A Systematic Review of Diagnostic Modalities and Strategies for the Assessment of Complications in Adult Patients with Neurofibromatosis Type 1
Abstract
Simple Summary
Abstract
1. Introduction
Aims of the Paper
2. Methods
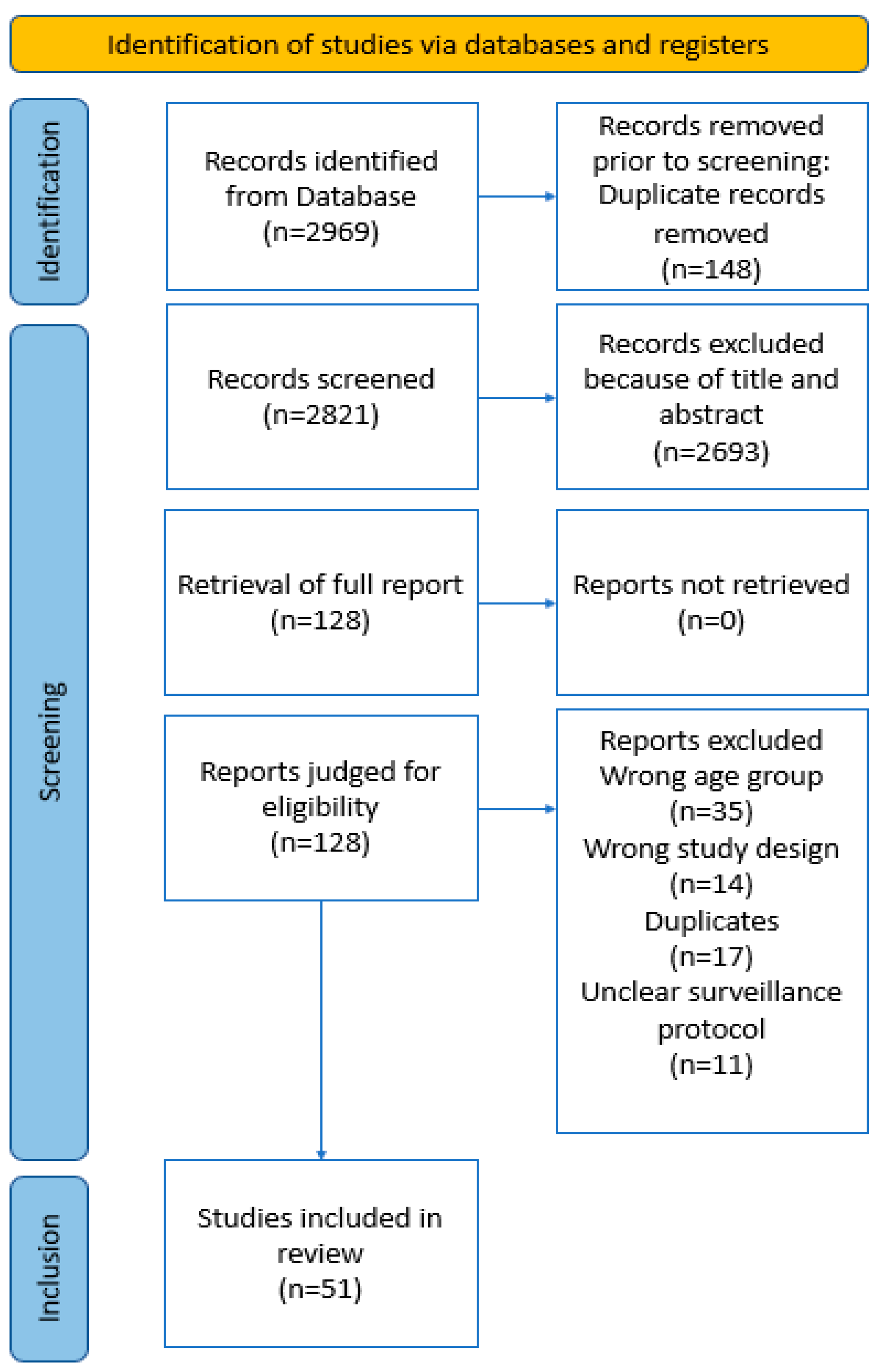
2.1. Protocol and Guidance
2.2. Data Sources and Search Strategy
2.3. Definitions
2.4. Study Selection: Inclusion and Exclusion Criteria
2.5. Data Extraction and Organisation
2.6. Risk of Bias Assessment
3. Results
3.1. Diagnostic Modalities
3.2. Socioeconomic Status
3.3. Outcomes
3.4. Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uusitalo, E.; Leppävirta, J.; Koffert, A.; Suominen, S.; Vahtera, J.; Vahlberg, T.; Pöyhönen, M.; Peltonen, J.; Peltonen, S. Incidence and mortality of neurofibromatosis: A total population study in Finland. J. Investig. Dermatol. 2015, 135, 904–906. [Google Scholar] [CrossRef] [PubMed]
- Legius, E.; Messiaen, L.; Wolkenstein, P.; Pancza, P.; Avery, R.A.; Berman, Y.; Blakeley, J.; Babovic-Vuksanovic, D.; Cunha, K.S.; Ferner, R.; et al. Revised diagnostic criteria for neurofibromatosis type 1 and Legius syndrome: An international consensus recommendation. Genet. Med. 2021, 23, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Neurofibromatosis. Available online: https://www.mayoclinic.org/diseases-conditions/neurofibromatosis/symptoms-causes/syc-20350490 (accessed on 1 March 2023).
- Stewart, D.R.; Korf, B.R.; Nathanson, K.L.; Stevenson, D.A.; Yohay, K. Care of adults with neurofibromatosis type 1: A clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2018, 20, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Carton, C.; Evans, D.G.; Blanco, I.; Friedrich, R.E.; Ferner, R.E.; Farschtschi, S.; Salvador, H.; Azizi, A.A.; Mautner, V.; Röhl, C.; et al. ERN GENTURIS tumour surveillance guidelines for individuals with neurofibromatosis type 1. EClinicalMedicine 2023, 56, 101818. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, C.; Servy, A.; Valeyrie-Allanore, L.; Ferkal, S.; Combemale, P.; Wolkenstein, P.; Adamski, H.; Adamski, H.; Baumann-Morel, C.; Bellanne, C.; et al. Neurofibromatosis 1 French national guidelines based on an extensive literature review since 1966. Orphanet J. Rare Dis. 2020, 15, 23. [Google Scholar] [CrossRef]
- Ferner, R.E.; Huson, S.M.; Thomas, N.; Moss, C.; Willshaw, H.; Evans, D.G.; Upadhyaya, M.; Towers, R.; Gleeson, M.; Steiger, C.; et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J. Med. Genet. 2007, 44, 81–88. [Google Scholar] [CrossRef]
- Kluwe, L.; Nguyen, R.; Vogt, J.; Bengesser, K.; Mussotter, T.; Friedrich, R.E.; Jett, K.; Kehrer-Sawatzki, H.; Mautner, V.F. Internal tumor burden in neurofibromatosis Type I patients with large NF1 deletions. Genes Chromosomes Cancer 2012, 51, 447–451. [Google Scholar] [CrossRef]
- Derlin, T.; Tornquist, K.; Münster, S.; Apostolova, I.; Hagel, C.; Friedrich, R.E.; Wedegärtner, U.; Mautner, V.F. Comparative Effectiveness of 18F-FDG PET/CT Versus Whole-Body MRI for Detection of Malignant Peripheral Nerve Sheath Tumors in Neurofibromatosis Type 1. Clin. Nucl. Med. 2013, 38, e19–e25. [Google Scholar] [CrossRef]
- Herness, D.; Posner, M.A.; Steiner, G. Malignant schwannoma. Hand 1975, 7, 300–302. [Google Scholar] [CrossRef]
- Hilabi, B.S.; Alghamdi, S.A.; Almanaa, M. Impact of Magnetic Resonance Imaging on Healthcare in Low- and Middle-Income Countries. Cureus 2023, 15, e37698. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- World Bank Country and Lending Groups. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 1 March 2023).
