Simple Summary
In this manuscript, the characteristics of cancer stem cells and their potential role in endometrial cancer are explored. An understanding of the mechanisms behind cancer stem cells in endometrial cancer is crucial because these cells potentially play a key role in how the cancer grows and spreads. The authors aim to review the most recent data concerning cancer stem cells in endometrial cancer, especially biomarkers which could enable identification and prognosis. The determination of signalling pathways crucial for the functioning of cancer stem cells could help with the design of effective treatment strategies.
Abstract
Endometrial cancer is one of most common types of gynaecological tumours in developing countries. It has been suggested that cancer stem cells play an important role in the development of endometrial cancer. These are a subset of highly tumorigenic cells with similar features to normal stem cells (unlimited proliferation, multi-potential differentiation, self-renewal, aggressiveness, invasion, recurrence, and chemo- and endocrine therapy resistance). Wnt/β-catenin, Hedghog, and Notch1 are the most frequently activated pathways in endometrial cancer stem cells. The presence of cancer stem cells is associated with the resistance to chemotherapy caused by different mechanisms. Various markers, including CD24, CD40, CD44, CD9, CD133, and CD 166, have been identified on the surface of these cells. A higher expression of such markers translates into enhanced tumorigenicity. However, there is no strong evidence showing that any of these identified markers can be used as the universal marker for endometrial cancer stem cells. Growing data from genomic and proteomic profiling shed some light on the understanding of the molecular basis of cancers in humans and the role of cancer stem cells. However, there is much left to discover. Therefore, more studies are needed to fully uncover their functional mechanisms in order to prevent the development and recurrence of cancer, as well as to enhance treatment effectiveness.
1. Introduction
Endometrial cancer is the most common type of gynaecological hormone-dependent tumour in developing countries [1]. According to American Cancer Society estimates, approximately 67,880 new cases of uterine (including endometrial) cancers will be diagnosed in 2024, and 13,250 women will die from this disease in United States [2]. In turn, according to GLOBOSCAN, the age-standardised incidence of endometrial cancer and patient mortality was 8.7 and 1.8 in 2020 [3].
The risk of endometrial cancer is increased among post-menopausal women [4,5]. The median age at diagnosis is 63 years [6,7]. Due to its characteristic symptoms, including post-menopausal uterine bleeding, endometrial cancer is usually diagnosed at an early stage [8]. Clinical data indicate that only one-third of patients have localised cancer at diagnosis [6]. The prognosis of early-stage (I and II) or localised endometrial cancer is favourable, and treatment can be limited to surgery, followed, in some cases, by brachytherapy and/or external beam radiation therapy [9]. High-risk patients with an early stage of endometrial cancer also undergo platinum-based chemotherapy [4]. However, endometrial cancer is frequently recurring and spreads to the vagina, pelvic and para-aortic lymph nodes, peritoneum, and lungs (distant metastases) [10,11]. Recurrences are reported in about 13% of high-risk patients, but also in 3% of low-risk patients [12,13]. The estimated five-year overall survival of patients with stage I disease is 95%, but drops to 69% in the case of patients with stage II disease [6]. The five-year survival rate of patients with advanced disease stages (III–IV) is as low as 15–17% [6,14]. The low survival in the advanced stages is due to the poor response of endometrial cancer to chemotherapy and radiotherapy. The standard first-line treatment involves surgery, followed by a combination of carboplatin and paclitaxel [15,16]. However, the quick development of treatment resistance and low response rates to single-agent chemotherapy, as well as to endocrine therapy with medroxyprogesterone acetate (MPA), limit the range of therapeutic options [4]. New drugs, including pembrolizumab alone (in microsatellite instability (MSI)-high tumours) or in combination with the angiogenesis inhibitor lenvatinib (in patients without MSI), have been approved by the US Food and Drug Administration (FDA) [17,18,19]. However, treatments that would considerably increase patient survival are awaited.
Cancer stem cells (CSCs) have been suggested to play an important role in the development of endometrial cancer [20]. Indeed, Hubbard et al. [20] identified a sparse population of endometrial cancer cells showing the ability differentiate and initiate tumours. CSCs were also found to express several genes with pluripotent features [21]. It is now believed that CSCs are at least partly responsible for drug resistance, tumour progression, invasiveness, and metastasis [10,22,23]. Therefore, novel approaches to treating tumours are based on inhibitors of the CSC signalling pathways, as well as adoptive cell therapy targeting specific CSC antigens and modulators of their epigenetic mechanisms [15,24,25,26,27].
