Screening Implications for Distribution of Colorectal Cancer Subsite by Age and Role of Flexible Sigmoidoscopy
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patient Cohort
2.2. Cancer Site and Screening Modality
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehta, S.J.; Morris, A.M.; Kupfer, S.S. Colorectal Cancer Screening Starting at Age 45 Years-Ensuring Benefits Are Realized by All. JAMA Netw. Open 2021, 4, e2112593. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-H.; Sanford, N.N.; Liang, P.S.; Singal, A.G.; Murphy, C.C. Persistent Disparities in Colorectal Cancer Screening: A Tell-Tale Sign for Implementing New Guidelines in Younger Adults. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Boland, R.C.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Levin, T.R.; Lieberman, D.; Robertson, D.J. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2017, 112, 1016–1030. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, A.; Zuppardo, R.A.; Rosati, R.; Leo, M.D.; Perea, J.; Cavestro, G.M. Colorectal cancer screening from 45 years of age: Thesis, antithesis and synthesis. World J. Gastroenterol. 2019, 25, 2565–2580. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, J.; Karanth, S.; Revere, F.L.; Agrawal, D. Cost Effectiveness of Screening Colonoscopy Depends on Adequate Bowel Preparation Rates—A Modeling Study. PLoS ONE 2016, 11, e0167452. [Google Scholar] [CrossRef] [PubMed]
- Azad, N.S.; Leeds, I.L.; Wanjau, W.; Shin, E.J.; Padula, W.V. Cost-utility of colorectal cancer screening at 40 years old for average-risk patients. Prev. Med. 2020, 133, 106003. [Google Scholar] [CrossRef] [PubMed]
- Lohsiriwat, V. Colonoscopic perforation: Incidence, risk factors, management and outcome. World J. Gastroenterol. 2010, 16, 425–430. [Google Scholar] [CrossRef]
- Shaukat, A.; Mongin, S.J.; Geisser, M.S.; Lederle, F.A.; Bond, J.H.; Mandel, J.S.; Church, T.R. Long-Term Mortality after Screening for Colorectal Cancer. N. Engl. J. Med. 2013, 369, 1106–1114. [Google Scholar] [CrossRef]
- Holme, Ø.; Løberg, M.; Kalager, M.; Bretthauer, M.; Hernán, M.A.; Aas, E.; Eide, T.J.; Skovlund, E.; Schneede, J.; Tveit, K.M.; et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: A randomized clinical trial. JAMA 2014, 312, 606–615. [Google Scholar] [CrossRef]
- Swartz, A.W.; Eberth, J.M.; Josey, M.J.; Strayer, S.M. Reanalysis of All-Cause Mortality in the U.S. Preventive Services Task Force 2016 Evidence Report on Colorectal Cancer Screening. Ann. Intern. Med. 2017, 167, 602–603. [Google Scholar] [CrossRef]
- Bretthauer, M.; Løberg, M.; Wieszczy, P.; Kalager, M.; Emilsson, L.; Garborg, K.; Rupinski, M.; Dekker, E.; Spaander, M.; Bugajski, M.; et al. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N. Engl. J. Med. 2022, 387, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- SEER*Stat Database: Incidence-SEER Research Plus 17 Data Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute, DCCPS. Available online: www.seer.cancer.gov (accessed on 19 April 2023).
