Incidence and Predictors of Textbook Outcome after Minimally Invasive Esophagectomy for Cancer: A Two-Center Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Inclusion/Exclusion Criteria
2.2. Neoadjuvant Therapy Protocol and Indication for Surgical Resection
2.3. Definition of Variables
2.4. Study Endpoints
2.5. Statistical Analysis
3. Results
3.1. Study Patients
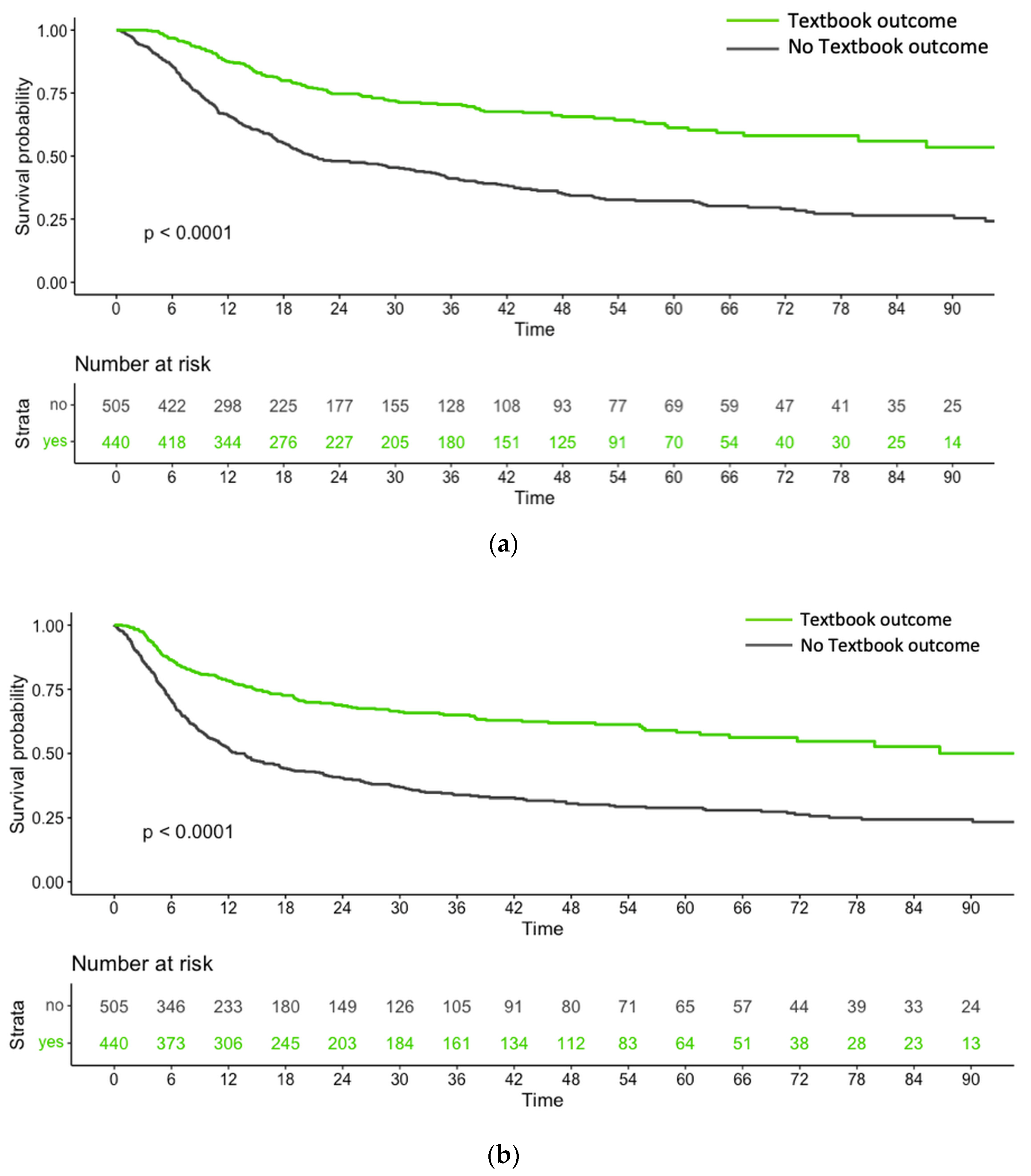
3.1.1. Survival Outcomes in Relation to the Achievement of TBO
3.1.2. Univariate and Multivariable Predictors of TBO
3.1.3. Associations of TBO with Different MIE Techniques (RE versus VATE)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Hagen, P.; Hulshof, M.C.; van Lanschot, J.J.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.; Richel, D.J.; Nieuwenhuijzen, G.A.; Hospers, G.A.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, H.; Chen, Y.; Zhu, C.; Fang, W.; Yu, Z.; Mao, W.; Xiang, J.; Han, Y.; Chen, Z. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): A phase III multicenter, randomized, open-label clinical trial. J. Clin. Oncol. 2018, 36, 2796. [Google Scholar] [CrossRef]
- Bhagat, R.; Bronsert, M.R.; Juarez-Colunga, E.; Weyant, M.J.; Mitchell, J.D.; Glebova, N.O.; Henderson, W.G.; Fullerton, D.; Meguid, R.A. Postoperative complications drive unplanned readmissions after esophagectomy for cancer. Ann. Thorac. Surg. 2018, 105, 1476–1482. [Google Scholar] [CrossRef] [PubMed]
- In, H.; Palis, B.E.; Merkow, R.P.; Posner, M.C.; Ferguson, M.K.; Winchester, D.P.; Pezzi, C.M. Doubling of 30-day mortality by 90 days after esophagectomy. Ann. Surg. 2016, 263, 286–291. [Google Scholar] [CrossRef] [PubMed]
- D’Journo, X.B.; Boulate, D.; Fourdrain, A.; Loundou, A.; van Berge Henegouwen, M.I.; Gisbertz, S.S.; O’Neill, J.R.; Hoelscher, A.; Piessen, G.; Van Lanschot, J. Risk prediction model of 90-day mortality after esophagectomy for cancer. JAMA Surg. 2021, 156, 836–845. [Google Scholar] [CrossRef]
- Kolfschoten, N.E.; Kievit, J.; Gooiker, G.A.; van Leersum, N.J.; Snijders, H.S.; Eddes, E.H.; Tollenaar, R.A.; Wouters, M.W.; Marang-van de Mheen, P.J. Focusing on desired outcomes of care after colon cancer resections; hospital variations in ‘textbook outcome’. Eur. J. Surg. Oncol. 2013, 39, 156–163. [Google Scholar] [CrossRef]
- Kalff, M.C.; Vesseur, I.; Eshuis, W.J.; Heineman, D.J.; Daams, F.; van der Peet, D.L.; van Berge Henegouwen, M.I.; Gisbertz, S.S. The association of textbook outcome and long-term survival after esophagectomy for esophageal cancer. Ann. Thorac. Surg. 2021, 112, 1134–1141. [Google Scholar] [CrossRef]
- Kulshrestha, S.; Bunn, C.; Patel, P.M.; Sweigert, P.J.; Eguia, E.; Pawlik, T.M.; Baker, M.S. Textbook oncologic outcome is associated with increased overall survival after esophagectomy. Surgery 2020, 168, 953–961. [Google Scholar] [CrossRef]
