A–Z of Epigenetic Readers: Targeting Alternative Splicing and Histone Modification Variants in Cancer
Abstract
Simple Summary
Abstract
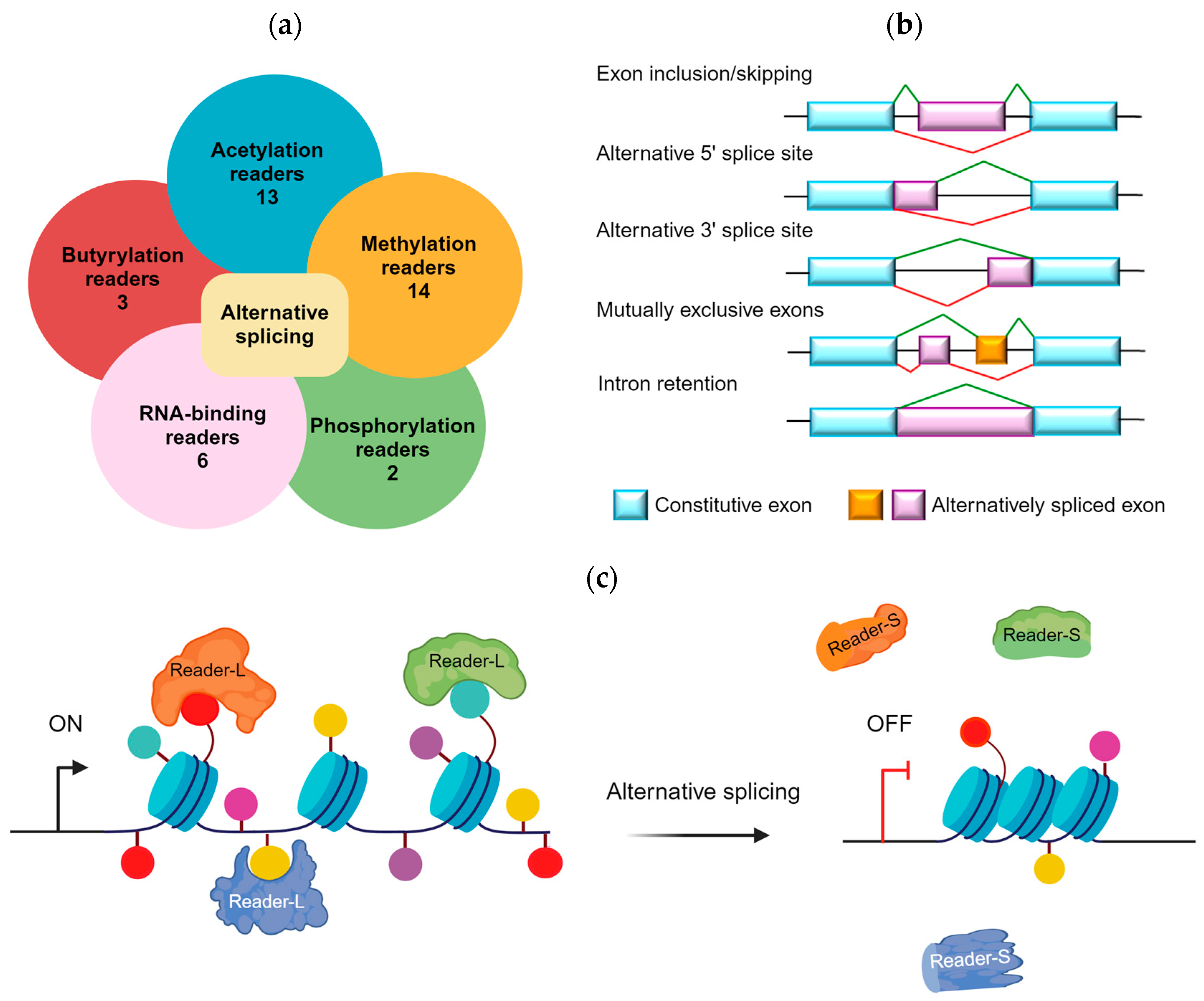
1. Introduction
2. Alternative Splicing of Acetyl Readers
2.1. Acetyl Readers in Cancer
2.2. Acetyl Readers in Other Developmental Processes
3. Alternative Splicing of Methylation Readers
3.1. Methyl Readers in Cancer
3.2. Methyl Readers in Other Developmental Processes
4. Alternative Splicing of Phosphoryl Readers
4.1. Phosphoryl Readers in Cancer
4.2. Phosphoryl Readers in Other Developmental Processes
5. Alternative Splicing in Butyryl Readers
Butyryl Readers in Cancer
6. Alternative Splicing of RNA-Binding Reader Proteins
6.1. RNA-Binding Readers in Cancer
6.2. RNA-Binding Readers as Modulators of Splicing
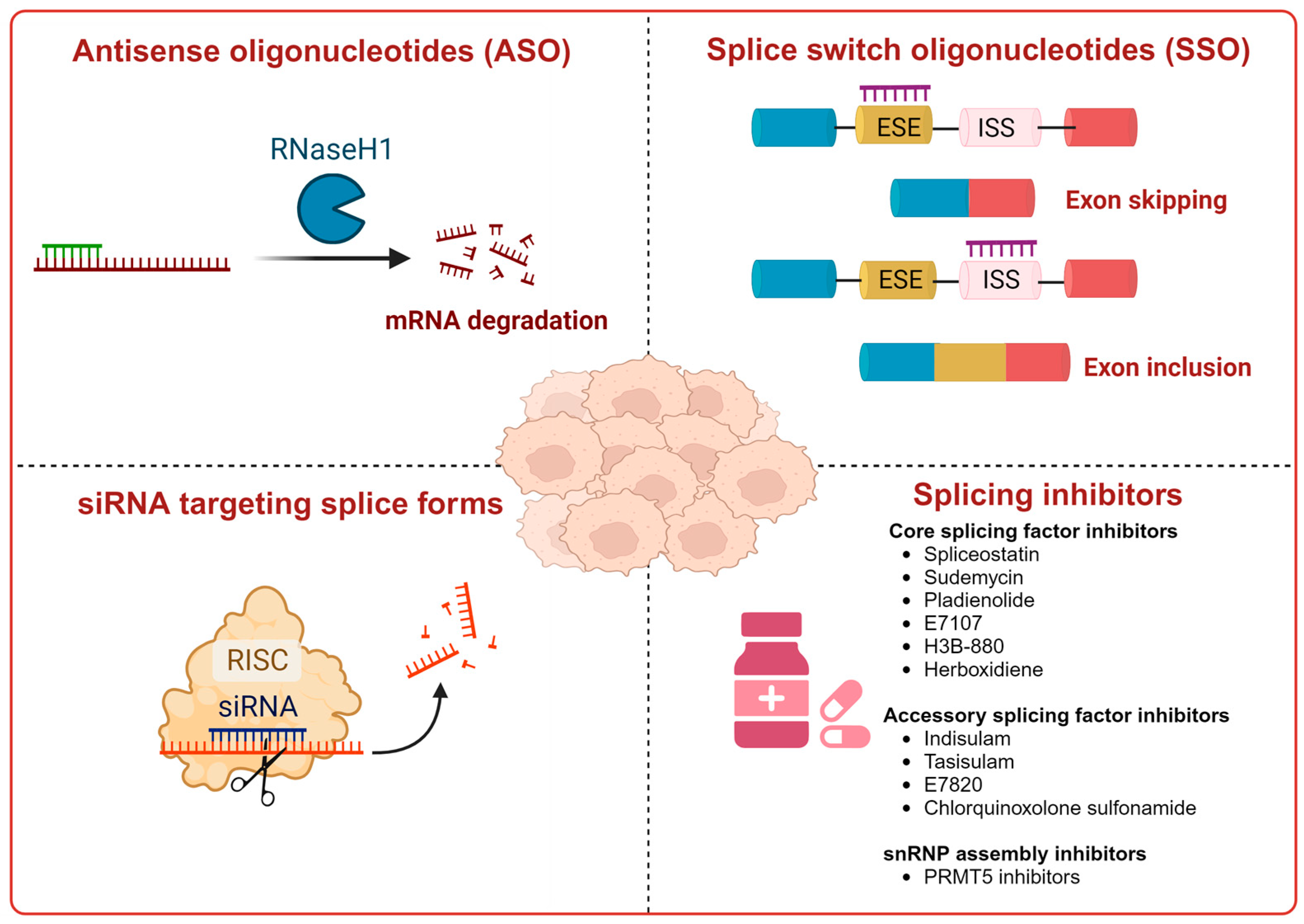
7. Therapeutic Modulation of Alternative Splicing in Cancer
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rothbart, S.B.; Strahl, B.D. Interpreting the language of histone and DNA modifications. Biochim. Biophys. Acta 2014, 1839, 627–643. [Google Scholar] [CrossRef]
- Badeaux, A.I.; Shi, Y. Emerging roles for chromatin as a signal integration and storage platform. Nat. Rev. Mol. Cell Biol. 2013, 14, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Gillette, T.G.; Hill, J.A. Readers, writers, and erasers: Chromatin as the whiteboard of heart disease. Circ. Res. 2015, 116, 1245–1453. [Google Scholar] [CrossRef]
- Delmore, J.E.; Issa, G.C.; Lemieux, M.E.; Rahl, P.B.; Shi, J.; Jacobs, H.M.; Kastritis, E.; Gilpatrick, T.; Paranal, R.M.; Qi, J.; et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 2011, 146, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Bai, G.; Zhao, J.; Wei, X.; Li, R.; Li, J.; Hu, S.; Peng, L.; Liu, P.; Mao, H. The BRD4 inhibitor JQ1 suppresses tumor growth by reducing c-Myc expression in endometrial cancer. J. Transl. Med. 2022, 20, 336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qian, J.; Gu, C.; Yang, Y. Alternative splicing and cancer: A systematic review. Signal Transduct. Target. Ther. 2021, 6, 78. [Google Scholar] [CrossRef]
- Pandya-Jones, A.; Black, D.L. Co-transcriptional splicing of constitutive and alternative exons. RNA 2009, 15, 1896–1908. [Google Scholar] [CrossRef] [PubMed]
- Haque, N.; Oberdoerffer, S. Chromatin and splicing. Methods Mol. Biol. 2014, 1126, 97–113. [Google Scholar] [PubMed]
- Mendenhall, E.M.; Bernstein, B.E. Chromatin state maps: New technologies, new insights. Curr. Opin. Genet. Dev. 2008, 18, 109–115. [Google Scholar] [CrossRef][Green Version]
- Weiner, A.; Hughes, A.; Yassour, M.; Rando, O.J.; Friedman, N. High-resolution nucleosome mapping reveals transcription-dependent promoter packaging. Genome Res. 2010, 20, 90–100. [Google Scholar] [CrossRef]
- Tilgner, H.; Nikolaou, C.; Althammer, S.; Sammeth, M.; Beato, M.; Valcárcel, J.; Guigó, R. Nucleosome positioning as a determinant of exon recognition. Nat. Struct. Mol. Biol. 2009, 16, 996–1001. [Google Scholar] [CrossRef]
- Kornblihtt, A.R.; Schor, I.E.; Alló, M.; Dujardin, G.; Petrillo, E.; Muñoz, M.J. Alternative splicing: A pivotal step between eukaryotic transcription and translation. Nat. Rev. Mol. Cell Biol. 2013, 14, 153–165. [Google Scholar] [CrossRef]
- Chen, M.; Manley, J.L. Mechanisms of alternative splicing regulation: Insights from molecular and genomics approaches. Nat. Rev. Mol. Cell Biol. 2009, 10, 741–754. [Google Scholar] [CrossRef]
- Pan, Q.; Shai, O.; Lee, L.J.; Frey, B.J.; Blencowe, B.J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 2008, 40, 1413–1415. [Google Scholar] [CrossRef]
- Wang, E.T.; Sandberg, R.; Luo, S.; Khrebtukova, I.; Zhang, L.; Mayr, C.; Kingsmore, S.F.; Schroth, G.P.; Burge, C.B. Alternative isoform regulation in human tissue transcriptomes. Nature 2008, 456, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Pinto, S.M.; Getnet, D.; Nirujogi, R.S.; Manda, S.S.; Chaerkady, R.; Madugundu, A.K.; Kelkar, D.S.; Isserlin, R.; Jain, S.; et al. A draft map of the human proteome. Nature 2014, 509, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Verdin, E.; Ott, M. 50 years of protein acetylation: From gene regulation to epigenetics, metabolism and beyond. Nat. Rev. Mol. Cell Biol. 2015, 16, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Histone modifications for human epigenome analysis. J. Hum. Genet. 2013, 58, 439–445. [Google Scholar] [CrossRef]