- Royston, P.; Parmar, M.K. Restricted mean survival time: An alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med. Res. Methodol. 2013, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Denz, R.; Klaaßen-Mielke, R.; Timmesfeld, N. A comparison of different methods to adjust survival curves for confounders. Stat. Med. 2023, 42, 1461–1479. [Google Scholar] [CrossRef] [PubMed]
- Conner, S.C.; Sullivan, L.M.; Benjamin, E.J.; LaValley, M.P.; Galea, S.; Trinquart, L. Adjusted restricted mean survival times in observational studies. Stat. Med. 2019, 38, 3832–3860. [Google Scholar] [CrossRef]
- Read, B.; Sylla, P. Aggressive Colorectal Cancer in the Young. Clin. Colon Rectal Surg. 2020, 33, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Surana, R.; Ng, K. The NordICC Trial: The Devil Is in the Details. ASCO Post, 25 November 2022. [Google Scholar]
- Inadomi, J.M.; Vijan, S.; Janz, N.K.; Fagerlin, A.; Thomas, J.P.; Lin, Y.V.; Muñoz, R.; Lau, C.; Somsouk, M.; El-Nachef, N.; et al. Adherence to colorectal cancer screening: A randomized clinical trial of competing strategies. Arch. Intern. Med. 2012, 172, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, A.B.; Rutter, C.M.; Peterse, E.F.P.; Lietz, A.P.; Seguin, C.L.; Meester, R.G.S.; Perdue, L.A.; Lin, J.S.; Siegel, R.L.; Doria-Rose, V.P.; et al. Colorectal Cancer Screening: An Updated Modeling Study for the US Preventive Services Task Force. JAMA 2021, 325, 1998. [Google Scholar] [CrossRef] [PubMed]
- Azar, I.; Al Masalmeh, N.; Esfandiarifard, S.; Virk, G.; Kiwan, W.; Frank Shields, A.; Mehdi, S.; Philip, P.A. The impact of primary tumor sidedness on survival in early-onset colorectal cancer by stage: A National Veterans Affairs retrospective analysis. Cancer Med. 2021, 10, 2987–2995. [Google Scholar] [CrossRef]