- Dal Cero, M.; Román, M.; Grande, L.; Yarnoz, C.; Estremiana, F.; Gantxegi, A.; Codony, C.; Gobbini, Y.; Garsot, E.; Momblan, D. Textbook outcome and survival after gastric cancer resection with curative intent: A population-based analysis. Eur. J. Surg. Oncol. 2022, 48, 768–775. [Google Scholar] [CrossRef]
- Kalagara, R.; Norain, A.; Chang, Y.-H.; Stucky, C.-C.; Wasif, N. Association of textbook outcome and surgical case volume with long-term survival in patients undergoing surgical resection for pancreatic cancer. J. Am. Coll. Surg. 2022, 235, 829–837. [Google Scholar] [CrossRef]
- Busweiler, L.; Schouwenburg, M.; van Berge Henegouwen, M.; Kolfschoten, N.; de Jong, P.; Rozema, T.; Wijnhoven, B.; van Hillegersberg, R.; Wouters, M.; van Sandick, J. Textbook outcome as a composite measure in oesophagogastric cancer surgery. J. Br. Surg. 2017, 104, 742–750. [Google Scholar] [CrossRef]
- Van Der Werf, L.R.; Wijnhoven, B.P.; Fransen, L.F.; van Sandick, J.W.; Nieuwenhuijzen, G.A.; Busweiler, L.A.; van Hillegersberg, R.; Wouters, M.W.; Luyer, M.D.; van Berge Henegouwen, M.I. A national cohort study evaluating the association between short-term outcomes and long-term survival after esophageal and gastric cancer surgery. Ann. Surg. 2019, 270, 868–876. [Google Scholar] [CrossRef] [PubMed]
- de Groot, E.; Goense, L.; Kingma, B.; Haverkamp, L.; Ruurda, J.; van Hillegersberg, R. Trends in surgical techniques for the treatment of esophageal and gastroesophageal junction cancer: The 2022 update. Dis. Esophagus 2023, 36, doac099. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, L.; Seesing, M.; Ruurda, J.; Boone, J. Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus 2017, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dyas, A.R.; Stuart, C.M.; Bronsert, M.R.; Schulick, R.D.; McCarter, M.D.; Meguid, R.A. Minimally invasive surgery is associated with decreased postoperative complications after esophagectomy. J. Thorac. Cardiovasc. Surg. 2023, 166, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Yerokun, B.A.; Sun, Z.; Yang, C.-F.J.; Gulack, B.C.; Speicher, P.J.; Adam, M.A.; D’Amico, T.A.; Onaitis, M.W.; Harpole, D.H.; Berry, M.F. Minimally invasive versus open esophagectomy for esophageal cancer: A population-based analysis. Ann. Thorac. Surg. 2016, 102, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Ising, M.S.; Smith, S.A.; Trivedi, J.R.; Martin, R.C.; Phillips, P.; Van Berkel, V.; Fox, M.P. Minimally invasive esophagectomy is associated with superior survival compared to open surgery. Am. Surg. 2023, 89, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Mariette, C.; Markar, S.; Dabakuyo-Yonli, T.S.; Meunier, B.; Pezet, D.; Collet, D.; D’journo, X.B.; Brigand, C.; Perniceni, T.; Carrere, N. Health-related