Clinicopathological and Genomic Identification of Breast Cancers with No Impact on Mortality
Abstract
Simple Summary
Abstract
1. Introduction
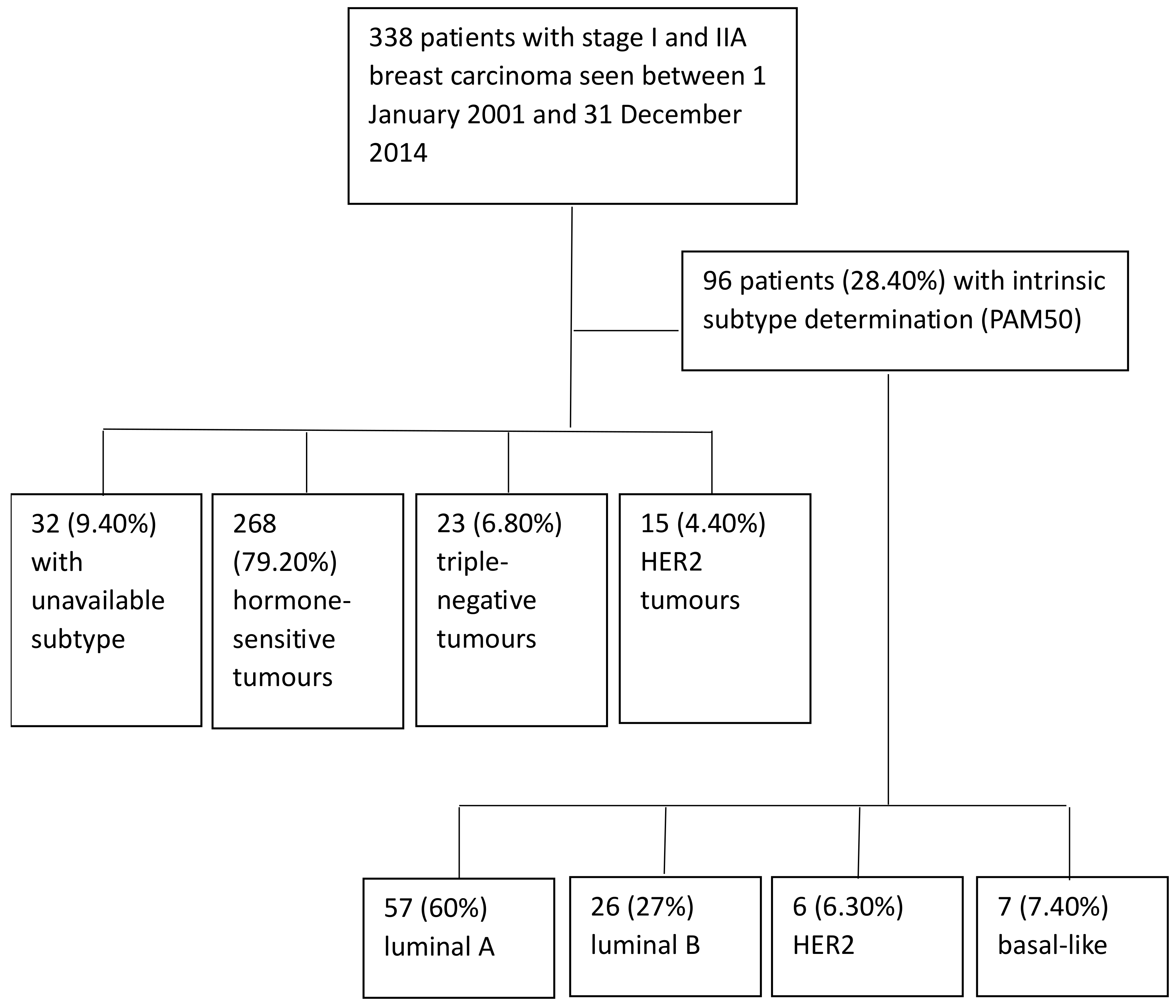
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Immunohistochemistry and Molecular Studies
2.4. Data Analysis
3. Results
3.1. Patient Characteristics
3.2. Event Analysis
3.3. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Srivastava, S.; Koay, E.J.; Borowsky, A.D.; De Marzo, A.M.; Ghosh, S.; Wagner, P.D.; Kramer, B.S. Cancer overdiagnosis: A biological challenge and clinical dilemma. Nat. Rev. Cancer 2019, 19, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Woloshin, S.; Schwartz, L.M.; Black, W.C.; Kramer, B.S. Cancer screening campaigns—Getting past uninformative persuasion. N. Engl. J. Med. 2012, 367, 1677–1679. [Google Scholar] [CrossRef]
- Dunn, B.K.; Woloshin, S.; Xie, H.; Kramer, B.S. Cancer overdiagnosis: A challenge in the era of screening. J. Natl. Cancer Cent. 2022, 2, 235–242. [Google Scholar] [CrossRef]
- Maas, C.C.H.M.; van Klaveren, D.; Visser, O.; Merkx, M.A.W.; Lingsma, H.F.; Lemmens, V.E.P.P.; Dinmohamed, A.G. Number of life-years lost at the time of diagnosis and several years post-diagnosis in patients with solid malignancies: A population-based study in the Netherlands, 1989–2019. eClinicalMedicine 2023, 60, 101994. [Google Scholar] [CrossRef]
- Marcadis, A.R.; Marti, J.L.; Ehdaie, B.; Hakimi, A.; Davies, L.; Morris, L.G.T. Characterizing relative and disease-specific survival in early-stage cancers. JAMA Intern. Med. 2020, 180, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Brouckaert, O.; Schoneveld, A.; Truyers, C.; Kellen, E.; Van Ongeval, C.; Vergote, I.; Moerman, P.; Floris, G.; Wildiers, H.; Christiaens, M.R.; et al. Breast cancer phenotype, nodal status and palpability may be useful in the detection of overdiagnosed screening-detected breast cancers. Ann. Oncol. 2013, 24, 1847–1852. [Google Scholar] [CrossRef] [PubMed]
- Welch, H.G.; Prorok, P.C.; O’Malley, A.J.; Kramer, B.S. Breast-Cancer Tumor Size, Overdiagnosis, and Mammography Screening Effectiveness. N. Engl. J. Med. 2016, 375, 1438–1447. [Google Scholar] [CrossRef]
- Lopes Cardozo, J.M.N.; Drukker, C.A.; Rutgers, E.J.T.; Schmidt, M.K.; Glas, A.M.; Witteveen, A.; Cardoso, F.; Piccart, M.; Esserman, L.J.; Poncet, C.; et al. Outcome of patients with an ultralow-risk 70-gene signature in the MINDACT Trial. J. Clin. Oncol. 2022, 40, 1335–1345. [Google Scholar] [CrossRef]
- Pu, M.; Messer, K.; Davies, S.R.; Vickery, T.L.; Pittman, E.; Parker, B.A.; Ellis, M.J.; Flatt, S.W.; Marinac, C.R.; Nelson, S.H.; et al. Research-based PAM50 signature and long-term breast cancer survival. Breast Cancer Res. Treat. 2020, 179, 197–206. [Google Scholar] [CrossRef]
