Simple Summary
There is a lack of literature on the role of molecular classification in patients with morphological low-risk EC. We aimed to evaluate the incidence and prognostic role of p53 mutations in this specific subgroup of patients. Our findings show that 4.9% of low-risk EC are p53abn; the OR for the recurrence of p53abn versus p53wt patients was 5.23—CI 95% 0.98–27.95, p = 0.053. No difference in OS was observed between the two groups. Recurrences were mostly local and occur two years after diagnosis. Our data might serve as a valuable tool for clinicians’ everyday practice, but larger prospective studies are urgently needed.
Abstract
No prospective study has validated molecular classification to guide adjuvant treatment in endometrial cancer (EC), and not even retrospective data are present for patients with morphological low-risk EC. We conducted a retrospective, multicenter, observational study including 370 patients with low-risk endometrioid EC to evaluate the incidence and prognostic role of p53 abnormal expression (p53abn) in this specific subgroup. Among 370 patients, 18 had abnormal expressions of p53 (4.9%). In 13 out of 370 patients (3.6%), recurrences were observed and two were p53abn. When adjusting for median follow-up time, the odds ratio (OR) for recurrence among those with p53abn versus p53 wild type (p53wt) was 5.23—CI 95% 0.98–27.95, p = 0.053. The most common site of recurrence was the vaginal cuff (46.2%). One recurrence occurred within the first year of follow-up, and the patient exhibited p53abn. Both 1-year and 2-year DFS rates were 94.4% and 100% in the p53abn and p53wt groups, respectively. One patient died from the disease and comprised p53wt. No difference in OS was registered between the two groups; the median OS was 21.9 months (16.4–30.1). Larger multicenter studies are needed to tailor the treatment of low-risk EC patients with p53abn. Performing molecular classification on all EC patients might be cost-effective, and despite the limits of our relatively small sample, p53abn patients seem to be at greater risk of recurrence, especially locally and after two years since diagnosis.
1. Introduction
Endometrial cancer (EC) is the most common gynecological malignancy in industrialized countries with a steadily increasing trend [1]. Although prognosis in early stages is mostly favorable with a 5-year overall survival of about 95% [2,3], around 20% of patients with early-stage disease have an unfavorable prognosis [4,5,6].
The new molecular classification introduced by the Cancer Genome Atlas (TGCA) defined four molecular risk groups based on mutational burden: ultramutated tumors with polymerase epsilon (POLE) mutations that have excellent prognoses, microsatellite instability (MSI), a low copy number (known as NSMP) with intermediate prognosis, and a high copy number with frequent tumor protein p53 (TP53) alterations and poor prognoses. This classification was added to the older morphological evaluation with major consequences on EC prognosis, diagnosis, and treatment [7,8,9,10].
Patients with TP53-mutated tumors, accounting for about 15% of all EC diagnoses and 10% of morphological low-risk cases, have the worst prognosis with up to 50–70% of all EC mortality [8,11]. According to the latest guidelines, this subgroup of high-risk patients benefits from adjuvant treatment with chemotherapy or sequential/concurrent radiotherapy [3].
To date, no prospective study has validated the use of molecular classification to guide adjuvant treatment in EC, and to our knowledge, not even retrospective data are present for patients with morphological low-risk EC [12,13]. Notably, the ongoing prospective randomized PORTEC-4 [14] and RAINBO [15] studies, designed with molecular-integrated risk profile-based recommendations, will introduce exceptional results that will shape the future management of EC, but the first one still does not include patients with very low-risk EC.
The main rationale of our study is to evaluate the incidence and prognostic role of molecular profiles, particularly the role of abnormal p53 immunohistochemical expression (p53abn) in patients with morphological low-risk EC.
2. Materials and Methods
2.1. Study Design
A retrospective, multicenter, observational study was conducted, including 370 patients with low-risk endometrioid EC treated from 1 January 2016 to 31 December 2020 in 10 different Italian gynecologic oncology departments (Santa Croce e Carle in Cuneo, Santa Maria della Misericordia in Udine, Policlinico Sant’Orsola in Bologna, Azienda Ospedaliera Universitaria Integrata di Verona, Ospedale Maggiore in Parma, Fondazione Giovanni Paolo II in Campobasso, Policlinico Martino in Messina, Sacro Cuore Don Calabria in Negrar, Ospedale di Polla S. Arsenio in Salerno, and Ospedale Martini in Torino).