- GNI per Capita, Atlas Method (Current US$). Available online: https://data.worldbank.org/indicator/NY.GNP.PCAP.CD (accessed on 1 March 2023).
- The Joanna Briggs Institute. The Joanna Briggs Institute Critical Appraisal Tools for Use in JBI Systematic Reviews Checklist for Systematic Reviews and Research Syntheses; Joanna Briggs Institute: Adelaide, SA, Australia, 2017. [Google Scholar]
- Debnam, J.M.; Mahfouz, Y.M.M.; Ketonen, L.; Slopis, J.M.; McCutcheon, I.E.; Guha-Thakurta, N. Multidetector CT with 3-dimensional volume rendering in the evaluation of the spine in patients with Neurofibromatosis type 1: A retrospective review in 73 patients. Scoliosis 2014, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Alkan, A.; Sigirci, A.; Kutlu, R.; Ozcan, H.; Erdem, G.; Aslan, M.; Ates, O.; Yakinci, C.; Egri, M. Neurofibromatosis type 1: Diffusion weighted imaging findings of brain. Eur. J. Radiol. 2005, 56, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Kassarjian, A.; Bredella, M.A.; Harris, G.J.; Yoshida, H.; Mautner, V.F.; Wenzel, R.; Plotkin, S.R. Tumor burden in patients with neurofibromatosis types 1 and 2 and schwannomatosis: Determination on whole-body MR images. Radiology 2009, 250, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, R.; Denecke, J.; Grenzebach, M.; Schuierer, G.; Weglage, J. Neurofibromatosis type 1: Motor and cognitive function and T2-weighted MRI hyperintensities. Neurology 2003, 61, 1725–1728. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.E.; Kluwe, L.; Fünsterer, C.; Mautner, V.F. Malignant peripheral nerve sheath tumors (MPNST) in neurofibromatosis type 1 (NF1): Diagnostic findings on magnetic resonance images and mutation analysis of the NF1 gene. Anticancer Res. 2005, 25, 1699–1702. [Google Scholar] [PubMed]
- Mautner, V.F.; Hartmann, M.; Kluwe, L.; Friedrich, R.E.; Fünsterer, C. MRI growth patterns of plexiform neurofibromas in patients with neurofibromatosis type 1. Neuroradiology 2006, 48, 160–165. [Google Scholar] [CrossRef]
- Sellmer, L.; Farschtschi, S.; Marangoni, M.; Heran, M.K.S.; Birch, P.; Wenzel, R.; Friedman, J.M.; Mautner, V.F. Non-optic glioma in adults and children with neurofibromatosis 1. Orphanet J. Rare Dis. 2017, 12, 34. [Google Scholar] [CrossRef]
- Jaremko, J.L.; MacMahon, P.J.; Torriani, M.; Merker, V.L.; Mautner, V.F.; Plotkin, S.R.; Bredella, M.A. Whole-body MRI in neurofibromatosis: Incidental findings and prevalence of scoliosis. Skelet. Radiol. 2012, 41, 917–923. [Google Scholar] [CrossRef]
- Koike, H.; Nishida, Y.; Ito, S.; Shimoyama, Y.; Ikuta, K.; Urakawa, H.; Sakai, T.; Shimizu, K.; Ito, K.; Imagama, S. Diffusion-Weighted Magnetic Resonance Imaging Improves the Accuracy of Differentiation of Benign from Malignant Peripheral Nerve Sheath Tumors. World Neurosurg. 2022, 157, e207–e214. [Google Scholar] [CrossRef]
- Matsumine, A.; Kusuzaki, K.; Nakamura, T.; Nakazora, S.; Niimi, R.; Matsubara, T.; Uchida, K.; Murata, T.; Kudawara, I.; Ueda, T.; et al. Differentiation between neurofibromas and malignant peripheral nerve sheath tumors in neurofibromatosis 1 evaluated by MRI. J. Cancer Res. Clin. Oncol. 2009, 135, 891–900. [Google Scholar] [CrossRef]
- Afridi, S.K.; Leschziner, G.D.; Ferner, R.E. Prevalence and clinical presentation of headache in a National Neurofibromatosis 1 Service and impact on quality of life. Am. J. Med. Genet. Part A 2015, 167, 2282–2285. [Google Scholar] [CrossRef]
- Chhabra, A.; Soldatos, T.; Durand, D.J.; Carrino, J.A.; McCarthy, E.F.; Belzberg, A.J. The role of magnetic resonance imaging in the diagnostic evaluation of malignant peripheral nerve sheath tumors. Indian. J. Cancer 2011, 48, 328–334. [Google Scholar] [CrossRef]
- Sellmer, L.; Farschtschi, S.; Marangoni, M.; Heran, M.K.S.; Birch, P.; Wenzel, R.; Mautner, V.F.; Friedman, J.M. Serial MRIs provide novel insight into natural history of optic pathway gliomas in patients with neurofibromatosis 1. Orphanet J. Rare Dis. 2018, 13, 9. [Google Scholar] [CrossRef]
- Curtis-Lopez, C.M.; Soh, C.; Ealing, J.; Evans, D.G.; Wright, E.; Vassallo, G.; Karabatsou, K.; George, K.J. Clinical and neuroradiological characterisation of spinal lesions in adults with Neurofibromatosis type 1. J. Clin. Neurosci. 2020, 77, 98–105. [Google Scholar] [CrossRef]
- Sheerin, U.M.; Holmes, P.; Childs, L.; Roy, A.; Ferner, R.E. Neurovascular complications in adults with Neurofibromatosis type 1: A national referral center experience. Am. J. Med. Genet. A 2022, 188, 3009–3015. [Google Scholar] [CrossRef]
- Well, L.; Salamon, J.; Kaul, M.G.; Farschtschi, S.; Herrmann, J.; Geier, K.I.; Hagel, C.; Bockhorn, M.; Bannas, P.; Adam, G.; et al. Differentiation of peripheral nerve sheath tumors in patients with neurofibromatosis type 1 using diffusion-weighted magnetic resonance imaging. Neuro Oncol. 2019, 21, 508–516. [Google Scholar] [CrossRef]
- Ramachandran, M.; Tsirikos, T.I.; Lee, J.; Saifuddin, A. Whole-spine magnetic resonance imaging in patients with neurofibromatosis type I and spinal deformity. J. Spinal Disord. Tech. 2004, 17, 483–491. [Google Scholar] [CrossRef]
- Pecoraro, A.; Arehart, E.; Gallentine, W.; Radtke, R.; Smith, E.; Pizoli, C.; Kansagra, S.; Abdelnour, E.; McLendon, R.; Mikati, M.A. Epilepsy in neurofibromatosis type 1. Epilepsy Behav. 2017, 73, 137–141. [Google Scholar] [CrossRef]