- Hudis, C.A.; Barlow, W.E.; Costantino, J.P.; Gray, R.J.; Pritchard, K.I.; Chapman, J.A.; Sparano, J.A.; Hunsberger, S.; Enos, R.A.; Gelber, R.D.; et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: The STEEP system. J. Clin. Oncol. 2007, 25, 2127–2132. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R.A. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Baena-Cañada, J.M.; Gámez-Casado, S.; Rodríguez-Pérez, L.; Bandera-López, C.; Mesas-Ruiz, A.; Campini-Bermejo, A.; Bernal-Gómez, M.; Zalabardo-Aguilar, M.; Calvete-Candenas, J.; Martínez-Bernal, G.; et al. Clinicopathological characteristics and survival results of patients with ultralow risk breast cancer. Med. Clin. 2022, 159, 351–358. [Google Scholar] [CrossRef]
- Baker, S.G.; Prorok, P.C.; Kramer, B.S. Lead Time and Overdiagnosis. J. Natl. Cancer Inst. 2014, 106, dju346. [Google Scholar] [CrossRef] [PubMed]
- Horii, R.; Akiyama, F.; Kasumi, F.; Koike, M.; Sakamoto, G. Spontaneous healing of breast cancer. Breast Cancer 2005, 12, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Bastien, R.R.; Rodríguez-Lescure, Á.; Ebbert, M.T.; Prat, A.; Munárriz, B.; Rowe, L.; Miller, P.; Ruiz-Borrego, M.; Anderson, D.; Lyons, B.; et al. PAM50 breast cancer subtyping by RT-qPCR and concordance with standard clinical molecular markers. BMC Med. Genom. 2012, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Köninki, K.; Tanner, M.; Auvinen, A.; Isola, J. HER-2 positive breast cancer: Decreasing proportion but stable incidence in Finnish population from 1982 to 2005. Breast Cancer Res. 2009, 11, R37. [Google Scholar] [CrossRef]
- Alanko, J.; Tanner, M.; Vanninen, R.; Auvinen, A.; Isola, J. Triple-negative and HER2-positive breast cancers found by mammography screening show excellent prognosis. Breast Cancer Res. Treat. 2021, 187, 267–274. [Google Scholar] [CrossRef]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Metcalfe, C.; Davis, M.; Turner, E.L.; Martin, R.M.; Young, G.J.; Walsh, E.I.; Bryant, R.J.; et al. Fifteen-year outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N. Engl. J. Med. 2023, 388, 1547–1558. [Google Scholar] [CrossRef]
- Weyers, W. Forward to the Past—Oncology between underdiagnosis and overtreatment. Am. J. Dermatopathol. 2016, 38, 517–528. [Google Scholar] [CrossRef]
- Lopes Cardozo, J.M.N.; Byng, D.; Drukker, C.A.; Schmidt, M.K.; Binuya, M.A.; van ‘t Veer, L.J.; Cardoso, F.; Piccart, M.; Smorenburg, C.H.; Poncet, C.; et al. Outcome without any adjuvant systemic treatment in stage I ER+/HER2- breast cancer patients included in the MINDACT trial. Ann. Oncol. 2022, 33, 310–320. [Google Scholar] [CrossRef]