Trained medical doctors reviewed operative room registers and gynecologic oncology databases to identify all low-risk endometrioid EC patients who underwent surgery and a subsequent follow-up of at least 24 months at each study center. Low-risk endometrioid EC was defined as FIGO stage IA, G1-G2 endometrioid, and lymph-vascular space invasion (LVSI) negative EC. The final pathology report of primary surgery was used for case classification, based on FIGO staging according to the 2009 revised classification system. POLE mutation testing was included only in a minority of cases due to the recent introduction of the method [3].
Among identified patients with low-risk endometrioid EC, those who underwent neoadjuvant or adjuvant treatment or had synchronous malignancy were excluded. Of the included patients, clinicopathological, surgical, and survival data were extracted from medical records. When follow-up information was updated in December 2022, telephone contact was made with the patients or their relatives. All patients attended follow-up visits at all study centers according to ESGO guidelines [3] (Figure 1).
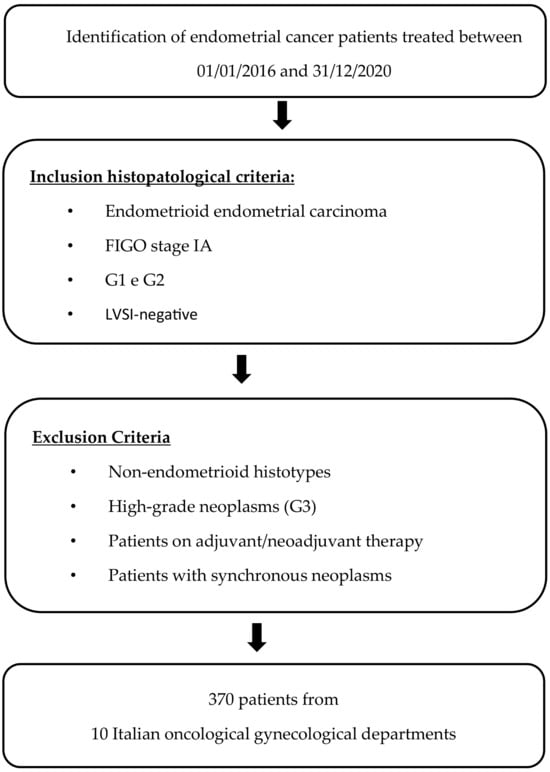
Figure 1.
Development process.
As a primary outcome, we evaluated the incidence of p53abn (immunohistochemical surrogate for TP53 molecular alterations). p53 immunohistochemistry patterns were defined as follows: (1) wild-type pattern (normal p53 IHC): when the distribution of nuclear staining in a “wild type” pattern ranges from a few positive cells to almost all cells being stained, but with variable intensity; (2) hyperexpression (abnormal p53 IHC): defined as strong nuclear staining in at least 80% of tumor cell nuclei; (3) complete absence or null pattern (abnormal): defined as no staining in tumor cell nuclei in the presence of the “wild-type”; (4) cytoplasmic (abnormal): defined as predominant cytoplasmic staining in the absence of strong nuclear staining in >80% of tumor cell nuclei, (5) subclonal expression (abnormal): defined as the combination of normal with one or more abnormal patterns;(6) inconclusive: when none of the last criteria are met [16,17,18].
All centers that participated in the study utilized the same analysis method for the study of p53.
As a secondary endpoint, we evaluated patients’ survival in terms of disease-free survival (DFS), defined as the time from the date of diagnosis to the detection of recurrence or the latest observation, and overall survival (OS), defined as the time from the date of primary surgery to death or the latest observation. Recurrence was defined as the histological evidence of the original disease after primary surgical treatment. We also analyzed the most common histopathological and clinical characteristics associated with the abnormal expression of p53.
All research activities were approved by the Institutional Review Board (16 March 2022—N° PROT. APROV. 51-2022), and written informed consent was waived by the IRB because patients’ data were collected anonymously.
2.2. Statistical Analysis
Descriptive statistical indicators were estimated to describe clinical and demographic characteristics. Quantitative variables will be summarized with the mean, standard deviation, min, and max. Qualitative variables will be described using frequency (absolute and percentage) tables. The incidence of the p53abn protein in patients with FIGO stage IA G1-G2, LVSI-negative EC, and response and recurrence rates were estimated and shown with relative 95%. confidence intervals. PFS and OS were analyzed using Kaplan–Meier curves and a relative 95% confidence interval. All statistical analyses were performed using SAS® and STATA Statistics Software (STATA 18 Version, year 2023) [19,20,21].