2. Endometrial Cancer
2.1. Classification
First, the historical endometrial cancer classification was based on clinical and hormonal features. According to Bokhman’s dualistic model, sporadic endometrial cancer was categorised into oestrogen-dependent highly-to-moderately differentiated carcinomas with an endometrioid morphology (Type 1) and oestrogen-independent non-endometrioid carcinomas (Type 2) [28]. Type 1 carcinomas occur more frequently and are less aggressive (80% of patients in this group have grade 1 or 2 tumours) [29,30]. Moreover, type 1 cancers show a high expression of oestrogen and progesterone receptors, and a low potential for lymphovascular invasion, and are associated with a favourable prognosis [28]. By contrast, up to 66% of women with type 2 endometrial cancer have a high-grade (grade 3) tumour with a high potential for lymphovascular invasion, little progesterone sensitivity, and a poor prognosis. The revised staging system for endometrial cancer which was published in 2023 incorporates diverse histological classifications, tumour patterns, and molecular categorisation to more accurately depict the intricate characteristics of different endometrial carcinoma types and their underlying biological dynamics [31]. According to this new system, stage I includes various subcategories from IA to IVC based on the extent and nature of the endometrial carcinoma. In turn, the Cancer Genome Atlas (TCGA) classification divides endometrial cancer into four types based on the overall mutational burden (including phosphatase and TENsin homolog (PTEN), p53, polymerase epsilon (POLE) mutations, MSI, and histology), which translates into different clinical outcomes [15,32]. A recently developed prognostic classification system for endometrial cancer that uses next-generation sequencing of the most discriminant genes shows a nearly 100% accuracy [33]. Endometrial cancers carrying POLE proofreading mutations show a favourable prognosis despite a strong association with high-grade cancers [34]. Moreover, most endometrioid tumours have been demonstrated to carry few somatic copy number alterations [32]. However, cancers burdened with extensive somatic copy number alterations show considerably worse progression-free survival compared with other groups.
2.2. Risk Factors
Various mutations in different genes have been linked with the development and progression of cancers [35]. Mutations in genes including TP53 (71%), PIK3CA (31%), PPP2R1A (25%), ABCC9, CYP4X1, CHD4, MAP3K4, FBXW7, and SPOP have been identified as key factors in the pathogenesis of endometrial cancer [35,36]. Moreover, CHD4 has been demonstrated to be specific to endometrial cancer [35]. This epigenetic factor contributes to metastasis via the modulation of the EZH2/β-catenin axis and is associated with a poor prognosis [37]. The identification of cancer-promoting alterations could contribute to the development of novel diagnostic and treatment methods. The activation of specific signalling pathways translates into tumour behaviour and patients’ outcomes. For example, the upregulation of genes linked to the epithelial-to-mesenchymal transition (EMT), such as SNAI1 and TWIST1, has been shown to enhance the tumorigenicity of endometrial CSCs [38]. In turn, the higher paclitaxel resistance in endometrial cancer cells was linked to PI3K/AKT activation mediated by human epidermal growth factor receptor 2 (HER2) [39].
Apart from genetic alterations, polycystic ovary syndrome, diabetes, obesity, early menarche, late menopause, Lynch syndrome (in women below the age of 50 years), and infertility are also well-known risk factors for endometrial cancer [40,41,42]. Additionally, exposure to air pollutants, including polycyclic aromatic hydrocarbons (PAHs), is a risk factor for endometrial cancer [43]. The dose or concentration and duration of exposure to environmental pollutants have been suggested to affect the initiation of chemical carcinogenesis. PAHs not only activate environmental carcinogens but also stimulate cancer cell proliferation [44,45]. Indeed, growing evidence supports a relationship between exposure to PAHs and the development of CSCs that is mediated by the aryl hydrocarbon receptor (AHR) pathway and the activation of the WNT/β-catenin pathway and the ABCG2 transporter [43,46,47,48,49]. Increased levels of AHR mRNA were observed in early-stage endometrial cancer due to an oestrogen-related mechanism compared to advanced disease stages and normal endometrium. Moreover, the expression of AHR was found to be higher in oestrogen-independent responsive grade 3 endometrioid adenocarcinomas than in oestrogen-dependent responsive grade 1 and 3 endometrioid adenocarcinomas [50].
The development of tumours is also promoted by hypoxia, since hypoxic tension stimulates the initiation and maintenance of CSC stemness and contributes to the formation of a microenvironment that favours the survival and thriving of tumour cells [51]. Hypoxia-inducible factor (HIF)-1α triggers a series of adaptive responses through decreasing oxygen consumption and the release of relevant stimulators including glycolytic enzymes, vascular endothelial growth factor (VEGF), and erythropoietin. It also induces the expression of CD44 and CD133, MYC, homeobox protein (NANOG), octamer binding transcription factor 4 (OCT4), and SRY-box transcription factor 2 (SOX2), and promotes EMT [51]. The interaction between HIF-1 and the Notch pathway has been found to enable the maintenance of tumour cells under hypoxia [52,53].