- Pedersen, R.N.; Esen, B.Ö.; Mellemkjær, L.; Christiansen, P.; Ejlertsen, B.; Lash, T.L.; Nørgaard, M.; Cronin-Fenton, D. The incidence of breast cancer recurrence 10–32 years after primary diagnosis. J. Natl. Cancer Inst. 2022, 114, 391–399. [Google Scholar] [CrossRef]
- Gentilini, O.D.; Botteri, E.; Sangalli, C.; Galimberti, V.; Porpiglia, M.; Agresti, R.; Luini, A.; Viale, G.; Cassano, E.; Peradze, N.; et al. Sentinel lymph node biopsy vs no axillary surgery in patients with small breast cancer and negative results on ultrasonography of axillary lymph nodes: The SOUND randomized clinical trial. JAMA Oncol. 2023, 9, e233759. [Google Scholar] [CrossRef]
- Whelan, T.J.; Smith, S.; Parpia, S.; Fyles, A.W.; Bane, A.; Liu, F.F.; Rakovitch, E.; Chang, L.; Stevens, C.; Bowen, J.; et al. Omitting radiotherapy after breast-conserving surgery in luminal A breast cancer. N. Engl. J. Med. 2023, 389, 612–619. [Google Scholar] [CrossRef]
- Hu, X.; Walker, M.S.; Stepanski, E.; Kaplan, C.M.; Martin, M.Y.; Vidal, G.A.; Schwartzberg, L.S.; Graetz, I. Racial differences in patient-reported symptoms and adherence to adjuvant endocrine therapy among women with early-stage, hormone receptor-positive breast cancer. JAMA Netw. Open 2022, 5, e2225485. [Google Scholar] [CrossRef] [PubMed]
- Sasada, S.; Kondo, N.; Hashimoto, H.; Takahashi, Y.; Terata, K.; Kida, K.; Sagara, Y.; Ueno, T.; Anan, K.; Suto, A.; et al. Prognostic impact of adjuvant endocrine therapy for oestrogen receptor-positive and HER2-negative T1a/bN0M0 breast cancer. Breast Cancer Res. Treat. 2023, 202, 473–483. [Google Scholar] [CrossRef]
- Pistilli, B.; Lohrisch, C.; Sheade, J.; Flemin, G.F. Personalizing adjuvant endocrine therapy for early-stage hormone receptor positive breast cancer. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 60–72. [Google Scholar] [CrossRef]
- DeCensi, A.; Puntoni, M.; Guerrieri-Gonzaga, A.; Caviglia, S.; Avino, F.; Cortesi, L.; Taverniti, C.; Pacquola, M.G.; Falcini, F.; Gulisano, M.; et al. Randomized placebo-controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia. J. Clin. Oncol. 2019, 37, 1629–1637. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, S.; Freedman, R.A.; Partridge, A.H. The impact of young age at diagnosis (age < 40 years) on prognosis varies by breast cancer subtype: A U.S. SEER database analysis. Breast 2022, 61, 77–83. [Google Scholar] [PubMed]
- Morris, E.; Feig, S.A.; Drexler, M.; Lehman, C. Implications of overdiagnosis: Impact on screening mammography practices. Popul. Health Manag. 2015, 18 (Suppl. S1), S3–S11. [Google Scholar] [CrossRef] [PubMed]


| Characteristics of the 338 Patients | N | % |
|---|---|---|
| Age, median (range) | 56 (38–71) | |
| Functional capacity (ECOG) 1 | ||
| 0 | 296 | 87.60 |
| 1 | 40 | 11.80 |
| 2 | 1 | 0.30 |
| 3 | 1 | 0.30 |
| Comorbidity 2 | ||
| 0 | 83 | 24.60 |
| 1 | 21 | 6.20 |
| 2 | 211 | 62.40 |