3. Results
3.1. General Demographic and Clinical Data
Between 1 January 2016 and 31 December 2020, 370 consecutive low-risk endometrioid EC patients underwent surgery at ten Italian centers. The median follow-up was 34.2 months (range of 32.3–36.1).
The average age of patients in the study population was 63. Most patients were postmenopausal (89.7%) and overweight (body mass index > 30 kg/m2) (31.9%). The family history of EC was reported by 2.9% of women. Vaginal bleeding was the main symptom at onset (74.1% of cases), while five patients (1.4%) presented with abnormal pap smear results. Fourteen patients (3.8%) had a positive remote pathological history for gynecological cancers, and preoperatively, 69.2% of women were correctly diagnosed with stage FIGO IA via ultrasound. In total, 162 patients (43.8%) underwent peritoneal washing cytology (PWC), radical hysterectomy (RH), and bilateral salpingo-oophorectomy (BSO), while all others underwent concomitant retroperitoneal staging, either by sentinel lymph node (SLN) evaluation and/or radical lymphadenectomy (Table 1).

Table 1.
Demographic and clinical characteristics of 370 women.
3.2. Histopathological and Immunohistochemical Characteristics
Histopathological and immunohistochemical characteristics of the 370 patients are reported in Table 2.

Table 2.
Histopathological and immunohistochemical characteristics of 370 women. WT = “wild type” expression; Abn hyp = abnormal hyperexpression; Abn null = abnormal “null” expression; Abn cyt = abnormal cytoplasmic expression; Abn sub = abnormal subclonal; ER = estrogen; PgR = progesterone.
Among p53abn patients, 16 (88.9%) had G1 tumors versus 201 (57.1%) in the p53wt group (p-value p = 0.007). The MELF pattern was present in 1 patient in the p53abn group (5.6%) versus 39 (11.1%) in the p53wt group (Table 3).

Table 3.
Comparison between the characteristics of the 18 p53abn patients and the 352 p53wt patients.
In both groups, the majority of women were p-MMR (15 (83.3%) for MSH-6 and MSH-2 and 18 (100%) for PMS-2 and MLH-1 in p53abn patients and 333 (94.6%) for MSH-6, 334 (97.7%) for MSH-2, 294 (83.5%) for PMS-2, and 297 (84.5%) for MLH-1 in p53wt patients).
The vast majority of patients in both groups had tumors expressing hormonal receptors (18 (94.4%) for both ER and PgR in p53abn patients and 343 (97.4%) and 335 (95.2%) for ER and PgR, respectively, in the p53wt group) (Table 3). A statistically significant difference was found between the two groups concerning MSH-2 expression (p = 0.006) and POLE mutation (p = 0.007).
In total, 216 patients (58.4%) had G1 tumors and 154 (41.6%) had G2. Microcystic elongated and fragmented (MELF) patterns were present in 10.5% of women. Regarding mismatched repair proteins, most patients were proficient (p-MMR): 93.5% for MSH-6, 93.2% for MSH-1, 70.3% for PMS-2, and 81.6% for MLH-1. In the majority of patients, ER was expressed in more than 50% of cells (72.7%), and PgR was also expressed in 56.8%. POLE analysis was carried out in 11.6% of cases, and no patient carried this mutation.
Most patients (95.1%) exhibited the normal IHC expression of p53 (wild-type—wt), while 18 patients exhibited abnormal expression (4.9%). Among these, seven had abn hyp expression (1.9%), eight had abn null (2.2%), two had abn cyt (0.5%), and one had abn sub (0.3%).
The average age of p53abn women was 65 versus 63 in p53wt patients. p53-mutated patients were postmenopausal in 100% of cases versus 86.4% in the p53wt group, and overweight was observed in 55.6% versus 79.5% in the p53wt group. In both groups, most patients underwent PWC, RH, BSO, and SLN biopsies (61.1% and 36.1% for p53abn and p53wt patients, respectively). Among clinical data, the only statistically significant data that differed between the two study groups were those about oncological family history (p = 0.0001) and the type of surgery (p = 0.0002).
3.3. Survival Analysis
Survival data are provided in Table 4.

Table 4.
Follow-up characteristics and survival data of 370 women.