- Filippakopoulos, P.; Qi, J.; Picaud, S.; Shen, Y.; Smith, W.B.; Fedorov, O.; Morse, E.M.; Keates, T.; Hickman, T.T.; Felletar, I.; et al. Selective inhibition of BET bromodomains. Nature 2010, 468, 1067–1073. [Google Scholar] [CrossRef]
- Fujisawa, T.; Filippakopoulos, P. Functions of bromodomain-containing proteins and their roles in homeostasis and cancer. Nat. Rev. Mol. Cell Biol. 2017, 18, 246–262. [Google Scholar] [CrossRef]
- Uppal, S.; Gegonne, A.; Chen, Q.; Thompson, P.S.; Cheng, D.; Mu, J.; Meerzaman, D.; Misra, H.S.; Singer, D.S. The Bromodomain protein 4 contributes to the regulation of alternative splicing. Cell Rep. 2019, 29, 2450–2460. [Google Scholar] [CrossRef] [PubMed]
- Hussong, M.; Kaehler, C.; Kerick, M.; Grimm, C.; Franz, A.; Timmermann, B.; Welzel, F.; Isensee, J.; Hucho, T.; Krobitsch, S.; et al. The bromodomain protein BRD4 regulates splicing during heat shock. Nucleic Acids Res. 2017, 45, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Stirnweiss, A.; McCarthy, K.; Oommen, J.; Crook, M.L.; Hardy, K.; Kees, U.R.; Wilton, S.D.; Anazodo, A.; Beesley, A.H. A novel BRD4-NUT fusion in an undifferentiated sinonasal tumor highlights alternative splicing as a contributing oncogenic factor in NUT midline carcinoma. Oncogenesis 2015, 4, e174. [Google Scholar] [CrossRef] [PubMed]
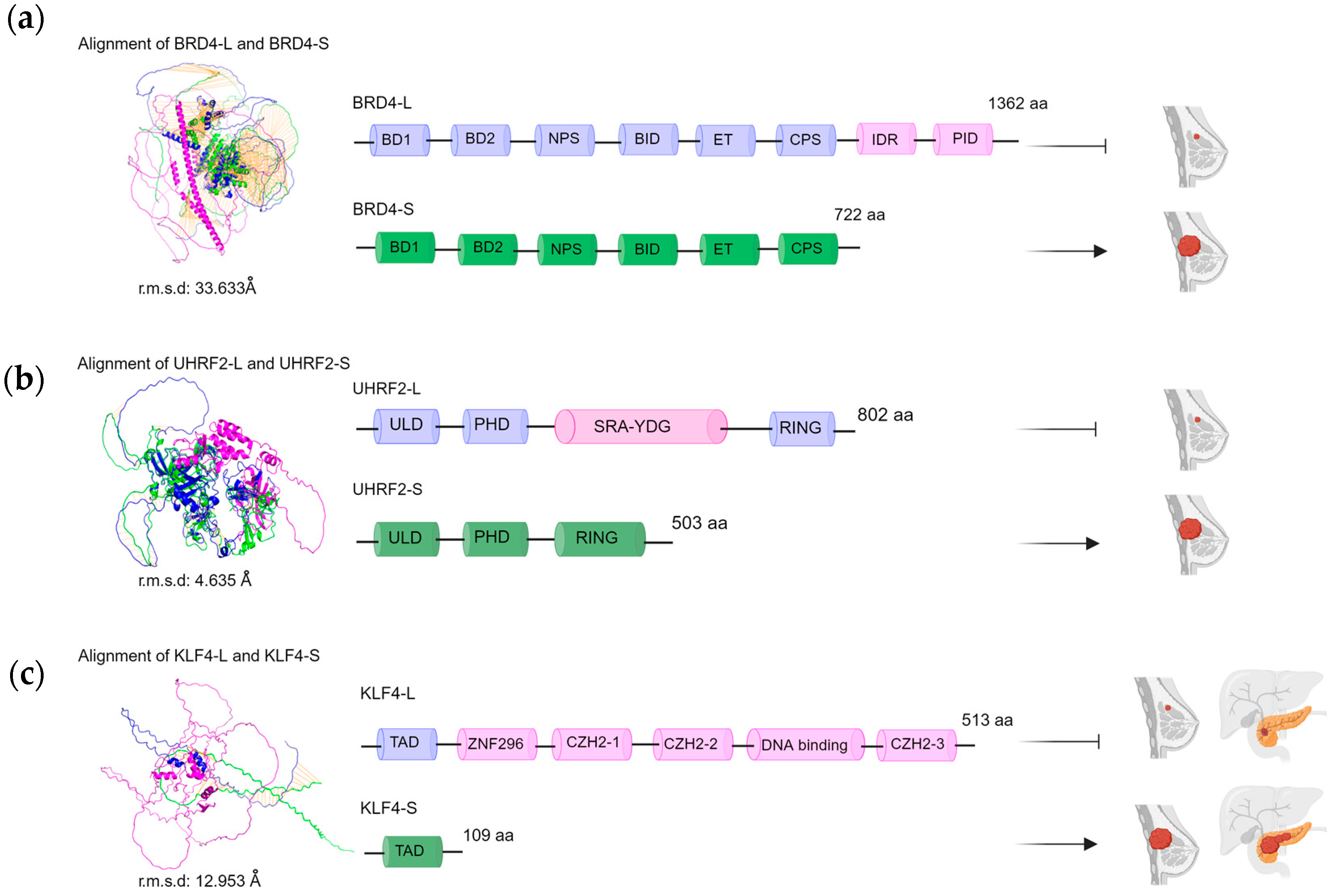
- Zhang, S.; Roeder, R.G. The Long and the short of BRD4: Two tales in breast cancer. Mol. Cell 2020, 78, 993–995. [Google Scholar] [CrossRef]
- Wu, S.Y.; Lee, C.F.; Lai, H.T.; Yu, C.T.; Lee, J.E.; Zuo, H.; Tsai, S.Y.; Tsai, M.J.; Ge, K.; Wan, Y.; et al. Opposing functions of BRD4 isoforms in breast cancer. Mol. Cell 2020, 78, 1114–1132. [Google Scholar] [CrossRef]
- Shao, Z.; Zhang, R.; Khodadadi-Jamayran, A.; Chen, B.; Crowley, M.R.; Festok, M.A.; Crossman, D.K.; Townes, T.M.; Hu, K. The acetyllysine reader BRD3R promotes human nuclear reprogramming and regulates mitosis. Nat. Commun. 2016, 7, 10869. [Google Scholar] [CrossRef]
- Yang, M.; Sun, Y.; Ma, L.; Wang, C.; Wu, J.M.; Bi, A.; Liao, D.J. Complex alternative splicing of the smarca2 gene suggests the importance of smarca2-B variants. J. Cancer 2011, 2, 386–400. [Google Scholar] [CrossRef]
- Szafranski, K.; Schindler, S.; Taudien, S.; Hiller, M.; Huse, K.; Jahn, N.; Schreiber, S.; Backofen, R.; Platzer, M. Violating the splicing rules: TG dinucleotides function as alternative 3′ splice sites in U2-dependent introns. Genome Biol. 2007, 8, R154. [Google Scholar] [CrossRef]
- Wong, A.K.; Shanahan, F.; Chen, Y.; Lian, L.; Ha, P.; Hendricks, K.; Ghaffari, S.; Iliev, D.; Penn, B.; Woodland, A.M.; et al. BRG1, a component of the SWISNF complex, is mutated in multiple human tumor cell lines. Cancer Res. 2000, 60, 6171–6177. [Google Scholar] [PubMed]
- Medina, P.P.; Carretero, J.; Fraga, M.F.; Esteller, M.; Sidransky, D.; Sanchez-Cespedes, M. Genetic and epigenetic screening for gene alterations of the chromatin-remodeling factor, SMARCA4/BRG1, in lung tumors. Genes Chromosomes Cancer 2004, 41, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Chiba, H.; Muramatsu, M.; Nomoto, A.; Kato, H. Two human homologues of Saccharomyces cerevisiae SWI2/SNF2 and Drosophila brahma are transcriptional coactivators cooperating with the estrogen receptor and the retinoic acid receptor. Nucleic Acids Res. 1994, 22, 1815–1820. [Google Scholar] [CrossRef] [PubMed]
- Medina, P.P.; Sanchez-Cespedes, M. Involvement of the chromatin-remodeling factor BRG1/SMARCA4 in human cancer. Epigenetics 2008, 3, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tsai, Y.H.; Tseng, S.H. Regulation of ZMYND8 to Treat Cancer. Molecules 2021, 26, 1083. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.E.; Xu, Y.; Lin, X.; Gao, X.D.; Qiu, Y.; Ahn, J.; Xiao, X.; Cheng, C. Coregulation of alternative splicing by hnRNPM and ESRP1 during EMT. RNA 2018, 24, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Siam, A.; Baker, M.; Amit, L.; Regev, G.; Rabner, A.; Najar, R.A.; Bentata, M.; Dahan, S.; Cohen, K.; Araten, S.; et al. Regulation of alternative splicing by p300-mediated acetylation of splicing factors. RNA 2019, 25, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zheng, L.; Park, J.W.; Lv, R.; Chen, H.; Jiao, F.; Xu, W.; Mu, S.; Wen, H.; Qiu, J.; et al. BS69/ZMYND11 reads and connects histone H3.3 lysine 36 trimethylation-decorated chromatin to regulated pre-mRNA processing. Mol. Cell 2014, 56, 298–310. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Xiao, H.; Wu, C.; Badenhorst, P. Alternative splicing of NURF301 generates distinct NURF chromatin remodeling complexes with altered modified histone binding specificities. PLoS Genet. 2009, 5, e1000574. [Google Scholar] [CrossRef] [PubMed]
- Berkovits, B.D.; Wang, L.; Guarnieri, P.; Wolgemuth, D.J. The testis-specific double bromodomain-containing protein BRDT forms a complex with multiple spliceosome components and is required for mRNA splicing and 3′-UTR truncation in round spermatids. Nucleic Acids Res. 2012, 40, 7162–7175. [Google Scholar] [CrossRef]
- Zheng, Y.; Yuan, W.; Zhou, Z.; Xu, M.; Sha, J.H. Molecular cloning and expression of a novel alternative splice variant of BRDT gene. Int. J. Mol. Med. 2005, 15, 315–321. [Google Scholar] [CrossRef]
- Katzenberger, R.J.; Marengo, M.S.; Wassarman, D.A. ATM and ATR pathways signal alternative splicing of Drosophila TAF1 pre-mRNA in response to DNA damage. Mol. Cell. Biol. 2006, 26, 9256–9267. [Google Scholar] [CrossRef]
- Hnilicová, J.; Hozeifi, S.; Stejskalová, E.; Dušková, E.; Poser, I.; Humpolíčková, J.; Hof, M.; Staněk, D. The C-terminal domain of Brd2 is important for chromatin interaction and regulation of transcription and alternative splicing. Mol. Biol. Cell 2013, 24, 3557–3568. [Google Scholar] [CrossRef] [PubMed]
- Shang, E.; Cui, Q.; Wang, X.; Beseler, C.; Greenberg, D.A.; Wolgemuth, D.J. The bromodomain-containing gene BRD2 is regulated at transcription, splicing, and translation levels. J. Cell. Biochem. 2011, 112, 2784–2793. [Google Scholar] [CrossRef] [PubMed]
- Banting, G.S.; Barak, O.; Ames, T.M.; Burnham, A.C.; Kardel, M.D.; Cooch, N.S.; Davidson, C.E.; Godbout, R.; McDermid, H.E.; Shiekhattar, R. CECR2, a protein involved in neurulation, forms a novel chromatin remodeling complex with SNF2L. Hum. Mol. Genet. 2005, 14, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.C.; Tallant, C.; Fedorov, O.; Witwicka, H.; Hwang, S.Y.; van Stiphout, R.G.; Lambert, J.P.; Rogers, C.; Yapp, C.; Gerstenberger, B.S.; et al. Selective Targeting of Bromodomains of the Bromodomain-PHD Fingers Family Impairs Osteoclast Differentiation. ACS Chem. Biol. 2017, 12, 2619–2630. [Google Scholar] [CrossRef] [PubMed]
- Riverso, M.; Montagnani, V.; Stecca, B. KLF4 is regulated by RAS/RAF/MEK/ERK signaling through E2F1 and promotes melanoma cell growth. Oncogene 2017, 36, 3322–3333. [Google Scholar] [CrossRef]
- Ferralli, J.; Chiquet-Ehrismann, R.; Degen, M. KLF4α stimulates breast cancer cell proliferation by acting as a KLF4 antagonist. Oncotarget 2016, 7, 45608–45621. [Google Scholar] [CrossRef]
- Scharnhorst, V.; Menke, A.L.; Attema, J.; Haneveld, J.K.; Riteco, N.; van Steenbrugge, G.J.; van der Eb, A.J.; Jochemsen, A.G. EGR-1 enhances tumor growth and modulates the effect of the Wilms’ tumor 1 gene products on tumorigenicity. Oncogene 2000, 19, 791–800. [Google Scholar] [CrossRef]
- Wu, J.; Liu, S.; Liu, G.; Dombkowski, A.; Abrams, J.; Martin-Trevino, R.; Wicha, M.S.; Ethier, S.P.; Yang, Z.-Q. Identification and functional analysis of 9p24 amplified genes in human breast cancer. Oncogene 2012, 31, 333–341. [Google Scholar] [CrossRef]
- Saraiva-Agostinho, N.; Barbosa-Morais, N.L. psichomics: Graphical application for alternative splicing quantification and analysis. Nucleic Acids Res. 2019, 47, e7. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, J.; Weng, L.; Wirbisky, S.E.; Freeman, J.L.; Liu, J.; Liu, Q.; Yuan, X.; Irudayaraj, J. Regulatory landscape and clinical implication of MBD3 in human malignant glioma. Oncotarget 2016, 7, 81698–81714. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Dong, Q.; Yuan, X.; Zeng, X.; Gao, Y.; Chiao, C.; Li, H.; Zhao, X.; Keles, S.; Wang, Z.; et al. Misregulation of Alternative Splicing in a Mouse Model of Rett Syndrome. PLoS Genet. 2016, 1, e1006129. [Google Scholar] [CrossRef]