quality of life following hybrid minimally invasive versus open esophagectomy for patients with esophageal cancer, analysis of a multicenter, open-label, randomized phase III controlled trial: The MIRO trial. Ann. Surg. 2020, 271, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Mariette, C.; Markar, S.R.; Dabakuyo-Yonli, T.S.; Meunier, B.; Pezet, D.; Collet, D.; D’Journo, X.B.; Brigand, C.; Perniceni, T.; Carrere, N.; et al. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N. Engl. J. Med. 2019, 380, 152–162. [Google Scholar] [CrossRef]
- Bolger, J.C.; Al Azzawi, M.; Whooley, J.; Bolger, E.M.; Trench, L.; Allen, J.; Kelly, M.E.; Brosnan, C.; Arumugasamy, M.; Robb, W.B. Surgery by a minimally invasive approach is associated with improved textbook outcomes in oesophageal and gastric cancer. Eur. J. Surg. Oncol. 2021, 47, 2332–2339. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Reynolds, J.V.; Donlon, N.; Elliott, J.A.; Donohoe, C.; Ravi, N.; Kuppusamy, M.K.; Low, D.E. Comparison of Esophagectomy outcomes between a National Center, a National Audit Collaborative, and an International database using the Esophageal Complications Consensus Group (ECCG) standardized definitions. Dis. Esophagus 2021, 34, doaa060. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Desai, R.J.; Franklin, J.M. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: A primer for practitioners. BMJ 2019, 367, l5657. [Google Scholar] [CrossRef]
- Heinze, G.; Jüni, P. An overview of the objectives of and the approaches to propensity score analyses. Eur. Heart J. 2011, 32, 1704–1708. [Google Scholar] [CrossRef]
- Kamel, M.K.; Sholi, A.N.; Rahouma, M.; Harrison, S.W.; Lee, B.; Stiles, B.M.; Altorki, N.K.; Port, J.L. National trends and perioperative outcomes of robotic oesophagectomy following induction chemoradiation therapy: A National Cancer Database propensity-matched analysis. Eur. J. Cardio-Thorac. Surg. 2021, 59, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Kingma, B.F.; Grimminger, P.P.; van der Sluis, P.C.; van Det, M.J.; Kouwenhoven, E.A.; Chao, Y.-K.; Tsai, C.-Y.; Fuchs, H.F.; Bruns, C.J.; Sarkaria, I.S. Worldwide techniques and outcomes in robot-assisted minimally invasive esophagectomy (RAMIE): Results from the multicenter international registry. Ann. Surg. 2022, 276, e386–e392. [Google Scholar] [CrossRef] [PubMed]
- Manigrasso, M.; Vertaldi, S.; Marello, A.; Antoniou, S.A.; Francis, N.K.; De Palma, G.D.; Milone, M. Robotic esophagectomy. A systematic review with meta-analysis of clinical outcomes. J. Pers. Med. 2021, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, D.; Cao, Y.; Huang, M.; Li, J.; Zhang, J.; Lin, J.; Sarkaria, I.S.; Toni, L.; David, R. Robotic versus conventional minimally invasive esophagectomy for esophageal cancer: A meta-analysis. Ann. Surg. 2023, 278, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Henckens, S.P.; Hagens, E.R.; van Berge Henegouwen, M.I.; Meijer, S.L.; Eshuis, W.J.; Gisbertz, S.S. Impact of increasing lymph node yield on staging, morbidity and survival after esophagectomy for esophageal adenocarcinoma. Eur. J. Surg. Oncol. 