Four patients were lost at follow-up. The mean follow-up time from initial diagnosis was 34.2 months (32.3–36.1). The median follow-up of patients with p53abn was 22.2 months (IC 95% 15.4–29.0) versus 34.9 months (IC 95% 32.95–36.79) with respect to patients with p53wt.
At the last follow-up (December 2022), 359 (98.1%) patients were alive without evidence of disease, 3 (0.8%) were alive with disease, 1 (0.3%) died due to disease, and 3 patients (0.8%) died from causes other than EC that had never previously recurred.
There were 13 recurrences among our cohort (3.6%). Among patients who recurred, two patients were p53abn, and the OR for recurrence for patients with p53abn was 3.83 (95% CI 0.78–18.74) (p = 0.09). When adjusting for median follow-up time, the OR for recurrence among those with p53abn versus p53 wild type (p53wt) was 5.23—CI 95% 0.98–27.95—which was at the limits and exhibited statistical significance (p = 0.053).
Only one recurrence was registered within the first year of follow-up; this patient was p53abn.
Both 1-year and 2-year DFS rates were 94.4% and 100% in the p53abn and p53wt groups, respectively, and the median DFS was 21.8 (IC 95% 15.4–29.0) and 34.2 months (IC 95% 32.6–36.6) for mutated and wt patients, respectively (Figure 2).
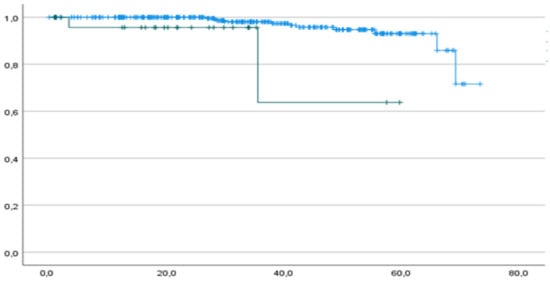
Figure 2.
DFS of p53abn patients (green curve) and p53wt patients (blue curve). Horizontal axis (x-axis): months since surgery. Vertical axis (y-axis): cumulative survival.
In Table 5, we reported the main characteristics of the two p53abn patients who recurred.

Table 5.
Main characteristics of p53abn patients who recurred. PT = patient; PL = pelvic lymphadenectomy; BMI = body mass index; AUB = abnormal uterine bleeding; DFS = disease-free survival; FUP = follow-up; PWC = peritoneal washing cytology; RH = radical hysterectomy; BSO = bilateral salpingo-oophorectomy; PA = para-aortic; G = grading; NED = note evidence of disease; ° = time calculated in months. * The patient underwent surgery at recurrence: histological examination of the lymphadenopathy showed high-grade tumors, p53 showed a mixed expression (mutated and wild-type areas), and PWC was positive. MMR proteins were proficient, and ER and PgR were only scarcely expressed (<1%). She underwent para-aortic radiotherapy. The last FUP was carried out with CT-PET in September 2023.
The most common site of recurrence was the vaginal cuff (six patients, 46.1%), followed by isolated pelvic peritoneal and isolated parenchymatous (two patients and 15.4% each). Eight patients (61.5%) underwent surgery, four (30.8%) received radiotherapy only, and only one patient (7.7%) was treated with chemotherapy at relapse
Only one patient died from the disease (0.3%), and she had p53wt. One-year OS was 100% and 99.7% in the p53abn and p53wt groups, respectively. Two-year OS was 100% and 99.1% in the p53abn and p53wt groups, respectively. The median OS was 21.9 months (IC 95% 16.4–30.1) for p53abn and 34.2 months (IC 95% 33.2–37.0) for p53wt patients (Figure 3).
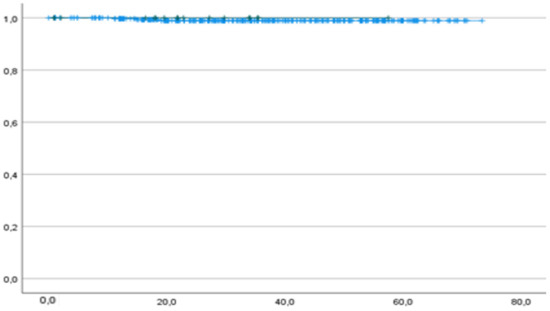
Figure 3.