- Ladabaum, U.; Shepard, J.; Mannalithara, A. Adenoma and Sessile Serrated Lesion Detection Rates at Screening Colonoscopy for Ages 45–49 Years vs Older Ages Since the Introduction of New Colorectal Cancer Screening Guidelines. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2022, 20, 2895–2904.e4. [Google Scholar] [CrossRef]
- Parikh, R.B.; Prasad, V. Blood-Based Screening for Colon Cancer: A Disruptive Innovation or Simply a Disruption? JAMA 2016, 315, 2519–2520. [Google Scholar] [CrossRef]
- Lin, J.S.; Perdue, L.A.; Henrikson, N.B.; Bean, S.I.; Blasi, P.R. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2021, 325, 1978–1998. [Google Scholar] [CrossRef]
- Diedrich, L.; Brinkmann, M.; Dreier, M.; Schramm, W.; Krauth, C. Additional offer of sigmoidoscopy in colorectal cancer screening in Germany: Rationale and protocol of the decision-analytic modelling approach in the SIGMO study. BMJ Open 2022, 12, e050698. [Google Scholar] [CrossRef]
- Farooq, A.; Keehn, A.R.; Xu, Y.; Kong, S.; Cheung, W.Y.; Quan, M.L.; MacLean, A.R. Patient and disease characteristics, treatment practices and oncologic outcomes among patients with colorectal cancer: A population-based analysis. Can. J. Surg. J. Can. Chir. 2023, 66, E71–E78. [Google Scholar] [CrossRef]
- Dominitz, J.A.; Robertson, D.J.; Ahnen, D.J.; Allison, J.E.; Antonelli, M.; Boardman, K.D.; Ciarleglio, M.; Del Curto, B.J.; Huang, G.D.; Imperiale, T.F.; et al. Colonoscopy vs. Fecal Immunochemical Test in Reducing Mortality From Colorectal Cancer (CONFIRM): Rationale for Study Design. Am. J. Gastroenterol. 2017, 112, 1736–1746. [Google Scholar] [CrossRef]