- Heffler, M.A.; Le, L.Q.; Xi, Y.; Chhabra, A. Tumor segmentation of whole-body magnetic resonance imaging in neurofibromatosis type 1 patients: Tumor burden correlates. Skelet. Radiol. 2017, 46, 93–99. [Google Scholar] [CrossRef]
- Tucker, T.; Wolkenstein, P.; Revuz, J.; Zeller, J.; Friedman, J.M. Association between benign and malignant peripheral nerve sheath tumors in NF1. Neurology 2005, 65, 205–211. [Google Scholar] [CrossRef]
- Ly, K.I.; Merker, V.L.; Cai, W.; Bredella, M.A.; Muzikansky, A.; Thalheimer, R.D.; Da, J.L.; Orr, C.C.; Herr, H.P.; Morris, M.E.; et al. Ten-Year Follow-up of Internal Neurofibroma Growth Behavior in Adult Patients with Neurofibromatosis Type 1 Using Whole-Body MRI. Neurology 2023, 100, e661–e670. [Google Scholar] [CrossRef]
- Well, L.; Jaeger, A.; Kehrer-Sawatzki, H.; Farschtschi, S.; Avanesov, M.; Sauer, M.; de Sousa, M.T.; Bannas, P.; Derlin, T.; Adam, G.; et al. The effect of pregnancy on growth-dynamics of neurofibromas in Neurofibromatosis type 1. PLoS ONE 2020, 15, e0232031. [Google Scholar] [CrossRef]
- Van Meerbeeck, S.F.; Verstraete, K.L.; Janssens, S.; Mortier, G. Whole body MR imaging in neurofibromatosis type 1. Eur. J. Radiol. 2009, 69, 236–242. [Google Scholar] [CrossRef]
- Well, L.; Careddu, A.; Stark, M.; Farschtschi, S.; Bannas, P.; Adam, G.; Mautner, V.F.; Salamon, J. Phenotyping spinal abnormalities in patients with Neurofibromatosis type 1 using whole-body MRI. Sci. Rep. 2021, 11, 16889. [Google Scholar] [CrossRef]
- Mautner, V.F.; Asuagbor, F.A.; Dombi, E.; Fünsterer, C.; Kluwe, L.; Wenzel, R.; Widemann, B.C.; Friedman, J.M. Assessment of benign tumor burden by whole-body MRI in patients with neurofibromatosis 1. Neuro Oncol. 2008, 10, 593–598. [Google Scholar] [CrossRef]
- Plotkin, S.R.; Bredella, M.A.; Cai, W.; Kassarjian, A.; Harris, G.J.; Esparza, S.; Merker, V.L.; Munn, L.L.; Muzikansky, A.; Askenazi, M.; et al. Quantitative assessment of whole-body tumor burden in adult patients with neurofibromatosis. PLoS ONE 2012, 7, e35711. [Google Scholar] [CrossRef]
- Zhang, L.; Dessouky, R.; Xi, Y.; Chhabra, A.; Le, L.Q. Clinical Value of Multiparametric Whole-Body Magnetic Resonance Imaging over Whole-Spine Magnetic Resonance Imaging in Patients with Neurofibromatosis Type I. World Neurosurg. 2017, 108, 729–737. [Google Scholar] [CrossRef]
- Byrne, S.; Connor, S.; Lascelles, K.; Siddiqui, A.; Hargrave, D.; Ferner, R.E. Clinical presentation and prognostic indicators in 100 adults and children with neurofibromatosis 1 associated non-optic pathway brain gliomas. J. Neuro Oncol. 2017, 133, 609–614. [Google Scholar] [CrossRef]
- Guillamo, J.S.; Creange, A.; Kalifa, C.; Grill, J.; Rodriguez, D.; Doz, F.; Barbarot, S.; Zerah, M.; Sanson, M.; Bastuji-Garin, S.; et al. Prognostic factors of CNS tumours in Neurofibromatosis 1 (NF1). Brain 2003, 126, 152–160. [Google Scholar] [CrossRef]
- Avanesov, M.; Well, L.; Laqmani, A.; Derlin, T.; Riccardi, V.M.; Adam, G.; Mautner, V.-F.; Salamon, J. Structural alteration of lung parenchyma in patients with NF1: A phenotyping study using multidetector computed tomography (MDCT). Orphanet J. Rare Dis. 2021, 16, 29. [Google Scholar] [CrossRef]
- Brahmi, M.; Thiesse, P.; Ranchere, D.; Mognetti, T.; Pinson, S.; Renard, C.; Decouvelaere, A.V.; Blay, J.Y.; Combemale, P. Diagnostic Accuracy of PET/CT-Guided Percutaneous Biopsies for Malignant Peripheral Nerve Sheath Tumors in Neurofibromatosis Type 1 Patients. PLoS ONE 2015, 10, e0138386. [Google Scholar] [CrossRef]
- Brenner, W.; Friedrich, R.E.; Gawad, K.A.; Hagel, C.; von Deimling, A.; de Wit, M.; Buchert, R.; Clausen, M.; Mautner, V.F. Prognostic relevance of FDG PET in patients with neurofibromatosis type-1 and malignant peripheral nerve sheath tumours. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 428–432. [Google Scholar] [CrossRef]
- Chirindel, A.; Chaudhry, M.; Blakeley, J.O.; Wahl, R. 18F-FDG PET/CT qualitative and quantitative evaluation in neurofibromatosis type 1 patients for detection of malignant transformation: Comparison of early to delayed imaging with and without liver activity normalization. J. Nucl. Med. 2015, 56, 379–385. [Google Scholar] [CrossRef]
- Cook, G.J.R.; Lovat, E.; Siddique, M.; Goh, V.; Ferner, R.; Warbey, V.S. Characterisation of malignant peripheral nerve sheath tumours in neurofibromatosis-1 using heterogeneity analysis of (18)F-FDG PET. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1845–1852. [Google Scholar] [CrossRef]
- Salamon, J.; Derlin, T.; Bannas, P.; Busch, J.D.; Herrmann, J.; Bockhorn, M.; Hagel, C.; Friedrich, R.E.; Adam, G.; Mautner, V.F. Evaluation of intratumoural heterogeneity on ¹⁸F-FDG PET/CT for characterization of peripheral nerve sheath tumours in neurofibromatosis type 1. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 685–692. [Google Scholar] [CrossRef]
- Salamon, J.; Mautner, V.F.; Adam, G.; Derlin, T. Multimodal Imaging in Neurofibromatosis Type 1-associated Nerve Sheath Tumors. Rofo 2015, 187, 1084–1092. [Google Scholar] [CrossRef]
- Salamon, J.; Veldhoen, S.; Apostolova, I.; Bannas, P.; Yamamura, J.; Herrmann, J.; Friedrich, R.E.; Adam, G.; Mautner, V.F.; Derlin, T. 18F-FDG PET/CT for detection of malignant peripheral nerve sheath tumours in neurofibromatosis type 1: Tumour-to-liver ratio is superior to an SUVmax cut-off. Eur. Radiol. 2014, 24, 405–412. [Google Scholar] [CrossRef]