2023, 49, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.-J.; Chen, H.-S.; Hung, W.-H.; Hsu, P.-K.; Wu, S.-C.; Chen, H.-C.; Wang, B.-Y. Survival impact of total resected lymph nodes in esophageal cancer patients with and without neoadjuvant chemoradiation. Ann. Surg. Oncol. 2018, 25, 3820–3832. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.M.; Yang, Y.S.; Kong, W.L.; Shang, Q.X.; Zhang, H.L.; Wang, W.P.; Yuan, Y.; Che, G.W.; Chen, L.Q. Effect of circumferential resection margin status on survival and recurrence in esophageal squamous cell carcinoma with neoadjuvant chemoradiotherapy. Front. Oncol. 2022, 12, 965255. [Google Scholar] [CrossRef] [PubMed]
- Hollertz, P.; Lindblad, M.; Sandström, P.; Halldestam, I.; Edholm, D. Outcome of microscopically non-radical oesophagectomy for oesophageal and oesophagogastric junctional cancer: Nationwide cohort study. BJS Open 2021, 5, zrab038. [Google Scholar] [CrossRef]
- Rebecchi, F.; Ugliono, E.; Allaix, M.E.; Morino, M. Why pay more for robot in esophageal cancer surgery? Updates Surg. 2023, 75, 367–372. [Google Scholar] [CrossRef]
- Kingma, B.F.; Hadzijusufovic, E.; Van der Sluis, P.C.; Bano, E.; Lang, H.; Ruurda, J.P.; van Hillegersberg, R.; Grimminger, P.P. A structured training pathway to implement robot-assisted minimally invasive esophagectomy: The learning curve results from a high-volume center. Dis. Esophagus 2020, 33, doaa047. [Google Scholar] [CrossRef] [PubMed]

| Variable | Entire Cohort | TBO(−) | TBO(+) | p |
|---|---|---|---|---|
| Number of patients (%) | 945 (100) | 505 (53.4) | 440 (46.6) | |
| Age, years (mean (SD)) | 59.8 (10.49) | 59.36 (10.36) | 60.30 (10.61) | 0.169 |
| Sex | 0.414 | |||
| Female | 104 (15.2) | 60 (11.9) | 44 (10.0) | |
| Male | 841 (84.8) | 445 (88.1) | 396 (90.0) | |
| BMI, kg/m2 (mean (SD)) | 24.30 (8.36) | 23.75 (4.43) | 24.93 (11.24) | 0.031 |
| Charlson comorbidity index | 0.043 | |||
| 0−1 | 647 (68.5) | 328 (65) | 319 (72.5) | |
| 2 | 177 (18.7) | 104 (20.6) | 73 (16.6) | |
| ≥3 | 121 (12.8) | 73(14.4) | 48 (10.9) | |
| Smoking | 0.674 | |||
| Never | 387 (41) | 201 (39.8) | 186 (42.3) | |
| Quit > 30 days | 127 (13.4) | 67 (13.3) | 60 (13.6) | |
| Active | 431 (45.6) | 237 (46.9) | 194 (44.1) | |
| Tumor location | 0.04 | |||
| Upper third | 117 (12.4) | 72 (14.3) | 45 (10.2) | |
| Middle third | 265 (28) | 157 (31.1) | 108 (24.5) | |
| Lower third | 563 (59.6) | 276 (54.7) | 287 (25.2) | |
| Histology | <0.001 | |||