- Zheng, Z.; Ambigapathy, G.; Keifer, J. MeCP2 regulates Tet1-catalyzed demethylation, CTCF binding, and learning-dependent alternative splicing of the BDNF gene in Turtle. eLife 2017, 6, e25384. [Google Scholar] [CrossRef]
- Martínez de Paz, A.; Khajavi, L.; Martin, H.; Claveria-Gimeno, R.; Tom Dieck, S.; Cheema, M.S.; Sanchez-Mut, J.V.; Moksa, M.M.; Carles, A.; Brodie, N.I.; et al. MeCP2-E1 isoform is a dynamically expressed, weakly DNA-bound protein with different protein and DNA interactions compared to MeCP2-E2. Epigenetics Chromatin 2019, 12, 63. [Google Scholar] [CrossRef]
- Osenberg, S.; Karten, A.; Sun, J.; Li, J.; Charkowick, S.; Felice, C.A.; Kritzer, M.; Nguyen, M.V.C.; Yu, P.; Ballas, N. Activity-dependent aberrations in gene expression and alternative splicing in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. USA 2018, 115, E5363–E5372. [Google Scholar] [CrossRef]
- Brito, D.V.C.; Gulmez Karaca, K.; Kupke, J.; Frank, L.; Oliveira, A.M.M. MeCP2 gates spatial learning-induced alternative splicing events in the mouse hippocampus. Mol. Brain 2020, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.F.; Ben-Porath, I.; Bird, A.P. Mbd1 is recruited to both methylated and nonmethylated CpGs via distinct DNA binding domains. Mol. Cell. Biol. 2004, 24, 3387–3395. [Google Scholar] [CrossRef] [PubMed]
- Laget, S.; Joulie, M.; Le Masson, F.; Sasai, N.; Christians, E.; Pradhan, S.; Roberts, R.J.; Defossez, P.A. The human proteins MBD5 and MBD6 associate with heterochromatin but they do not bind methylated DNA. PLoS ONE 2010, 5, e11982. [Google Scholar] [CrossRef]
- Camarena, V.; Cao, L.; Abad, C.; Abrams, A.; Toledo, Y.; Araki, K.; Araki, M.; Walz, K.; Young, J.I. Disruption of Mbd5 in mice causes neuronal functional deficits and neurobehavioral abnormalities consistent with 2q23.1 microdeletion syndrome. EMBO Mol. Med. 2014, 6, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Mullegama, S.V.; Elsea, S.H. Intragenic MBD5 familial deletion variant does not negatively impact MBD5 mRNA expression. Mol. Cytogenet. 2014, 7, 80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheon, S.; Culver, A.M.; Bagnell, A.M.; Ritchie, F.D.; Vacharasin, J.M.; McCord, M.M.; Papendorp, C.M.; Chukwurah, E.; Smith, A.J.; Cowen, M.H.; et al. Counteracting epigenetic mechanisms regulate the structural development of neuronal circuitry in human neurons. Mol. Psychiatry 2022, 27, 2291–2303. [Google Scholar] [CrossRef]
- Lu, Y.; Loh, Y.H.; Li, H.; Cesana, M.; Ficarro, S.B.; Parikh, J.R.; Salomonis, N.; Toh, C.X.; Andreadis, S.T.; Luckey, C.J.; et al. Alternative splicing of MBD2 supports self-renewal in human pluripotent stem cells. Cell Stem Cell 2014, 15, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.M.; Baker, R.D.; Bader, S.; Dunlop, M.G.; Nicholl, I.D. The identification of a novel alternatively spliced form of the MBD4 DNA glycosylase. Oncol. Rep. 2007, 17, 111–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, H.; Li, N.; Fu, D.; Ren, J.; Hui, J.; Peng, J.; Liu, Y.; Qiu, T.; Jiang, M.; Pan, Q.; et al. Histone methyltransferase SETD2 modulates alternative splicing to inhibit intestinal tumorigenesis. J. Clin. Investig. 2017, 127, 3375–3391. [Google Scholar] [CrossRef] [PubMed]
- Luco, R.F.; Pan, Q.; Tominaga, K.; Blencowe, B.J.; Pereira-Smith, O.M.; Misteli, T. Regulation of alternative splicing by histone modifications. Science 2010, 327, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Tauber, M.; Kreuz, S.; Lemak, A.; Mandal, P.; Yerkesh, Z.; Veluchamy, A.; Al-Gashgari, B.; Aljahani, A.; Cortés-Medina, L.V.; Azhibek, D.; et al. Alternative splicing and allosteric regulation modulate the chromatin binding of UHRF1. Nucleic Acids Res. 2020, 48, 7728–7747. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, K.; Hu, G.; Babarinde, I.A.; Li, Y.; Dong, X.; Chen, Y.S.; Shang, L.; Guo, W.; Wang, J.; et al. An alternative CTCF isoform antagonizes canonical CTCF occupancy and changes chromatin architecture to promote apoptosis. Nat. Commun. 2019, 10, 1535. [Google Scholar] [CrossRef]