- Van Der Gucht, A.; Zehou, O.; Djelbani-Ahmed, S.; Valeyrie-Allanore, L.; Ortonne, N.; Brugieres, P.; Wolkenstein, P.; Luciani, A.; Rahmouni, A.; Sbidian, E.; et al. Metabolic Tumour Burden Measured by F-18-FDG PET/CT Predicts Malignant Transformation in Patients with Neurofibromatosis Type-1. PLoS ONE 2016, 11, e0151809. [Google Scholar] [CrossRef]
- Bredella, M.A.; Torriani, M.; Hornicek, F.; Ouellette, H.A.; Palmer, W.E.; Williams, Z.; Fischman, A.J.; Plotkin, S.R. Value of PET in the assessment of patients with neurofibromatosis type I. Am. J. Roentgenol. 2007, 189, 928–935. [Google Scholar] [CrossRef]
- Nishida, Y.; Ikuta, K.; Ito, S.; Urakawa, H.; Sakai, T.; Koike, H.; Ito, K.; Imagama, S. Limitations and benefits of FDG-PET/CT in NF1 patients with nerve sheath tumors: A cross-sectional/longitudinal study. Cancer Sci. 2021, 112, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Combemale, P.; Valeyrie-Allanore, L.; Giammarile, F.; Pinson, S.; Guillot, B.; Goulart, D.M.; Wolkenstein, P.; Blay, J.Y.; Mognetti, T. Utility of 18F-FDG PET with a semi-quantitative index in the detection of sarcomatous transformation in patients with neurofibromatosis type 1. PLoS ONE 2014, 9, e85954. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahlawat, S.; Blakeley, J.O.; Rodriguez, F.J.; Fayad, L.M. Imaging biomarkers for malignant peripheral nerve sheath tumors in neurofibromatosis type 1. Neurology 2019, 93, e1076–e1084. [Google Scholar] [CrossRef] [PubMed]
- Ferner, R.E.; Lucas, J.D.; O’Doherty, M.J.; Hughes, R.A.; Smith, M.A.; Cronin, B.F.; Bingham, J. Evaluation of (18)fluorodeoxyglucose positron emission tomography ((18)FDG PET) in the detection of malignant peripheral nerve sheath tumours arising from within plexiform neurofibromas in neurofibromatosis 1. J. Neurol. Neurosurg. Psychiatry 2000, 68, 353–357. [Google Scholar] [CrossRef]
- Broski, S.M.; Johnson, G.B.; Howe, B.M.; Nathan, M.A.; Wenger, D.E.; Spinner, R.J.; Amrami, K.K. Evaluation of F-18-FDG PET and MRI in differentiating benign and malignant peripheral nerve sheath tumors. Skelet. Radiol. 2016, 45, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Seitz, S.; Schnabel, C.; Busse, B.; Schmidt, H.U.; Beil, F.T.; Friedrich, R.E.; Schinke, T.; Mautner, V.F.; Amling, M. High bone turnover and accumulation of osteoid in patients with neurofibromatosis 1. Osteoporos. Int. 2010, 21, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Heervä, E.; Leinonen, P.; Kuorilehto, T.; Peltonen, S.; Pöyhönen, M.; Väänänen, K.; Peltonen, J. Neurofibromatosis 1-Related Osteopenia Often Progresses to Osteoporosis in 12 Years. Calcif. Tissue Int. 2013, 92, 23–27. [Google Scholar] [CrossRef]
- Modica, R.; Altieri, B.; D’Aniello, F.; Benevento, E.; Cannavale, G.; Minotta, R.; Liccardi, A.; Colao, A.; Faggiano, A. Vitamin D and Bone Metabolism in Adult Patients with Neurofibromatosis Type 1. Metabolites 2023, 13, 255. [Google Scholar] [CrossRef]
- Arigon, V.; Binaghi, M.; Sabouret, C.; Zeller, J.; Revuz, J.; Soubrane, G.; Wolkenstein, P. Usefulness of systematic ophthalmologic investigations in neurofibromatosis 1: A cross-sectional study of 211 patients. Eur. J. Ophthalmol. 2002, 12, 413–418. [Google Scholar] [CrossRef]
- Khosrotehrani, K.; Bastuji-Garin, S.; Zeller, J.; Revuz, J.; Wolkenstein, P. Clinical risk factors for mortality in patients with neurofibromatosis 1: A cohort study of 378 patients. Arch. Dermatol. 2003, 139, 187–191. [Google Scholar] [CrossRef]
- Nishida, T.; Tsujimoto, M.; Takahashi, T.; Hirota, S.; Blay, J.Y.; Wataya-Kaneda, M. Gastrointestinal stromal tumors in Japanese patients with neurofibromatosis type I. J. Gastroenterol. 2016, 51, 571–578. [Google Scholar] [CrossRef]
- Drappier, J.C.; Khosrotehrani, K.; Zeller, J.; Revuz, J.; Wolkenstein, P. Medical management of neurofibromatosis 1: A cross-sectional study of 383 patients. J. Am. Acad. Dermatol. 2003, 49, 440–444. [Google Scholar] [CrossRef]
- Salamon, J.; Papp, L.; Tóth, Z.; Laqmani, A.; Apostolova, I.; Adam, G.; Mautner, V.F.; Derlin, T. Nerve Sheath Tumors in Neurofibromatosis Type 1: Assessment of Whole-Body Metabolic Tumor Burden Using F-18-FDG PET/CT. PLoS ONE 2015, 10, e0143305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Available online: https://data.oecd.org/healtheqt/magnetic-resonance-imaging-mri-units.htm (accessed on 1 March 2023).
- Angell, M. Privatizing health care is not the answer: Lessons from the United States. CMAJ 2008, 179, 916–919. [Google Scholar] [CrossRef][Green Version]
- Rosso, R.J. U.S. Health Care Coverage and Spending. 2023. Available online: https://crsreports.congress.gov/product/details?prodcode=IF10830 (accessed on 1 March 2023).
- Berenson, R.A.; Rich, E.C. US approaches to physician payment: The deconstruction of primary care. J. Gen. Intern. Med. 2010, 25, 613–618. [Google Scholar] [CrossRef]
- Tikkanen, R.; Osborn, B.; Mossialos, E.; Djordjevic, A.; Wharton, G.A. International Health Care System Profiles France. Available online: https://www.commonwealthfund.org/international-health-policy-center/countries/france (accessed on 1 March 2023).