OS of p53abn (green curve) and p53wt (blue curve) patients. p = 0.709. Horizontal axis (x-axis): months since surgery. Vertical axis (y-axis): cumulative survival.
4. Discussion
The study describes the prevalence of p53abn in a cohort of low-risk EC patients (4.9%) and aims to assess whether the p53 mutation is associated with survival outcomes in low-risk patients to help clinicians manage their patients in everyday clinical practice. The plausible poor prognostic significance of the p53 mutation was confirmed by the recent 2023 FIGO staging guidelines that upstaged p53abn FIGO IA (2009) tumors relative to FIGO stage IIC [22]. None of the studies from which such guidelines were formulated were included; however, very low-risk morphological patients and the major ongoing prospective studies investigating molecular profiles have not included such subgroups of low-risk patients either [14].
A reliable strategy to identify patients at high risk of recurrence despite low morphological risk is urgently needed. There are plenty of data in the literature regarding the prognostic role of molecular classifications on EC [9,23]; less clear is its role in guiding adjuvant treatment since prospective studies are missing to date.
Molecular profiling is recommended routinely in all patients with EC [3]. According to the current guidelines, POLE mutation should be performed as the first analysis [24]; however, as most patients are diagnosed with low morphological risk, it is questionable whether such an approach is cost-effective in clinical practice, especially since POLE analysis cannot routinely be performed for the paucity of laboratories and high costs [25]. We also need to consider the real use of molecular data in daily clinical practice in this particular subgroup of women in the absence of literature data that allows clinicians to tailor adjuvant treatments.
In a recent study by Vrede et al. [26], tumor molecular profiles were not associated with the outcomes in patients with low-grade EC, and the authors concluded that molecular classification could be omitted for this subgroup.
In our opinion, POLE analysis should be performed if not previously carried out in cases of low-risk p53abn EC to exclude multiple classifiers [27].
According to a recent study, the characteristics of POLEmut-p53abn resembled those of POLEmut, characterized by an excellent prognosis in the absence of adjuvant therapies [28].
We also strongly suggest analyzing p53 mutations in such settings using next-generation sequencing (NGS) and not only immunohistochemistry since this technique is more reliable in reducing the rate of false positives [29].
In our cohort, 2 out of 13 patients who recurred had p53abn (15%): Despite the limitations of a small sample, p53abn patients seem to be at a greater risk of recurrence, especially locally and two years after diagnosis. When adjusting for the median follow-up time, recurrence risk was higher for p53abn patients (OR 5.23–CI 95% 0.98–27.95; p = 0.053). The results remain consistent even at the limits of statistical significance, and this is probably due to an overestimation of the beta error caused by the low incidence of p53abn in the study group. The sample size of the two groups is actually very different, and the higher risk for the recurrence of p53abn should be considered in light of these data.
A worse DFS starts mainly after 40 months among our patients, but the curves already differ within the first 35 months (Figure 2). We registered one recurrence within the first year of follow-up, and this patient had p53abn; the remaining 12 recurrences occurred after 24 months, and only 1 had p53abn. Among patients who recurred, two had p53abn (Table 5).
In total, four patients within our cohort died (1.1%), and out of these, one (0.3%) was a result of EC. In particular, this 64-year-old patient comprised the p53 wild type, and her tumor was p-MMR; ER was positive in 60% of cells and was PgR (in 40%). She recurred in the vaginal cuff and pelvic lymph nodes after six months, and she finally died of the disease (OS 28 months).
There was no significant difference between the p53abn and p53wt groups regarding OS (Figure 3).
Our study is strengthened by being the only study in the literature that has so far investigated patients with p53abn and low morphological risks. Moreover, apart from giving a detailed description of the prevalence of such mutations in low-risk patients, we also concentrated on possible clinical, histopathological, and survival associations to understand the physiopathology of this disease and pave the way for tailored treatment more substantially.
The findings of this study must be observed while considering some limitations: The number of events was too low in the subgroups of analysis to extrapolate solid conclusions. Some ways to overcome this limitation may be conducting larger multicenter studies that include bigger cohorts comprising low-risk EC patients. Moreover, our study embraces a relatively long timeframe, which was necessary for recruiting more cases, but this resulted in more heterogeneity in the treatment, especially since the sentinel node algorithm [3] and POLE analysis were introduced. In accordance with ESGO guidelines, not all patients with very low-risk morphological endometrial cancer underwent retroperitoneal staging, and this could have upgraded the tumor stage [3,30]. Lastly, the median follow-up time differed significantly for the two groups (22.2 versus 34.9 months in p53abn versus p53wt patients).