- Aliperti, V.; Sgueglia, G.; Aniello, F.; Vitale, E.; Fucci, L.; Donizetti, A. Identification, Characterization, and Regulatory Mechanisms of a novel EGR1 splicing isoform. Int. J. Mol. Sci. 2019, 20, 1548. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Fang, F.; Tourtellotte, W.; Varga, J. Egr-1: New conductor for the tissue repair orchestra directs harmony (regeneration) or cacophony (fibrosis). J. Pathol. 2013, 229, 286–297. [Google Scholar] [CrossRef]
- Ulusan, A.M.; Rajendran, P.; Dashwood, W.M.; Yavuz, O.F.; Kapoor, S.; Gustafson, T.A.; Savage, M.I.; Brown, P.H.; Sei, S.; Mohammed, A.; et al. Optimization of Erlotinib Plus Sulindac Dosing Regimens for Intestinal Cancer Prevention in an Apc-Mutant Model of Familial Adenomatous Polyposis (FAP). Cancer Prev. Res. 2021, 14, 325–336. [Google Scholar] [CrossRef]
- Pilyugin, M.; André, P.A.; Ratajska, M.; Kuzniacka, A.; Limon, J.; Tournier, B.B.; Colas, J.; Laurent, G.; Irminger-Finger, I. Antagonizing functions of BARD1 and its alternatively spliced variant BARD1δ in telomere stability. Oncotarget 2017, 8, 9339–9353. [Google Scholar] [CrossRef] [PubMed]
- Mugabo, Y.; Sadeghi, M.; Fang, N.N.; Mayor, T.; Lim, G.E. Elucidation of the 14-3-3ζ interactome reveals critical roles of RNA-splicing factors during adipogenesis. J. Biol. Chem. 2018, 293, 6736–6750. [Google Scholar] [CrossRef]
- Inoue, D.; Chew, G.L.; Liu, B.; Michel, B.C.; Pangallo, J.; D’Avino, A.R.; Hitchman, T.; North, K.; Lee, S.C.; Bitner, L.; et al. Spliceosomal disruption of the non-canonical BAF complex in cancer. Nature 2019, 574, 432–436. [Google Scholar] [CrossRef]
- Mi, W.; Guan, H.; Lyu, J.; Zhao, D.; Xi, Y.; Jiang, S.; Andrews, F.H.; Wang, X.; Gagea, M.; Wen, H.; et al. YEATS2 links histone acetylation to tumorigenesis of non-small cell lung cancer. Nat. Commun. 2017, 8, 1088. [Google Scholar] [CrossRef]
- Nazim, M.; Lin, C.-H.; Feng, A.-C.; Yeom, K.-H.; Li, M.; Daly, A.E.; Tan, X.; Vu, H.; Ernst, J.; Carey, M.F.; et al. Alternative splicing of a chromatin modifier alters the transcriptional regulatory programs of stem cell maintenance and neuronal differentiation. bioRxiv 2023. [Google Scholar] [CrossRef]
- Huber, F.M.; Greenblatt, S.M.; Davenport, A.M.; Martinez, C.; Xu, Y.; Vu, L.P.; Nimer, S.D.; Hoelz, A. Histone-binding of DPF2 mediates its repressive role in myeloid differentiation. Proc. Natl. Acad. Sci. USA 2017, 114, 6016–6021. [Google Scholar] [CrossRef]
- Choi, Y.P.; Kang, S.; Hong, S.; Xie, X.; Cho, N.H. Proteomic analysis of progressive factors in uterine cervical cancer. Proteomics 2005, 5, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, L.; Collins, I.; Ge, H.; Libutti, D.; Li, J.; Egly, J.M.; Levens, D. The FBP interacting repressor targets TFIIH to inhibit activated transcription. Mol. Cell 2000, 5, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Malz, M.; Bovet, M.; Samarin, J.; Rabenhorst, U.; Sticht, C.; Bissinger, M.; Roessler, S.; Bermejo, J.L.; Renner, M.; Calvisi, D.F.; et al. Overexpression of far upstream element (FUSE) binding protein (FBP)-interacting repressor (FIR) supports growth of hepatocellular carcinoma. Hepatology 2014, 60, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Matalkah, F.; Jeong, B.; Sheridan, M.; Horstick, E.; Ramamurthy, V.; Stoilov, P. The Musashi proteins direct post-transcriptional control of protein expression and alternate exon splicing in vertebrate photoreceptors. Commun. Biol. 2022, 5, 1011. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, A.Q.; Zhou, S.L.; Lv, H.; Wei, P.; Yang, W.T. RNA-binding protein MSI2 isoforms expression and regulation in progression of triple-negative breast cancer. J. Exp. Clin. Cancer Res. 2020, 39, 92. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Zeng, P.; Zhou, X.; Zhao, X.; Chen, R.; Qiao, J.; Feng, L.; Zhu, Z.; Zhang, G.; Chen, C. RBMX suppresses tumorigenicity and progression of bladder cancer by interacting with the hnRNP A1 protein to regulate PKM alternative splicing. Oncogene 2021, 40, 2635–2650. [Google Scholar] [CrossRef] [PubMed]