- Chang, J.; Peysakhovich, F.; Wang, W.; Zhu, J. The UK Health Care System. Available online: http://assets.ce.columbia.edu/pdf/actu/actu-uk.pdf (accessed on 1 March 2023).
- Lynch, C.; Reguilon, I.; Langer, D.L.; Lane, D.; De, P.; Wong, W.-L.; McKiddie, F.; Ross, A.; Shack, L.; Win, T.; et al. A comparative analysis: International variation in PET-CT service provision in oncology—An International Cancer Benchmarking Partnership study. Int. J. Qual. Health Care 2021, 33, mzaa166. [Google Scholar] [CrossRef]
- Dreger, M.; Langhoff, H.; Henschke, C. Adoption of large-scale medical equipment: The impact of competition in the German inpatient sector. Eur. J. Health Econ. 2022, 23, 791–805. [Google Scholar] [CrossRef]
- Batista, P.B.; Bertollo, E.M.; Costa Dde, S.; Eliam, L.; Cunha, K.S.; Cunha-Melo, J.R.; Darrigo Junior, L.G.; Geller, M.; Gianordoli-Nascimento, I.F.; Madeira, L.G.; et al. Neurofibromatosis: Part 2—Clinical management. Arq. Neuropsiquiatr. 2015, 73, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Virk, J.; Holmes, D. Radiology in Brazil: A Country Report. J. Glob. Radiol. 2023, 9. [Google Scholar] [CrossRef]
- Darrigo Junior, L.G.; Ferraz, V.E.F.; Cormedi, M.C.V.; Araujo, L.H.H.; Magalhães, M.P.S.; Carneiro, R.C.; Sales, L.H.N.; Suchmacher, M.; Cunha, K.S.; Filho, A.B.; et al. Epidemiological profile and clinical characteristics of 491 Brazilian patients with neurofibromatosis type 1. Brain Behav. 2022, 12, e2599. [Google Scholar] [CrossRef]
- Frija, G.; Blažić, I.; Frush, D.P.; Hierath, M.; Kawooya, M.; Donoso-Bach, L.; Brkljačić, B. How to improve access to medical imaging in low- and middle-income countries? EClinicalMedicine 2021, 38, 101034. [Google Scholar] [CrossRef] [PubMed]
- Karia, A.; Zamani, R.; Akrami, M. Socio-Economic Disparities in Access to Diagnostic Neuroimaging Services in the United Kingdom: A Systematic Review. Int. J. Env. Res. Public. Health 2021, 18, 10633. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Country (GNI per Capita) | Other Modalities Included | Age (Mean (SD)) | Number of Subjects | Pathology | Study Methodology and Characteristics | |
|---|---|---|---|---|---|---|---|---|
| Studies Involving MRI as a Modality | ||||||||
| 1 | Debnam [16] | 2014 | United States (>USD 13,205) | MDCT, plain radiographs | 41 (16) | 73 | Spinal abnormalities | A cohort study involving NF1 patients who underwent MDCT scans for diagnostic, preoperative, or postoperative evaluation of spinal abnormalities. The study aimed to investigate how MDCT could be used for this patient population. |
| 2 | Alkan [17] | 2005 | Turkey (USD 4256 to USD 13,205) | - | 23 (15) | 30 | UBOs | A case-control study of NF1 patients who underwent MRI scans. The study aimed to evaluate the differences in ADC values between infra- and supratentorial UBOs in NF1 patient and control groups and to investigate the correlation between age and ADC values. |
| 3 | Cai [18] | 2009 | United States (>USD 13,205) | - | 42 (15) | 28 | Tumour burden | A cohort study of NF1 patients who underwent MRI scans. The study aimed to develop a 3D segmentation and computerised volumetry modality for use in the assessment of neurofibromatosis and to assess the ability of this modality to aid in the calculation of tumour burden in NF1 patients. |
| 4 | Feldmann [19] | 2003 | Germany (>USD 13,205) | - | 16 (9) | 200 | Motor and cognitive function | A case-control study of NF1 patients and age/sex/socioeconomic status-matched controls who underwent MRI scans of the brain. The study aimed to examine intelligence and school career, motor performance, and cranial MRI in a large series of 100 children and young adults with NF1. |
| 5 | Friedrich [20] | 2005 | Germany (>USD 13,205) | Ultrasound, physical examination (ophthalmological and neurological) | 34 (12) | 10 | MPNST | A cohort study of NF1 patients who underwent brain MRI, ultrasound, and physical examinations. The study aimed to compare the mutation type and MR imaging characteristics of MPNSTs. |
| 6 | Mautner [21] | 2006 | Germany (>USD 13,205) | Physical examination (dermatological, ophthalmological and neurological) | 28 (−) | 202 | PNFs | A cohort study of NF1 patients who underwent MRI and physical examinations. The study aimed to characterise growth patterns of PNFs and associated disfigurement and functional deficits. |
| 7 | Sellmer [22] | 2017 | Germany (>USD 13,205) | - | NR | 562 | Non-optic gliomas | A cohort study of NF1 patients who underwent brain MRIs. The study aimed to investigate progression, spontaneous regression, and the natural history of non-optic gliomas in adults and compare these findings to the results found in children. |
| 8 | Jaremko [23] | 2012 | United States (>USD 13,205) | - | 41 (−) | 141 | Scoliosis and other incidental findings | A cohort study of NF1 patients who underwent WBMRI. The study aimed to demonstrate incidental findings and scoliosis on WBMRI in patients with NF1. |
| 9 | Koike [24] | 2022 | Japan (>USD 13,205) | - | 35 (16) | 30 | MPNST | A cohort study of NF1 patients with PNFs or MPNSTs who underwent MRI. The study aimed to investigate the usefulness of DWI in differentiating PNFs and MPNST in NF1 patients. |
| 10 | Matsumine [25] | 2009 | Japan (>USD 13,205) | - | 43 (−) | 37 | MPNST | A cohort study of NF1 patients with neurogenic tumours in the extremities or on the trunk who underwent MRI. The study aimed to define the criteria for the differential diagnosis between NF and MPNST on MRI in NF1. |
| 11 | Afridi [26] | 2015 | United Kingdom (>USD 13,205) | Blood pressure measurement, physical examination (neurological) | 39 (10) | 115 | Headache | A cohort study of NF1 patients who received blood pressure measurements, neurological examinations, and MRI scans. The study aimed to characterise the phenotype and prevalence of headaches in NF1 patients and determine the quality-of-life impact. |
| 12 | Chhabra [27] | 2011 | United States (>USD 13,205) | - | 37 (18) | 56 | MPNST | A cohort study of NF1 patients with diagnoses of MPNST/BPNSTs who underwent MRI scans. The study aimed to determine the potential differentiating MRI features between NF-MPNSTs and non-NF-MPNSTs and between BPNSTs and MPNSTs. |