5. Conclusions
Given the rarity of p53abn EC in morphological low-risk EC, the literature is extremely limited. Since no data from prospective studies allow us to modify the adjuvant therapy of low-risk women based on molecular analysis so far, we at least suggest intensifying the follow-up of p53abn women. Based on our study, although within the described limits, we recommend that clinicians pay attention primarily to the local recurrence of p53abn tumors, even after two years since the primary treatment.
We believe that the incidence of p53abn in morphological low-risk EC patients (4.9%) is sufficiently high to make it advisable to investigate p53 protein statuses in all EC patients, particularly since the diagnosis of p53abn is possible in routine clinical practice with relatively low costs and high reproducibility. This analysis is even feasible preoperatively on endometrial biopsy specimens, and this may be particularly relevant for young patients who desire to preserve their fertility [31]. As mentioned, we need to keep in mind that the p53 status only has a prognostic role so far, and it is not yet sufficiently strong for modifying adjuvant treatment in low-risk EC patients.
Author Contributions
Conceptualization: A.P. (Andrea Puppo), M.B., G.F.O. and Y.M. Methodology: A.P. (Andrea Puppo) and M.B. Resources: A.P. (Andrea Puppo) and M.B. Supervision: A.P. (Andrea Puppo) and G.F.O. Writing—review and editing: M.B., V.C., A.P. (Andrea Puppo) and Y.M. Formal analysis: G.M. (Giuseppe Migliaretti). Visualization: A.P. (Andrea Puppo), M.B., G.M. (Giuseppe Migliaretti), V.C., C.A.Z., G.V., S.R., L.M., M.O., R.S., D.R., L.B., S.U., A.C., G.V.A., S.G., V.A.C., R.B., F.C., A.E., A.I., M.A., M.C. (Marco Camanni), A.P. (Anna Pesci), G.M. (Giulia Mantovani), F.B., G.R., P.Z., A.R., M.C. (Marcello Ceccaroni), E.M.D. and C.P. Data curation: A.P. (Andrea Puppo), M.B., G.M. (Giuseppe Migliaretti), V.C., C.A.Z., G.V., S.R., L.M., M.O., R.S., D.R., L.B., S.U., A.C., G.V.A., S.G., V.A.C., R.B., F.C., A.E., A.I., M.A., M.C. (Marco Camanni), A.P. (Anna Pesci), G.M. (Giulia Mantovani), F.B., G.R., P.Z., A.R., M.C. (Marcello Ceccaroni), E.M.D., C.P., G.V.T. and G.M.B. Investigation: A.P. (Andrea Puppo), M.B., G.M. (Giuseppe Migliaretti), V.C., C.A.Z., G.V., S.R., L.M., M.O., R.S., D.R., L.B., S.U., A.C., G.V.A., S.G., V.A.C., R.B., F.C., A.E., A.I., M.A., M.C. (Marco Camanni), A.P. (Anna Pesci), G.M. (Giulia Mantovani), F.B., G.R., P.Z., A.R., M.C. (Marcello Ceccaroni), E.M.D., C.P., G.V.T. and G.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. All research activities were approved by the Institutional Review Board (16 March 2022—N° PROT. APROV. 51-2022), and written informed consent was waived by the IRB because patients’ data were collected anonymously.