- Loerch, S.; Leach, J.R.; Horner, S.W.; Maji, D.; Jenkins, J.L.; Pulvino, M.J.; Kielkopf, C.L. The pre-mRNA splicing and transcription factor Tat-SF1 is a functional partner of the spliceosome SF3b1 subunit via a U2AF homology motif interface. J. Biol. Chem. 2019, 294, 2892–2902. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.B.; Saunders, K.O.; Tomaras, G.D.; Garcia-Blanco, M.A. Tat-SF1 is not required for Tat transactivation but does regulate the relative levels of unspliced and spliced HIV-1 RNAs. PLoS ONE 2009, 4, e5710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tran, N.T.; Su, H.; Wang, R.; Lu, Y.; Tang, H.; Aoyagi, S.; Guo, A.; Khodadadi-Jamayran, A.; Zhou, D.; et al. Cross-talk between PRMT1-mediated methylation and ubiquitylation on RBM15 controls RNA splicing. eLife 2015, 4, e07938. [Google Scholar] [CrossRef]
- Heinicke, L.A.; Nabet, B.; Shen, S.; Jiang, P.; van Zalen, S.; Cieply, B.; Russell, J.E.; Xing, Y.; Carstens, R.P. The RNA binding protein RBM38 (RNPC1) regulates splicing during late erythroid differentiation. PLoS ONE 2013, 8, e78031. [Google Scholar] [CrossRef]
- Muraoka, S.; Fukumura, K.; Hayashi, M.; Kataoka, N.; Mayeda, A.; Kaida, D. Rbm38 Reduces the Transcription Elongation Defect of the SMEK2 Gene Caused by Splicing Deficiency. Int. J. Mol. Sci. 2020, 21, 8799. [Google Scholar] [CrossRef]
- Ganaie, S.S.; Chen, A.Y.; Huang, C.; Xu, P.; Kleiboeker, S.; Du, A.; Qiu, J. RNA Binding Protein RBM38 Regulates Expression of the 11-Kilodalton Protein of Parvovirus B19, Which Facilitates Viral DNA Replication. J. Virol. 2018, 92, e02050-17. [Google Scholar] [CrossRef]
- Thonda, S.; Vinnakota, R.L.; Kona, S.V.; Kalivendi, S.V. Identification of RBMX as a splicing regulator in Parkinsonian mimetic induced alternative splicing of α-synuclein. Biochim. Biophys. Acta Gene Regul. Mech. 2022, 1865, 194825. [Google Scholar] [CrossRef]
- Cai, T.; Cinkornpumin, J.K.; Yu, Z.; Villarreal, O.D.; Pastor, W.A.; Richard, S. Deletion of RBMX RGG/RG motif in Shashi-XLID syndrome leads to aberrant p53 activation and neuronal differentiation defects. Cell Rep. 2021, 36, 109337. [Google Scholar] [CrossRef]
- Wang, B.D.; Lee, N.H. Aberrant RNA Splicing in Cancer and Drug Resistance. Cancers 2018, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Di, C.; Syafrizayanti; Zhang, Q.; Chen, Y.; Wang, Y.; Zhang, X.; Liu, Y.; Sun, C.; Zhang, H.; Hoheisel, J.D. Function, clinical application, and strategies of Pre-mRNA splicing in cancer. Cell Death Differ. 2019, 26, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, H.; Nishimura, K.; Yoshimi, A. Aberrant RNA splicing and therapeutic opportunities in cancers. Cancer Sci. 2022, 113, 373–381. [Google Scholar] [CrossRef]
- Renshaw, J.; Orr, R.M.; Walton, M.I.; Te Poele, R.; Williams, R.D.; Wancewicz, E.V.; Monia, B.P.; Workman, P.; Pritchard-Jones, K. Disruption of WT1 gene expression and exon 5 splicing following cytotoxic drug treatment: Antisense down-regulation of exon 5 alters target gene expression and inhibits cell survival. Mol. Cancer Ther. 2004, 3, 1467–1484. [Google Scholar] [CrossRef] [PubMed]
- Mogilevsky, M.; Shimshon, O.; Kumar, S.; Mogilevsky, A.; Keshet, E.; Yavin, E.; Heyd, F.; Karni, R. Modulation of MKNK2 alternative splicing by splice-switching oligonucleotides as a novel approach for glioblastoma treatment. Nucleic Acids Res. 2018, 46, 11396–11404. [Google Scholar] [CrossRef] [PubMed]
- Broering, R.; Real, C.I.; John, M.J.; Jahn-Hofmann, K.; Ickenstein, L.M.; Kleinehr, K.; Paul, A.; Gibbert, K.; Dittmer, U.; Gerken, G.; et al. Chemical modifications on siRNAs avoid Toll-like-receptor-mediated activation of the hepatic immune system in vivo and in vitro. Int. Immunol. 2014, 26, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Damiani, E.; Duran, M.N.; Mohan, N.; Rajendran, P.; Dashwood, R.H. Targeting Epigenetic ‘Readers’ with Natural Compounds for Cancer Interception. J. Cancer Prev. 2020, 25, 189–203. [Google Scholar] [CrossRef]
- Bashari, A.; Siegfried, Z.; Karni, R. Targeting splicing factors for cancer therapy. RNA 2023, 29, 506–515. [Google Scholar] [CrossRef]
- Murphy, A.J.; Li, A.H.; Li, P.; Sun, H. Therapeutic Targeting of Alternative Splicing: A New Frontier in Cancer Treatment. Front. Oncol. 2022, 12, 868664. [Google Scholar] [CrossRef]
- El Marabti, E.; Abdel-Wahab, O. Therapeutic Modulation of RNA Splicing in Malignant and Non-Malignant Disease. Trends Mol. Med. 2021, 27, 643–659. [Google Scholar] [CrossRef]
- Martinez-Montiel, N.; Rosas-Murrieta, N.H.; Anaya Ruiz, M.; Monjaraz-Guzman, E.; Martinez-Contreras, R. Alternative Splicing as a Target for Cancer Treatment. Int. J. Mol. Sci. 2018, 19, 545. [Google Scholar] [CrossRef]