| 13 | Sellmer [28] | 2018 | Germany (>USD 13,205) | - | NR | 562 | Optic pathway gliomas | A cohort study of NF1 patients who were offered WB and head MRIs. The study aimed to determine the natural history of OPGs in children and adults with NF1. |
| 14 | Curtis-Lopez [29] | 2020 | United Kingdom (>USD 13,205) | Plain radiograph | 38 (−) | 303 | Spinal lesions | A cohort study of NF1 patients with radiological evidence of spinal lesions seen on MRI spine or spinal X-ray. The study aimed to report the prevalence of different spinal lesions and attempt comparisons between the spinal phenotype and classic NF1 groups. |
| 15 | Sheerin [30] | 2022 | United Kingdom (>USD 13,205) | - | NR | 2068 | Neurovascular complications | A cohort study of NF1 patients who received cranial MRI imaging or were diagnosed with various neurovascular complications. The study aimed to assess the frequency and clinical and imaging spectrum of vascular complications in an adult cohort of NF1 patients. |
| 16 | Salamon [31] | 2019 | Germany (>USD 13,205) | - | 34 (−) | 26 | MPNST | A cohort study of NF1 patients with clinical suspicion of MPNST who received DW-MRI. The study aimed to determine the value of DW-MRI for the characterisation of BPNSTs and MPNSTs in NF1 patients. |
| 17 | Ramachandran [32] | 2004 | United Kingdom (>USD 13,205) | Plain radiograph | 16 (−) | 27 | Spinal deformity | A cohort study of NF1 patients who underwent WSMRI and plain radiographs. The study aimed to determine the role of whole-spine MRI in the classification and management of patients with NF-1 and spinal deformity. |
| 18 | Pecoraro [33] | 2017 | United States (>USD 13,205) | - | 18 (16) | 184 | Epilepsy | A cohort study of NF1 patients with epilepsy/epilepsy syndromes who underwent MRI. The study aimed to describe the characteristics of epilepsy in patients with NF1. |
| 19 | Heffler [34] | 2017 | United States (>USD 13,205) | - | 42 (14) | 15 | Tumour burden | A cohort study of NF1 patients who underwent WBMRI with segmentation. The study aimed to perform segmentation of WBMRI to assess the feasibility and quantitate the total tumour volume (tumour burden) in NF1 patients and examine associations with demographic, disease-related and anthropomorphic features. |
| 20 | Tucker [35] | 2005 | France (>USD 13,205) | Physical examination (ophthalmologic), plain radiograph, ultrasound, CT, 24 h urinary catecholamine analysis | 35 (13) | 476 | MPNST | A cohort study of NF1 patients who underwent routine clinical assessment. The study aimed to determine whether NF1 patients who have benign neurofibromas of various kinds are at greater risk of developing MPNSTs than patients with NF1 who lack these benign tumours. |
| 21 | Ly [36] | 2023 | United States (>USD 13,205) | - | 43 (12) | 47 | Internal neurofibromas | A cohort study of NF1 patients with a baseline WBMRI who underwent a follow-up WBMRI. The study aimed to evaluate the long-term growth behaviour of internal neurofibromas in adults with NF1. |
| 22 | Well [37] | 2020 | United States (>USD 13,205) | - | 25 (6) | 13 | Neurofibroma growth during pregnancy | A case-control study of pregnant NF1 patients and an age-matched female control group. The study aimed to quantify the growth of cutaneous and plexiform neurofibromas in NF1 patients during pregnancy and to assess NF1-related clinical symptoms. |
| 23 | Van Meerbeeck [38] | 2009 | Belgium (>USD 13,205) | - | 37 (11) | 24 | Neurofibromas | A cohort study of NF1 patients who underwent WBMRI. The study aimed to assess the value of WBMRI in NF1 patients. |
| 24 | Well [39] | 2021 | Germany (>USD 13,205) | - | 27 (−) | 537 | Spinal abnormalities | A case-control study of NF1 patients and age/sex-matched non-NF1 patients who underwent WBMRI. The study aimed to quantify the prevalence of spinal abnormalities in NF1 patients, associate the co-appearance of spinal abnormalities with both NF1 and clinical symptoms, and investigate whether different mutations of the NF1 gene affect the prevalence of these abnormalities. |
| 25 | Mautner [40] | 2008 | France (>USD 13,205) | - | 31 (18) | 39 | MPNST | A case-control study of NF1 patients with MPNSTs and controls without MPNST who underwent WBMRI. The study aimed to evaluate the relationship of the total body burden of internal neurofibromas to MPNSTs. |
| 26 | Plotkin [41] | 2012 | United States (>USD 13,205) | - | 39 (−) | 247 | Internal tumour burden | A cohort study of neurofibromatosis patients who underwent WBMRI. The study aimed to establish an international cohort of patients with quantified whole-body internal tumour burden and to correlate tumour burden with clinical features of the disease. |
| 27 | Zhang [42] | 2017 | United States (>USD 13,205) | - | 39 (14) | 30 | Internal tumour burden | A cohort study of NF1 patients who underwent WBMRI and WSMRI. The study aimed to determine the incremental value of multiparametric WBMRI over WSMRI in NF1 patients. |
| 28 | Byrne [43] | 2017 | United Kingdom (>USD 13,205) | - | 16 (−) | 100 | Non-optic pathway gliomas | A cohort study of NF1 patients with a history of non-OPG who underwent serial neuroimaging. The study aimed to characterise the clinical presentation, management, progression, and outcomes in a non-OPG NF1 cohort. |
| 29 | Guillamo [44] | 2003 | France (>USD 13,205) | CT | 32 (9) | 16 | Optic pathway and extra optic pathway CNS tumours | A cohort study of NF1 patients with presence of a CNS tumour who underwent imaging. The study aimed to identify prognostic factors for patients with CNS tumours and NF1. |
| Studies Involving FDG PET/CT as a Modality | ||||||||
| 1 | Avanesov [45] | 2021 | Germany (>USD 13,205) | MDCT | 33 (14) | 71 | MPNST | A cohort study involving NF1 patients who had undergone 18 FDG PET/CT for exclusion of MPNST, who received MDCT for assessment of lung manifestations. The study aimed to evaluate the smoking history, patients’ age, genetics, and the presence of MPNSTs as potential influencing factors for lung pathologies. |