| Adenocarcinoma | 351 (37.1) | 161 (31.9) | 190 (43.2) | |
| Squamous cell carcinoma | 594 (62.9) | 344 (68.1) | 250 (56.8) | |
| cT-stage | 0.232 | |||
| cT1 | 102 (10.8) | 57 (11.3) | 45 (10.2) | |
| cT2−3 | 769 (81.4) | 402 (79.6) | 367 (83.4) | |
| cT4 | 74 (7.8) | 46 (9.1) | 28 (6.4) | |
| cN-stage | 0.046 | |||
| cN− | 245 (25.9) | 117 (23.2) | 128 (29.1) | |
| cN+ | 700 (74.1) | 388 (76.8) | 312 (70.9) | |
| Preoperative radiotherapy | 0.001 | |||
| No | 385 (40.7) | 180 (35.6) | 205 (46.6) | |
| Yes | 560 (59.3) | 325 (64.4) | 235 (53.4) | |
| Preoperative chemotherapy | 0.568 | |||
| No | 189 (20) | 97 (19.2) | 92 (20.9) | |
| Yes | 756 (80) | 408 (80.8) | 348 (79.1) | |
| Medical center | 0.001 | |||
| Center A | 426 (45.1) | 201 (39.8) | 225 (51.1) | |
| Center B | 519 (54.9) | 304 (60.2) | 215 (48.9) | |
| Operative procedure | <0.001 | |||
| Ivor Lewis | 412 (43.6) | 193 (38.2) | 219 (49.8) | |
| McKeown | 533 (56.4) | 312 (61.8) | 221 (60.2) | |
| Technique used for MIE | <0.001 | |||
| VATE | 537 (56.8) | 319 (63.2) | 218 (49.5) | |
| RE | 408 (43.2) | 186 (36.8) | 222 (50.5) | |
| Abdominal part | 0.01 | |||
| Open surgery | 122 (12.9) | 79 (15.6) | 43 (9.8) | |
| Minimally invasive surgery | 823 (87.1) | 426 (84.4) | 397 (90.2) |
| Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | p | OR (95% CI) | p |
| Age | 1.009 (0.996–1.021) | 0.169 | ||
| Sex | ||||
| Female | Reference | 0.357 | ||
| Male | 1.213 (0.804–1.832) | |||
| BMI | 1.036 (1.006–1.066) | 0.019 | 1.016 (0.990–1.044) | 0.235 |
| Charlson comorbidity index | ||||
| 0−1 | Reference | 0.044 | Reference | 0.015 |
| 2 | 0.722 (0.515–1.011) | 0.058 | 0.687 (0.483–0.977) | 0.037 |
| ≥3 | 0.676 (0.455–1.004) | 0.052 | 0.604 (0.399–0.915) | 0.017 |
| Tumor location | ||||
| Upper third | Reference | 0.004 | Reference | 0.660 |
| Middle third | 1.101 (0.705–1.719) | 0.673 | 0.989 (0.626–1.561) | 0.962 |
| Lower third | 1.664 (1.107–2.501) | 0.014 | 1.168 (0.720–1.894) | 0.530 |
| Histology | ||||
| Squamous cell carcinoma | Reference | <0.001 | Reference | 0.917 |
| Adenocarcinoma | 1.624 (1.245–2.118) | 0.977 (0.625–1.525) | ||
| Preoperative radiotherapy | ||||
| No | Reference | 0.001 | Reference | 0.053 |
| Yes | 0.635 (0.489–0.824) | 0.729 (0.530–1.004) | ||
| Preoperative chemotherapy | ||||
| No | Reference | 0.514 | ||
| Yes | 0.899 (0.654–1.237) | |||
| Medical center | ||||
| Center A | Reference | <0.001 | Reference | 0.878 |
| Center B | 0.632 (0.488–0.818) | 1.090 (0.361–3.293) | ||
| Operative procedure | ||||
| Ivor Lewis | Reference | <0.001 | Reference | 0.780 |
| McKeown | 0.624 (0.482–0.809) | 0.851 (0.274–2.641) | ||
| Technique used for MIE | ||||
| VATE | Reference | <0.001 | Reference | 0.004 |
| RE | 1.747 (1.347–2.265) | 1.527 (1.149–2.028) | ||