- Seiler, M.; Yoshimi, A.; Darman, R.; Chan, B.; Keaney, G.; Thomas, M.; Agrawal, A.A.; Caleb, B.; Csibi, A.; Sean, E.; et al. H3B-8800, an orally available small-molecule splicing modulator, induces lethality in spliceosome-mutant cancers. Nat. Med. 2018, 24, 497–504. [Google Scholar] [CrossRef]
- Ting, T.C.; Goralski, M.; Klein, K.; Wang, B.; Kim, J.; Xie, Y.; Nijhawan, D. Aryl Sulfonamides Degrade RBM39 and RBM23 by Recruitment to CRL4-DCAF15. Cell Rep. 2019, 29, 1499–1510. [Google Scholar] [CrossRef]
- Hwang, J.W.; Cho, Y.; Bae, G.U.; Kim, S.N.; Kim, Y.K. Protein arginine methyltransferases: Promising targets for cancer therapy. Exp. Mol. Med. 2021, 53, 788–808. [Google Scholar] [CrossRef]
- Janisiak, J.; Kopytko, P.; Tkacz, M.; Rogińska, D.; Perużyńska, M.; Machaliński, B.; Pawlik, A.; Tarnowski, M. Protein Arginine Methyltransferase (PRMT) Inhibitors-AMI-1 and SAH Are Effective in Attenuating Rhabdomyosarcoma Growth and Proliferation in Cell Cultures. Int. J. Mol. Sci. 2021, 22, 8023. [Google Scholar] [CrossRef]
- Sauter, C.; Simonet, J.; Guidez, F.; Dumétier, B.; Pernon, B.; Callanan, M.; Bastie, J.N.; Aucagne, R.; Delva, L. Protein Arginine Methyltransferases as Therapeutic Targets in Hematological Malignancies. Cancers 2022, 14, 5443. [Google Scholar] [CrossRef] [PubMed]
- Tunyasuvunakool, K.; Adler, J.; Wu, Z.; Green, T.; Zielinski, M.; Žídek, A.; Bridgland, A.; Cowie, A.; Meyer, C.; Laydon, A.; et al. Highly accurate protein structure prediction for the human proteome. Nature 2021, 596, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]

| Acetylation Reader | Splicing Event | Functional Implication | References |
|---|---|---|---|
| BRD2 | Inclusion of exon 2a; Exon 7 and intron inclusion in IL17RC; Intron retention in DUSP2 | Neural development; Signal transduction modulation | [41,42] |
| BRD4 | Intron retention; Fusion of exon 15 (BRD4) with exon 2 (NUT); Cassette exons in CD45, Arhgef1, and Picalm | Heat stress response; Oncogenic fusion; Signal transduction; Cell communication | [21,22,23] |
| BRD3 | Exon skipping | Nuclear reprogramming | [26] |
| BRDT | Intron 6 retention | Spermatogenesis | [39] |
| BRPF1 | Cassette exon 9 | Osteoclastogenesis | [44] |
| CECR2 | Exon 2–8 skipping | Neural development | [43] |
| NURF | Intron retention (partial exon 6) | Spermatocyte differentiation | [37] |
| P300 | Exon skipping/inclusions in CD44 | Signal transduction | [35] |
| SMARCA2 | Alternative 3′ splice site in exon 1; Exon 2–5 skipping | Tumorigenesis | [27] |
| SMARCA4 | Alternative TG splice site in intron 28; Extra exon between exons 26 and 27 | Spermatogenesis | [29,30,31,32] |
| TAF1 | Cassette exons 12a and 13a | Spermatogenesis | [40] |
| ZMYND8 | Exon 22 inclusion | Breast cancer | [34] |
| ZMYND11 | Intron retention | Chromatin regulation in pre-mRNA processing | [36] |
| Methylation Reader | Splicing Event | Functional Implication | References |
|---|---|---|---|
| ASH1L | Exon 3–5 inclusion | Neuronal morphogenesis | [60] |
| CTCF | Exon 3–4 skipping | Chromatin regulation | [66] |
| EGR1 | Exon 2 skipping | Transcriptional activation alteration | [67] |
| KLF4 | Exon 3 deletion | Oncogenic activity in various cancers | [45,46] |
| MBD1 | Exon 10 skipping, Alternative 3′ end | Transcriptional regulation | [56] |
| MBD2 | Alternative 3′ end | Stem cell differentiation, Chromatin remodeler interaction | [61] |
| MBD3 | Exon 9–11 skipping | Breast tumorigenesis | [50] |
| MBD4 | Exon 3 skipping | Metabolic activity alteration | [62] |
| MBD5 | Intron 9 retention, Exon 10–15 skipping, Intron 11 retention, Exon 12 and 14 skipping, Inherited intronic deletion in 5′-UTR | Neurodevelopment, Cellular localization, and Abundance changes | [57,58,59] |
| MeCP2 | Exon 2 skipping, Intron retention, Flop exon inclusion | Neurodevelopment, Cognition, and Synaptic transmission | [51,52,53,54,55] |
| SETD2 | Exon inclusion | Chromatin regulation | [63,64] |
| UHRF1 | Intron retention | Chromatin regulation | [65] |
| UHRF2 | Exon 10 inclusion | Breast tumor suppression | [49] |
| WT1 | Intron retention | Tumorigenesis | [47] |
| Phosphoryl Reader | Splicing Event | Functional Implication | References |
