.880 |
| positive | 7% | 3% | 5% | 4% | 7% | 3% | |||
| pStage * | |||||||||
| I | 8% | 20% | <0.001 | 14% | 14% | 0.658 | 16% | 12% | 0.013 |
| II | 17% | 5% | 11% | 11% | 17% | 5% | |||
| III | 20% | 10% | 18% | 12% | 24% | 7% | |||
| IVA | 15% | 4% | 10% | 9% | 16% | 3% | |||
| PD-L1 Stroma | PD-L1 Tumor | PD-L1 Stroma + Tumor | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Low N = 65 | High N = 20 | p-Value | Low N = 52 | High N = 33 | p-Value | Low N = 79 | High N = 6 | p-Value |
| Lymphogenous metastasis | |||||||||
| negative | 22% | 10% | 0.248 | 23% | 12% | 0.339 | 20% | 0% | 0.135 |
| positive | 78% | 90% | 77% | 88% | 80% | 100% | |||
| Hematogenous metastasis | |||||||||
| negative | 35% | 50% | 0.241 | 40% | 36% | 0.247 | 38% | 50% | 0.127 |
| positive | 65% | 50% | 60% | 64% | 62% | 50% | |||
| Locoregional recurrence | |||||||||
| negative | 86% | 100% | 0.078 | 88% | 91% | 0.386 | 89% | 100% | 0.289 |
| positive | 14% | 0% | 12% | 9% | 11% | 0% | |||
| Pleural/peritoneal dissemination | |||||||||
| negative | 78% | 75% | 0.745 | 79% | 76% | 0.739 | 78% | 67% | 0.503 |
| positive | 22% | 25% | 21% | 24% | 22% | 33% | |||
| Total recurrence types | |||||||||
| 1 | 45% | 55% | 0.515 | 46% | 48% | 0.139 | 43% | 50% | 0.742 |
| 2 | 37% | 25% | 38% | 27% | 32% | 17% | |||
| 3 | 14% | 20% | 15% | 15% | 13% | 33% | |||
| 4 | 5% | 0% | 0% | 9% | 3% | 0% | |||
| Total metastatic sites | |||||||||
| 1 | 40% | 55% | 0.352 | 44% | 42% | 0.318 | 41% | 50% | 0.115 |
| 2 | 22% | 10% | 25% | 9% | 19% | 0% | |||
| 3 | 23% | 20% | 19% | 27% | 19% | 17% | |||
| 4 | 9% | 5% | 8% | 9% | 8% | 17% | |||
| 5 | 6% | 5% | 4% | 9% | 4% | 17% | |||
| 6 | 0% | 5% | 0% | 3% | 0% | 0% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murakami, T.; Booka, E.; Furuhashi, S.; Sakai, Y.; Sekimori, K.; Haneda, R.; Fujihiro, M.; Matsumoto, T.; Morita, Y.; Kikuchi, H.; et al. Contrasting Roles of Programmed Death-Ligand 1 Expression in Tumor and Stroma in Prognosis of Esophageal Squamous Cell Carcinoma. Cancers 2024, 16, 1135. https://doi.org/10.3390/cancers16061135
Murakami T, Booka E, Furuhashi S, Sakai Y, Sekimori K, Haneda R, Fujihiro M, Matsumoto T, Morita Y, Kikuchi H, et al. Contrasting Roles of Programmed Death-Ligand 1 Expression in Tumor and Stroma in Prognosis of Esophageal Squamous Cell Carcinoma. Cancers. 2024; 16(6):1135. https://doi.org/10.3390/cancers16061135
Chicago/Turabian StyleMurakami, Tomohiro, Eisuke Booka, Satoru Furuhashi, Yuki Sakai, Kenichi Sekimori, Ryoma Haneda, Mayu Fujihiro, Tomohiro Matsumoto, Yoshifumi Morita, Hirotoshi Kikuchi, and et al. 2024. "Contrasting Roles of Programmed Death-Ligand 1 Expression in Tumor and Stroma in Prognosis of Esophageal Squamous Cell Carcinoma" Cancers 16, no. 6: 1135. https://doi.org/10.3390/cancers16061135
APA StyleMurakami, T., Booka, E., Furuhashi, S., Sakai, Y., Sekimori, K., Haneda, R., Fujihiro, M., Matsumoto, T., Morita, Y., Kikuchi, H., Hiramatsu, Y., Baba, S., & Takeuchi, H. (2024). Contrasting Roles of Programmed Death-Ligand 1 Expression in Tumor and Stroma in Prognosis of Esophageal Squamous Cell Carcinoma. Cancers, 16(6), 1135. https://doi.org/10.3390/cancers16061135