| 2 | Brahmi [46] | 2015 | France (>USD 13,205) | Biopsy | 35 (12) | 26 | MPNST | A cohort study involving NF1 patients with a clinical suspicion of MPNST and a suspect lesion from a PET/CT scan, who received PET/CT-guided percutaneous biopsy for a pathological diagnosis. The study aimed to investigate the effectiveness of, and complications associated with PET/CT-guided percutaneous biopsies for an NF1-related MPNST diagnosis. |
| 3 | Brenner [47] | 2006 | Germany (>USD 13,205) | - | 32 (12) | 16 | MPNST | A cohort study involving NF1 patients with MPNSTs who underwent PET imaging with FDG during routine preoperative staging, and underwent wide local excision of the tumour which was then histologically examined. The study aimed to evaluate the potential of 18F-fluorodeoxyglucose positron emission tomography (FDG PET) for the prediction of patient outcomes in MPNST. |
| 4 | Chirindel [48] | 2015 | United States (>USD 13,205) | - | 39 (16) | 41 | MPNST | A cohort study involving NF1 patients presenting new symptoms or enlarging lesions, who were clinically evaluated with early and delayed 18F-FDG PET/CT imaging. The study aimed to compare the effectiveness of early (1 h) and delayed (4 h) 18F-FDG PET/CT imaging in differentiating MPNSTs from BNFs. |
| 5 | Cook [49] | 2017 | United Kingdom (>USD 13,205) | - | 35 (−) | 54 | MPNST | A cohort study involving NF1 patients with clinical suspicion of MPNSTs who received F-18-FDG PET scans. The study aimed to determine whether measurements of 18F-FDG heterogeneity could improve the differentiation of BNFs and MPNSTs. |
| 6 | Salamon [50] | 2013 | Germany (>USD 13,205) | - | 33 (15) | 50 | MPNST | A cohort study involving NF1 patients who had been referred for an 18F-FDG PET/CT scan for exclusion of MPNST and underwent an 18F-FDG PET/CT scan. The study aimed to evaluate the potential usefulness of intratumoural tracer uptake heterogeneity on F-18-FDG PET/CT as compared to SUVmax for PNST characterisation. |
| 7 | Salamon [51] | 2015 | Germany (>USD 13,205) | - | 37(12) | 36 | MPNST | A case-control study involving NF1 patients with MPNSTs and age/sex-matched patients with BPNSTs who underwent F-18-FDG PET/CT scans. The study aimed to determine WB-MTV via F-18-FDG PET/CT and compare WB-MTV between patients with BPNSTs and MPNSTs. |
| 8 | Salamon [52] | 2014 | Germany (>USD 13,205) | - | 33 (15) | 49 | MPNST | A cohort study involving NF1 patients who underwent F-18-FDG PET/CT for exclusion of MPNSTs. The study aimed to evaluate the usefulness of normalising intratumour tracer accumulation on F-18-FDG PET/CT to reference tissue uptake for characterisation of PNSTs compared with the established SUVmax cutoff of >3.5. |
| 9 | Van Der Gucht [53] | 2016 | France (>USD 13,205) | - | 33 (12) | 49 | MPNST | A cohort study involving NF1 patients with clinical suspicion of MPNST who underwent F-18-FDG PET/CT scans. The aim of the study was to investigate the diagnostic and prognostic performances of 18F-FDG PET/CT measures of metabolic tumour burden in NF1 patients with suspicion of malignant transformation. |
| 10 | Bredella [54] | 2007 | United States (>USD 13,205) | - | 37 (−) | 45 | MPNST | A cohort study involving NF1 patients with clinical suspicion of MPNST who underwent F-18-FDG PET scans. The aim of the study was to investigate the use of PET scans in the diagnosis of MPNSTs in NF1 patients. |
| 11 | Nishida [55] | 2021 | Japan (>USD 13,205) | F-18-FDG PET/CT | 32 (13) | 35 | MPNST | A cross-sectional/longitudinal study of NF1 patients with clinical suspicion of tumour presence who received F-18-FDG PET/CT scans. The study aimed to re-confirm the usefulness of PET/CT in the differentiation of benignity/malignancy of neurogenic tumours in NF1 patients and to analyse the natural course of PNFs and clarify whether PET/CT is also useful for detecting non-neurogenic tumours. |
| 12 | Combemale [56] | 2014 | France (>USD 13,205) | F-18-FDG PET | 35 (15) | 113 | MPNST | A cohort study of NF1 patients with suspected MPNSTs who received F-18-FDG PET scans. The study aimed to evaluate a semi-quantitative index for the reproducible detection of MPNST with FDG PET. |
| 13 | Ahlawat [57] | 2019 | United States (>USD 13,205) | MRI | 30 (−) | 20 | MPNST | A cohort study of NF1 patients with PNSTs who received MRI or F-18-FDG PET/CT. The study aimed to determine the utility of quantitative metrics obtained from fMRI using DWI/ADC mapping compared with F-18-FDG PET/CT imaging in NF1 patients for the characterisation of BPNST/MPNST. |
| 14 | Derlin [9] | 2003 | Germany (>USD 13,205) | WBMRI | 30 (15) | 31 | MPNST | A cohort study of NF1 patients referred for the exclusion of MPNST who underwent MRI and F-18-FDG PET/CT. The study aimed to compare the diagnostic performance of F-18-FDG PET/CT and WBMRI for MPNST detection in NF1 patients. |
| 15 | Ferner [58] | 2000 | United Kingdom (>USD 13,205) | MRI | 30 (16) | 18 | MPNST | A cohort study of NF1 patients with clinical suspicion of MPNST who underwent MRI and F-18-FDG PET scans. The study aimed to evaluate the ability of F-18-FDG PET to detect MPNSTs in NF1 patients. |
| 16 | Broski [59] | 2016 | United States (>USD 13,205) | MRI | 38 (16) | 38 | MPNST | A cohort study of NF1 patients with BPNSTs or MPNSTs who underwent F-18-FDG PET and MRI. The study aimed to compare 18F-FDG PET/CT and MRI for differentiating BPNSTs and MPNSTs and correlating imaging characteristics with histopathology. |
| Studies Involving DXA Scan | ||||||||
| 1 | Seitz [60] | 2010 | Germany (>USD 13,205) | Bone densitometry (DXA scan) | 47 (16) | 56 | Bone health | A case-control study involving NF1 patients and age/sex-matched controls who underwent DXA osteodensitometry. The study aimed to perform a systematic clinical and histomorphometric analysis of decreased BMD and vitamin D deficiency in NF1 patients. |