|---|---|---|---|
| BARD1 | Exon 2–6 skipping | Tumor suppression | [70] |
| YWHAQ | Exon 3 skipping | Lipogenesis | [71] |
| Butyryl Reader | Splicing Event | Functional Implication | References |
|---|---|---|---|
| BRD9 | SF3B1 mutation-induced exonization of introns | Impairment of non-canonical BAF complex activity | [72] |
| DPF2 | Exon 7 skipping | Neural cell differentiation | [74] |
| YEATS2 | Exon inclusion | Downregulation of splicing genes | [73] |
| RNA Reader | Splicing Event | Biological Implication | References |
|---|---|---|---|
| FIR | Exon-2 skipping | Oncogenesis in liver cancer | [78] |
| MSI2 | Promotion of photoreceptor-specific alternate exons, Promotion of TP53INP1 mRNA stability | Photoreceptor survival, cancer cell invasion | [79,80] |
| RBM15 | Interactions with intronic regions of pre-mRNA | Gene expression in blood cell development | [84] |
| RBM38 | Splicing activation, ISE-2 interaction | Hematopoietic splicing regulation | [85,86,87] |
| RBMX | Intron-4 interaction, Disruption of hnRNPA1 and PKM interaction, Exon-6 exclusion in MDM4 | Neurological disease modulation, splicing regulation | [81,88,89] |
| TAT-SF1 | Intron inclusion | Spliceosome function, viral RNA processing | [82,83] |
| Drug Name | Chemical Structure | Target(s) |
|---|---|---|
| Spliceostatin | 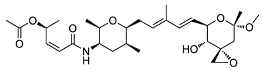 | Inhibits the SF3B1 complex, affecting spliceosome function |
| Sudemycin | 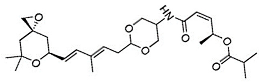 | Same as Above |
| Pladienolide | 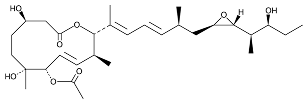 | Same as Above |
| H3B-8800 | 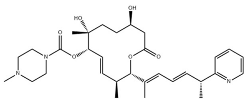 | Same as Above |
| Herboxidiene | 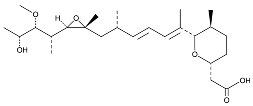 | Same as Above |
| E7107 |  | Disrupts spliceosome assembly by targeting spliceosome-associated protein-130 |
| Indisulam |  | Targets RNA Binding Motif 39 (RBN39) for degradation |
| Tasisulam |  | Apoptosis induction via the intrinsic pathway in cancer |
| E7820 |  | Interferes with cell adhesion and metastasis by targeting Integrin alpha2 |
| Chloroquinoxaline sulfonamides | 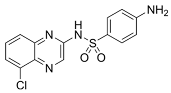 | Inhibits Topoisomerase II alpha/beta, affecting DNA replication/cell division |
| GSK3368715 | 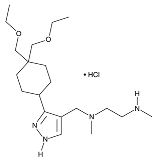 | Inhibits protein arginine methyltransferases (PRMTs), affecting RNA splicing |
| MS023 | 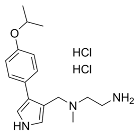 | Same as Above |
| SGC707 | 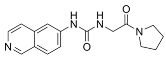 | Same as Above |
| TP-064 | 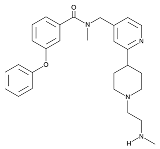 | Same as Above |
| SGC6870 | 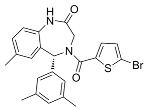 | Same as Above |
| AMI-1 |  | Same as Above |
| EZM2302 | 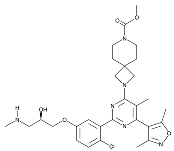 | Same as Above |
| EPZ0220411 | 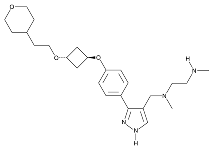 | Same as Above |
| MS049 |  | Same as Above |
| Allantodapsone | 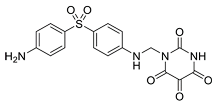 | Targets bacterial adhesins like Staphylococcus aureus ClfA and ClfB |
| EPZ-15666 | 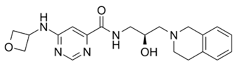 | Specifically inhibits PRMT5, affecting RNA splicing |
| GSK3326595 | 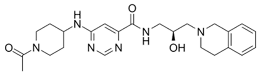 | Same as Above |
| LLY-283 | 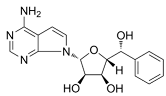 | Same as Above |
| JNJ-64619178 |  | Same as Above |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohan, N.; Dashwood, R.H.; Rajendran, P. A–Z of Epigenetic Readers: Targeting Alternative Splicing and Histone Modification Variants in Cancer. Cancers 2024, 16, 1104. https://doi.org/10.3390/cancers16061104
Mohan N, Dashwood RH, Rajendran P. A–Z of Epigenetic Readers: Targeting Alternative Splicing and Histone Modification Variants in Cancer. Cancers. 2024; 16(6):1104. https://doi.org/10.3390/cancers16061104
Chicago/Turabian StyleMohan, Nivedhitha, Roderick H. Dashwood, and Praveen Rajendran. 2024. "A–Z of Epigenetic Readers: Targeting Alternative Splicing and Histone Modification Variants in Cancer" Cancers 16, no. 6: 1104. https://doi.org/10.3390/cancers16061104
APA StyleMohan, N., Dashwood, R. H., & Rajendran, P. (2024). A–Z of Epigenetic Readers: Targeting Alternative Splicing and Histone Modification Variants in Cancer. Cancers, 16(6), 1104. https://doi.org/10.3390/cancers16061104