| Abdominal part | ||||
| Open surgery | Reference | 0.008 | Reference | 0.357 |
| Minimally invasive surgery | 1.712 (1.153–2.544) | 1.227 (0.794–1.897) | ||
| Characteristic | Original Cohort | IPTW Cohort | ||||
|---|---|---|---|---|---|---|
| RE (n = 408) | VATE (n = 537) | SMD | RE | VATE | SMD | |
| Men, n (%) | 360 (88.2%) | 481 (89.6%) | 0.043 | 90.0% | 89.2% | 0.028 |
| Age, years | 61.76 ± 10.835 | 58.31 ± 9.968 | 0.331 | 59.26 ± 11.02 | 59.93 ± 10.46 | 0.063 |
| Body mass index, kg/m2 | 25.15 ± 11.686 | 23.66 ± 4.297 | 0.169 | 24.30 ± 9.97 | 24.27 ± 4.52 | 0.004 |
| Smoking, n (%) | 0.506 | 0.079 | ||||
| No | 222 (54.41%) | 165 (30.73%) | 39.63% | 41.36% | ||
| Quit > 30 days | 50 (12.25%) | 77 (14.34%) | 11.28% | 13.12% | ||
| Active smoker | 136 (33.34) | 295 (54.93%) | 49.09% | 45.52% | ||
| CCI | 0.110 | 0.101 | ||||
| 0–1 | 280 (68.63%) | 367 (68.34%) | 63.31% | 68.12% | ||
| 2 | 69 (16.91%) | 108 (20.11%) | 21.74% | 18.86% | ||
| 3 | 59 (14.46%) | 62 (11.55%) | 14.95% | 13.02% | ||
| Histology | 0.575 | 0.003 | ||||
| Squamous cell carcinoma | 194 (47.55%) | 400 (74.49%) | 62.07% | 62.20% | ||
| Adenocarcinoma | 214 (52.45) | 137 (25.51%) | 37.93% | 37.80% | ||
| Clinical AJCC stage | 0.198 | 0.080 | ||||
| I | 38 (9.31%) | 54 (10.06%) | 11.34% | 10.04% | ||
| II | 120 (29.41%) | 118 (21.97%) | 26.02% | 25.62% | ||
| III | 191 (46.81%) | 259 (48.23%) | 48.15% | 47.18% | ||
| IV | 59 (14.47%) | 106 (19.74) | 14.49% | 17.16% | ||
| Preop Radiotherapy | 214 (52.45%) | 346 (64.43%) | 0.245 | 58.05% | 57.60% | 0.009 |
| Preop Chemotherapy | 328 (80.39%) | 428 (79.70%) | 0.017 | 78.17% | 78.35% | 0.004 |
| Center | 0.727 | 0.015 | ||||
| A | 263 (64.46%) | 163 (30.35%) | 44.44% | 45.21% | ||
| B | 145 (35.54%) | 374 (69.65%) | 55.56% | 54.79% | ||
| Abdominal surgical technique | 0.631 | 0.034 | ||||
| Laparoscopy | 400 (98.04%) | 423 (78.77%) | 85.92% | 87.08% | ||
| Laparotomy | 8 (1.96%) | 114 (21.23%) | 14.08% | 12.92% | ||
| Type of resection | 0.698 | 0.016 | ||||
| Ivor Lewis | 254 (62.25%) | 158 (29.42%) | 42.97% | 43.76% | ||
| McKeown | 154 (37.75%) | 379 (70.58%) | 57.03% | 56.24% | ||
| Before IPTW | p | After IPTW | p | |||
|---|---|---|---|---|---|---|
| n (%) | RE | VATE | RE | VATE | ||
| TBO items | ||||||
| (1) No intraop complication | 98.5% | 96.5% | 0.05 | 98.7% | 96.8% | 0.071 |
| (2) Lymph node yield ≥ 15 | 388 (95.1%) | 475 (88.5%) | <0.001 | 95.8% | 90.4% | 0.001 |
| (3) LOS ≤ 21 days | 81.9% | 75% | 0.012 | 78.0% | 76.6% | 0.729 |
| (4) No need of reintervention | 78.2% | 65% | <0.001 | 74.2% | 67.0% | 0.086 |
| (5) Margin-negative resection | 93.9% | 87.2% | 0.001 | 94.2% | 88.6% | 0.005 |
| (6) No readmission to an ICU | 92.2% | 90.9% | 0.493 | 91.0% | 91.9% | 0.692 |