Informed Consent Statement
Written informed consent was waived by the IRB due to the retrospective nature of the study and because patients’ data were collected anonymously.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Visser, N.C.; Bulten, J.; van der Wurff, A.A.; Boss, E.A.; Bronkhorst, C.M.; Feijen, H.W.; Haartsen, J.E.; van Herk, A.D.M.; de Kievit, I.M.; Klinkhamer, P.J.J.M.; et al. PIpelle Prospective ENDOmetrial carcinoma (PIPENDO) study, pre-operative recognition of high risk endometrial carcinoma: A multicentre prospective cohort study. BMC Cancer 2015, 15, 487. [Google Scholar] [CrossRef] [PubMed]
- TTortorella, L.; Restaino, S.; Zannoni, G.F.; Vizzielli, G.; Chiantera, V.; Cappuccio, S.; Gioè, A.; La Fera, E.; Dinoi, G.; Angelico, G.; et al. Substantial lymph-vascular space invasion (LVSI) as predictor of distant relapse and poor prognosis in low-risk early-stage endometrial cancer. J. Gynecol. Oncol. 2021, 32, e11. [Google Scholar] [CrossRef] [PubMed]
- Restaino, S.; Tortorella, L.; Dinoi, G.; Zannoni, G.F.; Baroni, A.; Capasso, I.; Distefano, E.; Sozzi, G.; Chiantera, V.; Scambia, G.; et al. Semiquantitative evaluation of lymph-vascular space invasion in patients affected by endometrial cancer: Prognostic and clinical implications. Eur. J. Cancer. 2021, 142, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.A.; The Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Jamieson, A.; Thompson, E.F.; Huvila, J.; Gilks, C.B.; McAlpine, J.N. p53abn Endometrial Cancer: Understanding the most aggressive endometrial cancers in the era of molecular classification. Int. J. Gynecol. Cancer 2021, 31, 907–913. [Google Scholar] [CrossRef]
- Leon-Castillo, A.; Horeweg, N.; Peters, E.E.; Rutten, T.; Ter Haar, N.; Smit, V.T.; Kroon, C.D.; Boennelycke, M.; Hogdall, E.; Hogdall, C.; et al. Prognostic relevance of the molecular classification in high-grade endometrial cancer for patients staged by lymphadenectomy and without adjuvant treatment. Gynecol. Oncol. 2022, 164, 577–586. [Google Scholar] [CrossRef]
- Restaino, S.; Paglietti, C.; Arcieri, M.; Biasioli, A.; Della Martina, M.; Mariuzzi, L.; Andreetta, C.; Titone, F.; Bogani, G.; Raimondo, D.; et al. Management of Patients Diagnosed with Endometrial Cancer: Comparison of Guidelines. Cancers 2023, 15, 1091. [Google Scholar] [CrossRef]
- Kurnit, K.C.; Kim, G.N.; Fellman, B.M.; Urbauer, D.L.; Mills, G.B.; Zhang, W.; Broaddus, R.R. CTNNB1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod. Pathol. 2017, 30, 1032–1041. [Google Scholar] [CrossRef]
- Wortman, B.G.; Creutzberg, C.L.; Putter, H.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.H.W.; van der Steen-Banasik, E.M.; Mens, J.W.M.; Slot, A.; Kroese, M.C.S.; et al. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: Improving patient selection for adjuvant therapy. Br. J. Cancer 2018, 119, 1067–1074. [Google Scholar] [CrossRef]
- León-Castillo, A.; De Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef] [PubMed]
- Van Den Heerik, A.S.V.; Horeweg, N.; Nout, R.A.; Lutgens, L.C.; Van Der Steen-Banasik, E.M.; Westerveld, G.H.; van den Berg, H.A.; Slot, A.; Koppe, F.L.A.; Kommoss, S.; et al. PORTEC-4a: International randomized trial of molecular profile-based adjuvant treatment for women with high-intermediate risk endometrial cancer. Int. J. Gynecol. Cancer 2020, 30, 2002–2007. [Google Scholar] [CrossRef] [PubMed]