| 3 | 20 | 5.90 |
| 4 | 1 | 0.30 |
| 6 | 2 | 0.60 |
| Menopause status | ||
| Premenopausal | 65 | 19.20 |
| Postmenopausal | 273 | 80.80 |
| Breast screening mammogram | ||
| Public | 302 | 89.30 |
| Opportunistic | 36 | 10.70 |
| Histological type | ||
| Ductal | 279 | 82.50 |
| Lobular | 21 | 6.20 |
| Others | 38 | 11.20 |
| Stage | ||
| I | 261 | 77.20 |
| IIA | 75 | 22.20 |
| Unknown | 2 | 0.60 |
| Tumor (pT) | ||
| pT1mi | 8 | 2.40 |
| pT1a | 21 | 6.20 |
| pT1b | 101 | 29.90 |
| pT1c | 161 | 47.60 |
| pT2 | 44 | 13 |
| Unknown | 3 | 0.90 |
| Tumor size (cm), median (range) | 1.20 (0.06–5) | |
| Histological grade | ||
| 1 | 106 | 31.40 |
| 2 | 167 | 49.40 |
| 3 | 49 | 14.50 |
| Unknown | 16 | 4.70 |
| Ki67 proliferative index, median (range) | 12 (1–90) | |
| Estrogen receptors | ||
| Positive | 299 | 88.50 |
| Negative | 34 | 10.10 |
| Unknown | 5 | 1.50 |
| Progesterone receptors | ||
| Positive | 258 | 76.30 |
| Negative | 75 | 22.20 |
| Unknown | 5 | 1.50 |
| HER2 | ||
| Positive | 15 | 4.40 |
| Negative | 318 | 94.10 |
| Unknown | 5 | 1.50 |
| Breast surgery | ||
| Conserving | 300 | 88.80 |
| Mastectomy | 38 | 11.20 |
| Axillary surgery | ||
| Sentinel lymph node biopsy | 232 | 68.60 |
| Axillary lymphadenectomy | 98 | 29 |
| None | 8 | 2.40 |
| Adjuvant systemic treatment | ||
| Hormone therapy | 152 | 45 |
| Chemotherapy | 28 | 8.30 |
| Chemotherapy-hormone therapy | 95 | 28.10 |
| None | 58 | 17.20 |
| Unknown | 5 | 1.50 |
| Hormone therapy | ||
| Tamoxifen | 106 | 43.60 |
| Aromatase inhibitor | 59 | 24.30 |
| Tamoxifen-aromatase inhibitor | 76 | 31.30 |
| Ovarian ablation | 2 | 0.80 |
| Chemotherapy | ||
| Anthracyclines | 67 | 54 |
| Anthracyclines and taxanes | 37 | 29.80 |
| Others | 20 | 16.10 |
| Events | N | % |
|---|---|---|
| Local recurrence | 16 | 4.70 |
| After conserving surgery | 13 | 3.80 |
| After mastectomy | 3 | 0.90 |
| Regional recurrence | 3 | 0.90 |
| Metastasis | 18 | 5.30 |
| Liver | 6 | 1.80 |
| Bone | 5 | 1.50 |
| Lung | 5 | 1.50 |
| Central nervous system | 2 | 0.60 |
| Skin | 1 | 0.30 |
| Peritoneum | 1 | 0.30 |
| Others | 2 | 0.60 |
| Secondary invasive primaries | 31 | 9.30 |
| Breast | 10 | 3 |
| Ipsilateral | 3 | 0.90 |
| Contralateral | 7 | 2.10 |
| Non-breast | 21 | 6.30 |
| Colorectal | 4 | 1.20 |
| Ovarian | 3 | 0.90 |
| Endometrial | 3 | 0.90 |
| Pancreatic | 2 | 0.60 |
| Lung | 1 | 0.30 |
| Thyroid | 1 | 0.30 |
| Bladder | 1 | 0.30 |
| Vulva | 1 | 0.30 |
| Carcinoid tumor | 1 | 0.30 |
| Soft tissue sarcoma | 1 | 0.30 |
| Non-Hodgkin’s lymphoma | 1 | 0.30 |
| Multiple myeloma | 1 | 0.30 |
| Oncocytoma | 1 | 0.30 |
| Secondary non-invasive primaries | 7 | 2.10 |
| Ductal carcinoma in situ of the ipsilateral breast | 5 | 1.50 |
| Ductal carcinoma in situ of the contralateral breast | 2 | 0.60 |