| 2 | Heervä [61] | 2013 | Finland (>USD 13,205) | Bone densitometry (DXA scan) | 46 (18) | 19 | Bone health | A longitudinal study involving NF1 patients, who had initial BMD measurements taken in a 1999 study, and who then underwent DXA scans to measure BMD, 12 years after the initial study. The study aimed to reach the 35 NF1 patients examined in 1999 and assess their bone health after 12 years. |
| 3 | Modica [62] | 2023 | Italy (>USD 13,205) | MRI, physical examination, biochemical testing | 41 (11) | 31 | Bone health | A cross-sectional, case-control study involving NF1 patients and sex/age/BMI- matched controls who underwent bone densitometry (DXA scan), Physical examination, MRI, and biochemical testing for assessment of clinical phenotype. The study aimed to assess vitamin D levels and bone metabolism in NF1 patients, analysing potential correlations with clinical phenotype. |
| Studies involving physical examination | ||||||||
| 1 | Arigon [63] | 2002 | France (>USD 13,205) | - | 32 (14) | 232 | Ophthalmologic complications | A cohort study of NF1 patients who received an ophthalmologic examination. The study aimed to evaluate the usefulness of ophthalmologic examination for the diagnosis and detection of complications in adult patients with neurofibromatosis type 1. |
| 2 | Khosrotehrani [64] | 2003 | France (>USD 13,205) | Blood pressure measurement | 33 (13) | 378 | Multiple manifestations (cutaneous, neurofibromas, Lisch nodules, skeletal abnormalities, hypertension) | A cohort study of NF1 patients who received a full clinical examination. The study aimed to identify the main clinical features associated with mortality in NF1 patients. |
| Studies involving CT | ||||||||
| 1 | Nishida [65] | 2016 | Japan (>USD 13,205) | MDCT | 47 (13) | 118 | GISTs | A cohort study of NF1 patients who received screening, and of NF1 patients surveyed in Japan who underwent MDCT. The study aimed to determine the risk, clinicopathologic features, and prognosis of NF1-GIST. |
| Country | Studies (n) | NF1 Complication Assessed, n (%) | Modalities (n) | Modality, n (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumour Burden | MPNST | BMD | OPG | Spinal Abnormalities | Others | MRI | FDG PET/CT | CT | Physical Examination | Blood Pressure Measurement | DXA | |||
| All countries | 51 | 8 (15.7) | 23 (45.1) | 3 (5.9) | 2 (3.9) | 5 (9.8) | 10 (19.6) | 67 | 34 (50.7) | 16 (23.8) | 5 (7.5) | 7 (10.4) | 2 (3.0) | (4.5) |
| Germany | 14 | 1 (7.1) | 8 (57.1) | 1 (7.1) | 1 (7.1) | 1 (7.1) | 2 (21.4) | 18 | 8 (44.4) | 6 (33.3) | 1 (5.6) | 2 (11.1) | - | 1 (5.6) |
| United States | 14 | 6 (42.9) | 5 (35.7) | - | - | 2 (14.3) | 1 (7.1) | 17 | 12 (70.6) | 4 (23.5) | 1 (5.9) | - | - | - |
| France | 8 | - | 5 (62.5) | - | 1 (12.5) | - | 2 (25) | 12 | 3(25) | 3 (25) | 2 (16.7) | 3 (25) | 1 (8.3) | - |
| United Kingdom | 7 | - | 2 (28.6) | - | - | 2 (28.6) | 3 (42.9) | 10 | 6 (60) | 2 (20) | - | 1 (10) | 1 (10) | - |
| Japan | 4 | - | 3 (75) | - | - | - | 1 (25) | 4 | 2 (50) | 1 (25) | 1 (25) | - | - | - |
| Finland | 1 | - | - | 1 (100) | - | - | - | 1 | - | - | - | - | - | 1 (100) |
| Italy | 1 | - | - | 1 (100) | - | - | - | 3 | 1 (33.3) | - | - | 1 (33.3) | - | 1 (33.3) |
| Belgium | 1 | 1 (100) | - | - | - | - | - | 1 | 1 (100) | - | - | - | - | |
| Türkiye | 1 | - | - | - | - | - | 1 (100) | 1 | 1 (100) | - | - | - | - | - |
| Authors | Number of Patients | Number of Lesions (MPNST) | Quantified Parameter | Threshold Value | Mean SUVmax | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|---|---|
| MPNST | Benign NF | |||||||
| Brenner [47] | 16 | N/A (16) | SUV | 3.0 | 5.7 | N/A | 75 | 100 |
| Chirindel [48] | 41 | 93(24) | SUL | 3.2 | 6.5 | 2.0 | 91 | 84 |
| Salamon (2013) [50] | 50 | 159 (19) | SUV | 3.5 | 8.4 ±3.2 | 2.6 ±1.2 | 100 | 68.9 |
| Salamon (2015) [51] | 18 | 74 (19) | SUV | 3.5 | 10.3 ± 4.2 | 4.2 ± 1.6 | 94.1 | 75 |
| Salamon (2014) [52] | 49 | 31 (18) | SUV | 3.5 | 8.61 | 2.56 | 100 | 79.8 |
| Van der Gucht [53] | 49 | 40 (16) | SUV | 4.5 | 8.8 | 2.9 | 94 | 88 |
| Bredella [54] | 45 | 50(24) | SUV | 3.0 | 8.5 ± 0.63 | 1.5 ± 0.37 | 95 | 72 |
| Nishida [55] | 36 | 57(14) | SUV | 4.1 | 7.43 ± 1.84 | 4.575 ± 1.69 | 92.9 | 88.9 |
| Combemale [56] | 113 | 145 (41) | T/L Ratio | 1.5 | N/A | N/A | 97 | 76 |
| Ahlawat [57] | 21 | 55 (19) | SUV | 3.2 | 8.0 ± 3.9 | 3.2 ± 1.8 | 100 | 83 |
| Ferner [58] | 18 | 20 (5) | SUV | 2.5 | 5.4 ± 2.4 | 1.54 ± 0.7 | N/A | N/A |
| Broski [59] | 38 | 43 (20) | SUV | 3.0 | 10.1 ± 1.0 | 4.2 ± 0.4 | 90 | 78 |
| Number of Patients | Normal BMD, n (%) | Osteopenia (BMD between 1.0 and 2.5 SD), n(%) | Osteoporosis (BMD ≤ 2.5 SD), n (%) | |
|---|---|---|---|---|
| Heerva [61] | 19 | 6 (31.6) | 5 (26.3) | 8 (42.1) |
| Seitz [60] | 14 | - | 6 (42.9) | 8 (57.1) |
| Modica [62] | 24 | 12 (50) | 9 (37.5) | 3 (12.5) |
| ACMG Guidelines [4] | French Guidelines [6] | ERN GENTURIS Guidelines [5] | United Kingdom Guidelines [7] | |
|---|---|---|---|---|
| Tumour burden |
|
|
|
|
| MPNSTs |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, S.; Low, C.E.; Karthikeyan, M.; Koh, M.J.A.; Ngeow, J.; Chiang, J. A Systematic Review of Diagnostic Modalities and Strategies for the Assessment of Complications in Adult Patients with Neurofibromatosis Type 1. Cancers 2024, 16, 1119. https://doi.org/10.3390/cancers16061119
Rana S, Low CE, Karthikeyan M, Koh MJA, Ngeow J, Chiang J. A Systematic Review of Diagnostic Modalities and Strategies for the Assessment of Complications in Adult Patients with Neurofibromatosis Type 1. Cancers. 2024; 16(6):1119. https://doi.org/10.3390/cancers16061119
Chicago/Turabian StyleRana, Sounak, Chen Ee Low, Manasadevi Karthikeyan, Mark Jean Aan Koh, Joanne Ngeow, and Jianbang Chiang. 2024. "A Systematic Review of Diagnostic Modalities and Strategies for the Assessment of Complications in Adult Patients with Neurofibromatosis Type 1" Cancers 16, no. 6: 1119. https://doi.org/10.3390/cancers16061119
APA StyleRana, S., Low, C. E., Karthikeyan, M., Koh, M. J. A., Ngeow, J., & Chiang, J. (2024). A Systematic Review of Diagnostic Modalities and Strategies for the Assessment of Complications in Adult Patients with Neurofibromatosis Type 1. Cancers, 16(6), 1119. https://doi.org/10.3390/cancers16061119