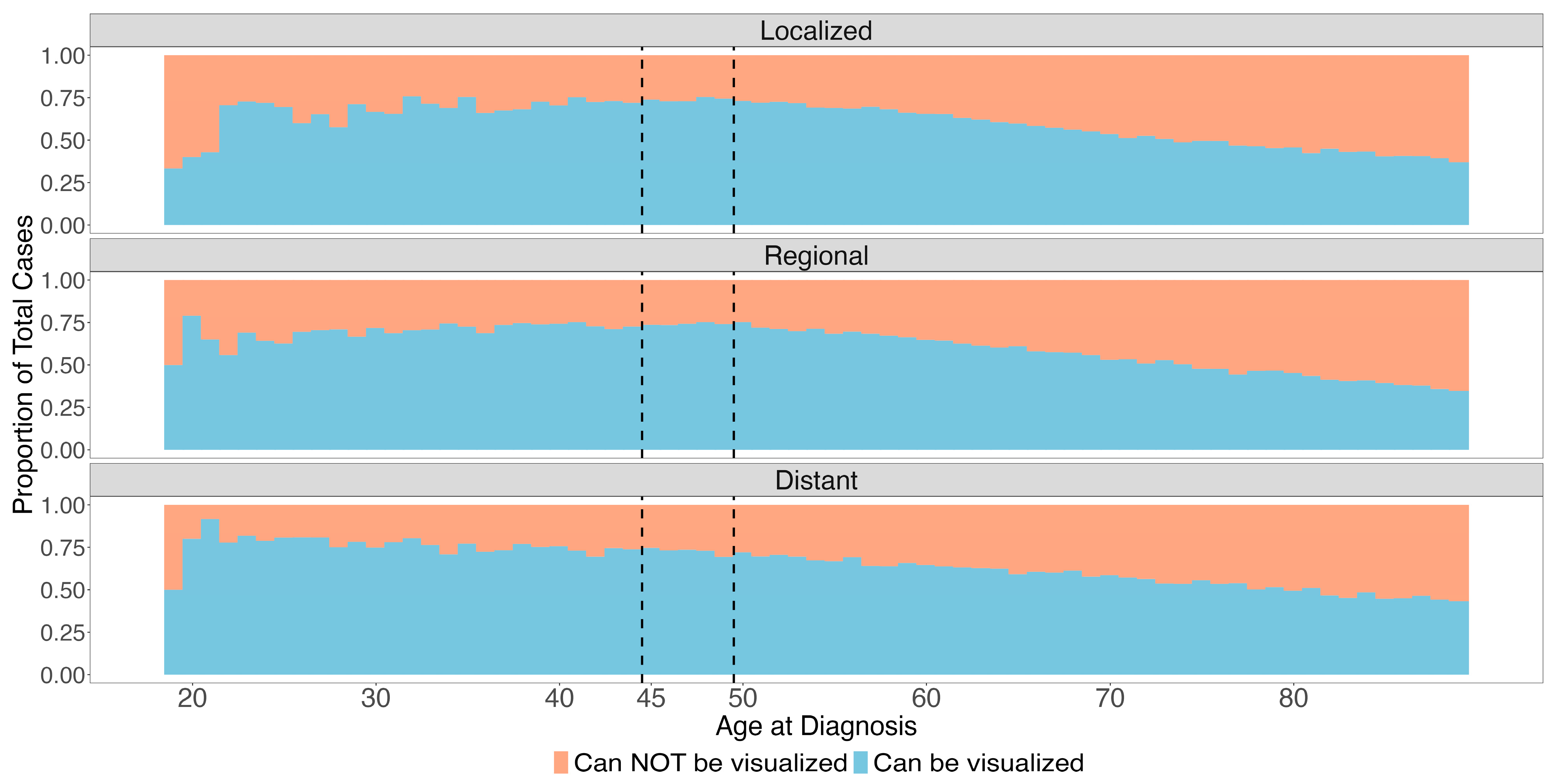

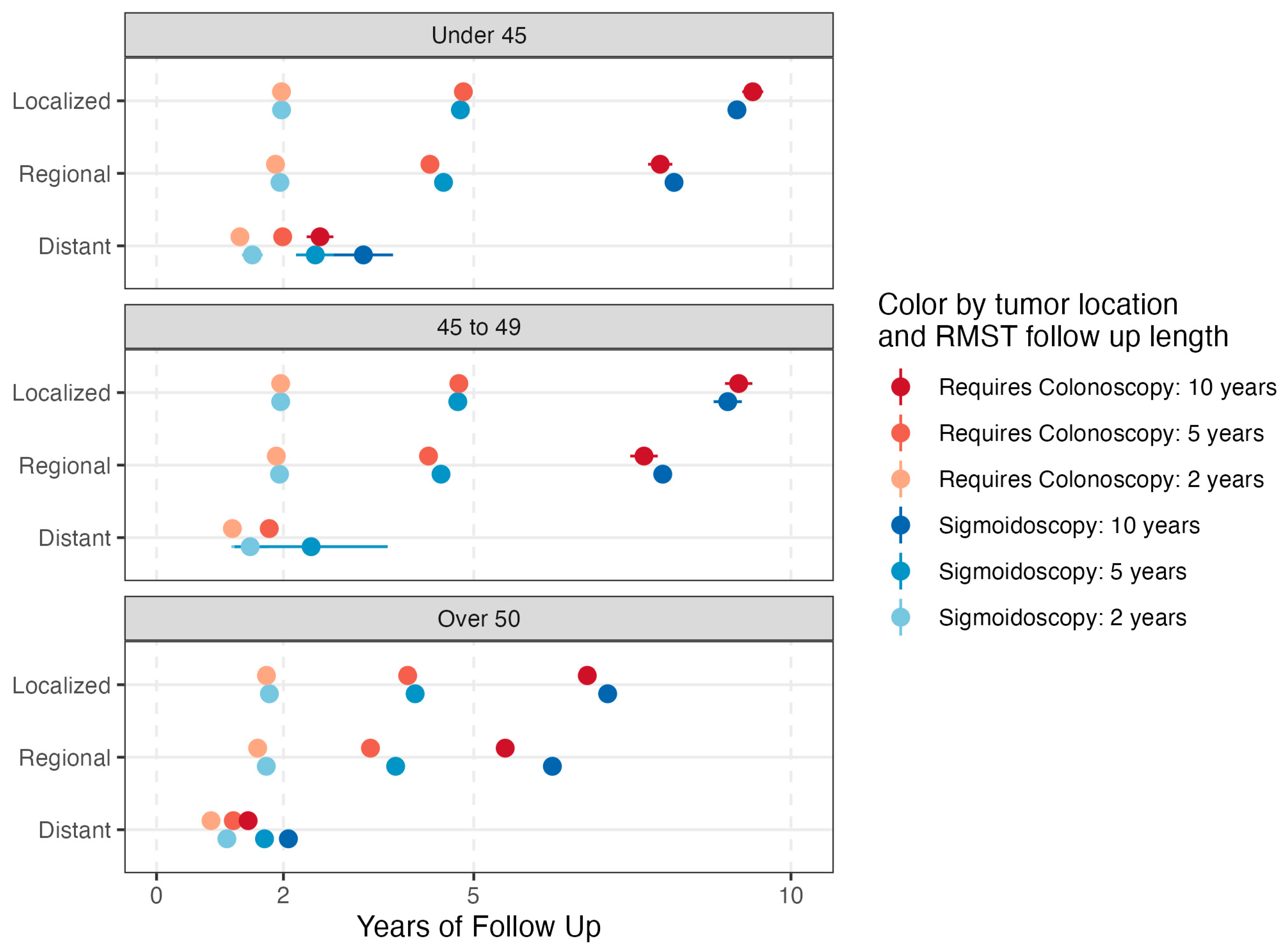
| N (%) Can Be Visualized | N Total | Univariable OR (95% CI) | Multivariable OR (95% CI) | |
|---|---|---|---|---|
| Overall | 179,986 (58.2) | 309,466 | - | - |
| Age Group | ||||
| Over 50 | 151,015 (56.0) | 269,861 | ref | ref |
| 45–49 | 13,922 (73.6) | 18,906 | 2.20 (2.13, 2.27) | 2.16 (2.09, 2.23) |
| Under 45 | 15,049 (72.7) | 20,699 | 2.10 (2.03, 2.16) | 2.05 (1.99, 2.12) |
| Sex | ||||
| Female | 78,119 (52.7) | 148,136 | ref | ref |
| Male | 101,867 (63.1) | 161,330 | 1.54 (1.51–1.56) | 1.52 (1.50, 1.54) |
| Cancer Stage | ||||
| Localized | 54,769 (56.6) | 96,974 | ref | ref |
| Regional | 77,596 (57.6) | 134,694 | 1.05 (1.03, 1.06) | 1.01 † (0.99, 1.03) |
| Distant | 47,621 (61.2) | 77,798 | 1.22 (1.19, 1.24) | 1.16 (1.14, 1.19) |
| Race | ||||
| Non-Hispanic White | 117,647 (57.1) | 206,185 | ref | ref |
| Hispanic (All Races) | 23,089 (62.9) | 36,736 | 1.27 (1.24, 1.30) | 1.18 (1.15, 1.21) |
| Non-Hispanic American Indian/Alaska Native | 1481 (61.9) | 2394 | 1.22 (1.12, 1.33) | 1.17 (1.07, 1.27) |
| Non-Hispanic Asian or Pacific Islander | 19,070 (68.0) | 28,041 | 1.60 (1.56, 1.64) | 1.56 (1.52, 1.60) |
| Non-Hispanic Black | 18,018 (51.4) | 35,067 | 0.80 (0.78, 0.81) | 0.76 (0.75, 0.78) |
| Non-Hispanic Unknown Race | 681 (65.3) | 1043 | 1.42 (1.25, 1.61) | 1.35 (1.19, 1.54) |
| Year of Diagnosis | ||||
| 2000–2004 | 38,201 (57.2) | 66,774 | ref | ref |
| 2005–2009 | 39,821 (57.2) | 69,575 | 1.00 (0.98, 1.02) | 0.98 (0.96, 1.00) |
| 2010–2014 | 40,625 (58.0) | 70,071 | 1.03 (1.01, 1.05) | 0.99 (0.97. 1.01) |
| 2015–2020 | 61,339 (60.0) | 103,046 | 1.10 (1.08, 1.12) | 1.03 (1.01, 1.05) |