| Deaths | 30 | 9 |
| From breast carcinoma | 16 | 4.80 |
| From other causes | 12 | 3.60 |
| Due to unknown causes | 2 | 0.60 |
| Intrinsic Subtype and Tumor Size | N and % | Events (N and %) | Events Not Related to Breast Cancer Mortality | Events Related to Breast Cancer Mortality |
|---|---|---|---|---|
| Luminal A | 56/95 (58.90%) | 9/56 (16.10%) | 7/56 (12.50%) | 2/56 (3.50%) |
| <1 cm | 22 (39.30%) | 3/56 (5.30%) | 3/56 (5.30%) | 0/56 (0%) |
| >1 cm | 34 (60.70%) | 6/56 (10.70%) | 4/56 (7.10%) | 2/56 (3.60%) |
| Luminal B | 26/95 (27.30%) | 6/26 (23%) | 5/26 (19.20%) | 1/26 (3.80%) |
| <1 cm | 9 (34.60%) | 3/26 (11.50%) | 3/26 (11.50%) | 0/26 (0%) |
| >1 cm | 17 (65.40%) | 3/26 (11.50%) | 2/26 (7.70%) | 1/26 (3.80%) |
| HER2 Enrichment | 6/95 (6.30%) | 1/6 (16.70%) | 1/6 (16.70%) | 0/6 (0%) |
| <1 cm | 0 (0%) | |||
| >1 cm | 6 (100%) | 1/6 (16.70%) | 1/6 (16.70%) | 0/6 (0%) |
| Basal like | 7/95 (7.30%) | 1/7 (14.30%) | 1/7 (14.30%) | 0/7 (0%) |
| <1 cm | 4 (57.10%) | 0/7 (0%) | 0/7 (0%) | 0/7 (0%) |
| >1 cm | 3 (42.90%) | 1/7 (14.30%) | 1/7 (14.30%) | 0/7 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gámez-Casado, S.; Rodríguez-Pérez, L.; Bandera-López, C.; Mesas-Ruiz, A.; Campini-Bermejo, A.; Bernal-Gómez, M.; Zalabardo-Aguilar, M.; Calvete-Candenas, J.; Martínez-Bernal, G.; Atienza-Cuevas, L.; et al. Clinicopathological and Genomic Identification of Breast Cancers with No Impact on Mortality. Cancers 2024, 16, 1086. https://doi.org/10.3390/cancers16061086
Gámez-Casado S, Rodríguez-Pérez L, Bandera-López C, Mesas-Ruiz A, Campini-Bermejo A, Bernal-Gómez M, Zalabardo-Aguilar M, Calvete-Candenas J, Martínez-Bernal G, Atienza-Cuevas L, et al. Clinicopathological and Genomic Identification of Breast Cancers with No Impact on Mortality. Cancers. 2024; 16(6):1086. https://doi.org/10.3390/cancers16061086
Chicago/Turabian StyleGámez-Casado, Salvador, Lourdes Rodríguez-Pérez, Cristina Bandera-López, Andrés Mesas-Ruiz, Alicia Campini-Bermejo, Marta Bernal-Gómez, Manuel Zalabardo-Aguilar, Julio Calvete-Candenas, Gala Martínez-Bernal, Lidia Atienza-Cuevas, and et al. 2024. "Clinicopathological and Genomic Identification of Breast Cancers with No Impact on Mortality" Cancers 16, no. 6: 1086. https://doi.org/10.3390/cancers16061086
APA StyleGámez-Casado, S., Rodríguez-Pérez, L., Bandera-López, C., Mesas-Ruiz, A., Campini-Bermejo, A., Bernal-Gómez, M., Zalabardo-Aguilar, M., Calvete-Candenas, J., Martínez-Bernal, G., Atienza-Cuevas, L., García-Rojo, M., Benítez-Rodríguez, E., Pajares-Hachero, B., Bermejo-Pérez, M. J., & Baena-Cañada, J. M. (2024). Clinicopathological and Genomic Identification of Breast Cancers with No Impact on Mortality. Cancers, 16(6), 1086. https://doi.org/10.3390/cancers16061086