- RAINBO Research Consortium. Refining adjuvant treatment in endometrial cancer based on molecular features: The RAINBO clinical trial program. Int. J. Gynecol. Cancer 2023, 33, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Köbel, M.; Ronnett, B.M.; Singh, N.; Soslow, R.A.; Gilks, C.B.; McCluggage, W.G. Interpretation of p53 immunohistochemistry in endometrial carcinomas: Toward increased reproducibility. Int. J. Gynecol. Pathol. 2019, 38 (Suppl. S1), S123–S131. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Piskorz, A.M.; Bosse, T.; Jimenez-Linan, M.; Rous, B.; Brenton, J.D.; Gilks, C.B.; Köbel, M. p53 immunohistochemistry is an accurate surrogate for TP53 mutational analysis in endometrial carcinoma biopsies. J. Pathol. 2020, 250, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Huvila, J.; Thompson, E.F.; Vanden Broek, J.; Lum, A.; Senz, J.; Leung, S.; Gilks, C.B.; Köbel, M.; McAlpine, J.N.; Jamieson, A. Subclonal p53 immunostaining in the diagnosis of endometrial carcinoma molecular subtype. Histopathology 2023, 83, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Armitage, P.; Berry, G.; Matthews, J.N.S. Statistical Methods in Medical Research, 1st ed.; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar] [CrossRef]
- Ashby, D. Practical statistics for medical research. Douglas G. Altman, Chapman and Hall, London, 1991. Stat. Med. 1991, 10, 1635–1636. [Google Scholar] [CrossRef]
- Parman, M.K.B.; Machin, D. Survival Analysis: A Practical Approach; John Wiley: Chichester, UK, 1995. [Google Scholar]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N.; Endometrial Cancer Staging Subcommittee; et al. FIGO staging of endometrial cancer: 2023. Int. J. Gynecol. Obstet. 2023, 162, 383–394. [Google Scholar] [CrossRef]
- Betella, I.; Fumagalli, C.; Raviele, P.R.; Schivardi, G.; De Vitis, L.A.; Achilarre, M.T.; Aloisi, A.; Garbi, A.; Maruccio, M.; Zanagnolo, V.; et al. A novel algorithm to implement the molecular classification according to the new ESGO/ESTRO/ESP 2020 guidelines for endometrial cancer. Int. J. Gynecol. Cancer 2022, 32. [Google Scholar] [CrossRef]
- Church, D.N.; Stelloo, E.; Nout, R.A.; Valtcheva, N.; Depreeuw, J.; Haar, N.T.; Noske, A.; Amant, F.; Tomlinson, I.P.M.; Wild, P.J.; et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J. Natl. Cancer Inst. 2015, 107, 402. [Google Scholar] [CrossRef] [PubMed]
- León-Castillo, A.; Britton, H.; McConechy, M.K.; McAlpine, J.N.; Nout, R.; Kommoss, S.; Brucker, S.Y.; Carlson, J.W.; Epstein, E.; Rau, T.T.; et al. Interpretation of somatic POLE mutations in endometrial carcinoma. J. Pathol. 2020, 250, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Vrede, S.W.; Kasius, J.; Bulten, J.; Teerenstra, S.; Huvila, J.; Colas, E.; Gil-Moreno, A.; Boll, D.; Vos, M.C.; van Altena, A.M.; et al. Relevance of Molecular Profiling in Patients With Low-Grade Endometrial Cancer. JAMA Netw. Open 2022, 5, e2247372. [Google Scholar] [CrossRef] [PubMed]
- León-Castillo, A.; Gilvazquez, E.; Nout, R.; Smit, V.T.; McAlpine, J.N.; McConechy, M.; Kommoss, S.; Brucker, S.Y.; Carlson, J.W.; Epstein, E.; et al. Clinicopathological and molecular characterisation of ‘multiple-classifier’ endometrial carcinomas. J. Pathol. 2020, 250, 312–322. [Google Scholar] [CrossRef] [PubMed]
- De Vitis, L.A.; Schivardi, G.; Caruso, G.; Fumagalli, C.; Vacirca, D.; Achilarre, M.T.; Aloisi, A.; Garbi, A.; Zanagnolo, V.; Aletti, G.; et al. Clinicopathological characteristics of multiple-classifier endometrial cancers: A cohort study and systematic review. Int. J. Gynecol. Cancer 2024, 34, 229–238. [Google Scholar] [CrossRef]
- Streel, S.; Salmon, A.; Dheur, A.; Bours, V.; Leroi, N.; Habran, L.; Delbecque, K.; Goffin, F.; Pleyers, C.; Kakkos, A.; et al. Diagnostic Performance of Immunohistochemistry Compared to Molecular Techniques for Microsatelli8te Instability and p53 Mutation Detection in Endometrial Cancer. Int. J. Mol. Sci. 2023, 24, 4866. [Google Scholar] [CrossRef] [PubMed]
- Restaino, S.; Buda, A.; Puppo, A.; Capozzi, V.A.; Sozzi, G.; Casarin, J.; Gallitelli, V.; Murgia, F.; Vizzielli, G.; Baroni, A.; et al. Anatomical distribution of sentinel lymph nodes in patients with endometrial cancer: A multicenter study. Int. J. Gynecol. Cancer. 2022, 32, 517–524. [Google Scholar] [CrossRef]
- Fan, Z.; Li, H.; Hu, R.; Liu, Y.; Liu, X.; Gu, L. Fertility-preserving treatment in young women with grade 1 presumed stage Ia endometrial adenocarcinoma: A meta-analysis. Int. J. Gynecol. Cancer 2018, 28, 385–393. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).