| Primary Tumor Site | Age Group | |||
|---|---|---|---|---|
| <45 | 45–49 | >50 | All Ages | |
| Rectum | 6152 | 5807 | 56,988 | 68,947 (22.3%) |
| Rectosigmoid Junction | 2333 | 2186 | 23,762 | 28,281 (9.14%) |
| Sigmoid | 5380 | 4976 | 58,308 | 68,664 (22.2%) |
| Descending Colon | 1184 | 953 | 11,957 | 14,094 (4.55%) |
| Splenic Flexure | 571 | 443 | 6922 | 7936 (2.56%) |
| Transverse Colon | 1146 | 878 | 18,595 | 20,619 (6.66%) |
| Hepatic Flexure | 563 | 418 | 9887 | 10,868 (3.51%) |
| Ascending Colon | 1615 | 1493 | 38,933 | 42,041 (13.6%) |
| Cecum | 1755 | 1752 | 44,509 | 48,016 (15.5%) |
| All Sites | 20,699 (6.69%) | 18,906 (6.11%) | 269,861 (87.2%) | 309,466 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, G.; Hein, D.M.; Liu, P.-H.; Singal, A.G.; Sanford, N.N. Screening Implications for Distribution of Colorectal Cancer Subsite by Age and Role of Flexible Sigmoidoscopy. Cancers 2024, 16, 1110. https://doi.org/10.3390/cancers16061110
Lin G, Hein DM, Liu P-H, Singal AG, Sanford NN. Screening Implications for Distribution of Colorectal Cancer Subsite by Age and Role of Flexible Sigmoidoscopy. Cancers. 2024; 16(6):1110. https://doi.org/10.3390/cancers16061110
Chicago/Turabian StyleLin, Gloria, David M. Hein, Po-Hong Liu, Amit G. Singal, and Nina N. Sanford. 2024. "Screening Implications for Distribution of Colorectal Cancer Subsite by Age and Role of Flexible Sigmoidoscopy" Cancers 16, no. 6: 1110. https://doi.org/10.3390/cancers16061110
APA StyleLin, G., Hein, D. M., Liu, P.-H., Singal, A. G., & Sanford, N. N. (2024). Screening Implications for Distribution of Colorectal Cancer Subsite by Age and Role of Flexible Sigmoidoscopy. Cancers, 16(6), 1110. https://doi.org/10.3390/cancers16061110