| (7) No readmissions within 30 days from discharge | 87.5% | 84.9% | 0.256 | 88.6% | 84.7% | 0.098 |
| (8) N0 major complications | 73.3% | 60% | <0.001 | 70.6% | 61.1% | 0.024 |
| (9) No 90-day postoperative mortality | 97.1% | 95.5% | 0.224 | 97.6% | 95.2% | 0.056 |
| Overall TBO rates | 54.4% | 40.6% | <0.001 | 53.3% | 42.2% | 0.008 |
| Non-TBO items | ||||||
| (1) Number of harvested nodes (mean (SD)) | 32.73 ± 13.343 | 28.80 ± 13.086 | <0.001 | 33.33 ± 14.07 | 29.62 ± 12.78 | 0.001 |
| (2) LOS, days (mean (SD)) | 17.06 ± 13.381 | 21.47 ± 19.037 | <0.001 | 17.77 ± 13.11 | 20.87 ± 19.57 | 0.017 |
| (3) Blood loss, mL (mean (SD)) | 132.43 ± 94.556 | 170.82 ± 187.386 | <0.001 | 120.92 ± 89.49 | 169.31 ± 170.16 | <0.001 |
| (4) Total operation time, min (mean (SD)) | ||||||
| Thoracic | 189.63 ± 54.02 | 226.32 ± 727.29 | 0.312 | 176.35 ± 51.31 | 218.96 ± 637.59 | 0.147 |
| Abdomen | 139.42 ± 42.43 | 133.58 ± 53.95 | 0.177 | 145.34 ± 44.41 | 126.59 ± 49.44 | <0.001 |
| Authors (Year of Publication) | Study Design | Sample Size | Minimally Invasive Surgery, n (%) | RE, n (%) | TBO Rate | Survival Impact |
|---|---|---|---|---|---|---|
| Busweiler et al. (2017) [11] | Nationwide study | 2748 | 1347 (49%) | N/A | 29.7% | N/A |
| Van der Werf et al. (2019) [12] | Nationwide study | 4414 | 2595 (58.8%) | N/A | 33% | Yes |
| Bolger et al. (2021) [20] | Single-center study | 269 | 130 (48.3%) | N/A | 32.3% | Yes |
| Kalff et al. (2021) [7] | Two-center study | 1065 | 676 (63.5%) | N/A | 30.7% | Yes |
| Current study | Two-center study | 945 | 945 (100%) | 408 (43.2%) | 46.6% | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagkalos, E.; Grimminger, P.; Gao, X.; Chiu, C.-H.; Uzun, E.; Lang, H.; Wen, Y.-W.; Chao, Y.-K. Incidence and Predictors of Textbook Outcome after Minimally Invasive Esophagectomy for Cancer: A Two-Center Study. Cancers 2024, 16, 1109. https://doi.org/10.3390/cancers16061109
Tagkalos E, Grimminger P, Gao X, Chiu C-H, Uzun E, Lang H, Wen Y-W, Chao Y-K. Incidence and Predictors of Textbook Outcome after Minimally Invasive Esophagectomy for Cancer: A Two-Center Study. Cancers. 2024; 16(6):1109. https://doi.org/10.3390/cancers16061109
Chicago/Turabian StyleTagkalos, Evangelos, Peter Grimminger, Xing Gao, Chien-Hung Chiu, Eren Uzun, Hauke Lang, Yu-Wen Wen, and Yin-Kai Chao. 2024. "Incidence and Predictors of Textbook Outcome after Minimally Invasive Esophagectomy for Cancer: A Two-Center Study" Cancers 16, no. 6: 1109. https://doi.org/10.3390/cancers16061109
APA StyleTagkalos, E., Grimminger, P., Gao, X., Chiu, C.-H., Uzun, E., Lang, H., Wen, Y.-W., & Chao, Y.-K. (2024). Incidence and Predictors of Textbook Outcome after Minimally Invasive Esophagectomy for Cancer: A Two-Center Study. Cancers, 16(6), 1109. https://doi.org/10.3390/cancers16061109