3. Stem Cells and Endometrium
The human endometrium contains populations of epithelial and stromal colony-forming cells and has an exceptional regenerative capacity [54,55]. The endometrium undergoes a monthly cycle of growth, proliferation, differentiation, shedding, and regeneration under the influence of the circulating ovarian steroid hormones oestrogen and progesterone [56,57,58]. Endometrium regeneration with each menstrual cycle as well as the rapid enlargement and adjustment of the uterus to accommodate the developing foetus are possible, owing to the presence of stem cells.
Stem cells represent unspecialised cells within the human body, possessing the capacity for differentiation into diverse cellular lineages observed throughout the continuum of life in both embryonic and adult tissues [59]. They play essential roles in neonatal development and tissue regeneration following injury or disease by serving as the primary source for specific cell types within tissues and organs due to the ability to migrate freely throughout different tissues [60,61]. Stem cells undergo a series of specialisation stages during which their developmental potential diminishes progressively. Totipotent stem cells, with the highest differentiation potential, can generate cells for the entire organism. Pluripotent stem cells, such as embryonic stem cells (ESCs), form germ layer cells but not extraembryonic structures. Multipotent stem cells, including haematopoietic stem cells, specialise in specific cell lineages but possess a broader differentiation range than oligopotent stem cells, which can differentiate into several cell types. Unipotent stem cells, with their restricted differentiation capabilities, possess the unique ability to repeatedly divide, rendering them promising candidates for regenerative medicine applications [59]. Endometrial stem cells are distinguished by their extraordinary capacity for self-renewal and differentiation into assorted cell phenotypes, including stromal, epithelial, and vascular lineages [62]. Endometrial tissue harbours diverse stem cell populations, including epithelial-like, stromal-like, and perivascular endometrial stem cells, each characterised by distinct molecular and functional attributes [62].
The fundamental traits of stem cells involve their capacity for self-renewal, proliferation, and high differentiation capacity (pluripotency), ensuring a continual generation of progeny aimed at replacing aging, senescent, or impaired cells [61,63]. These characteristics distinguish them from other types of cells. Stem cells have been demonstrated to be involved in the telomere maintenance mechanism. Telomeres, comprised of repetitive DNA sequences, undergo gradual attrition with each successive cellular division as a result of DNA polymerases’ inability to completely replicate a linear template [64]. Upon reaching a critical threshold of shortening, telomeres activate a DNA damage response pathway, culminating in cell cycle arrest [65]. However, pluripotent stem cells possess mechanisms for telomere elongation, thereby protecting their prolonged proliferation and self-renewal capabilities [66]. However, excessive telomere elongation can prove detrimental, prompting the activation of a rapid telomere deletion process known as telomere trimming [67]. This delicate balance between telomere elongation and trimming mechanisms is essential for maintaining genomic stability and cellular homeostasis in pluripotent stem cells [68].
Since stem cells exhibit an extended lifespan compared to somatic cells, the likelihood of accumulating genetic alterations, potentially predisposing them to malignant transformation, is increased. The accrual of abnormalities and mutations within endometrial stem cells may trigger the initiation and progression of various endometrial pathologies, including endometrial cancers [69]. The occurrence of a few mutations can disrupt the regulatory mechanisms governing self-renewal and proliferation, fostering the emergence of cancerous phenotypes. Epithelial-like endometrial stem cells have been suggested to contribute to the formation and progression of endometrial malignancies not only as a result of genetic instability but also as a result of the enhanced proliferative capacity [70]. The accumulation of genetic mutations within epithelial-like stem cells was suggested to trigger the transformation of normal endometrial stem cells into malignant ones—cancer stem cells [71].
4. Cancer Stem Cells
CSCs are a subset of highly tumorigenic cells that show similar features to normal stem cells including unlimited proliferation, self-renewal, aggressiveness, multi-potential differentiation, recurrence, invasion, metastasis, chemoresistance, and endocrine therapy resistance [10]. Self-renewal means that CSCs are able to regenerate and give rise to a subset of cells displaying an abnormal differentiation and survival potential despite apoptotic signals, which is crucial for tumour maintenance [72]. The predisposition of endometrial CSCs to spread to other organs is associated with their ability to migrate, stimulate angiogenesis, and release the extracellular matrix [72]. CSCs were identified for the first time in acute myeloid leukaemia but were later also observed in other types of tumours [73]. Subsequent studies demonstrated that CSCs form spherical colonies expressing surface markers and executing biological functions [74]. Endometrial CSCs with the ability to form sphere-like structures were found to have a greater self-renewal potential and capacity for chemoresistance. Moreover, their presence favours tumour initiation [75]. The results of one study demonstrated that endometrial CSCs account for 0.02–0.08% of cells in endometrial cancer cell lines and 3.4% in primary tumours [20].
CSCs contribute to tumour initiation and development since they constantly give rise to numerous descendant cancer cells [16]. They are involved in every step of tumour formation, development, and metastasis. These cells are capable of switching between higher oxidative mitochondrial metabolism and anaerobic glycolysis [76]. They are also capable of undergoing phenotypic transition following stimulation (plasticity) [77]. The results of some studies have revealed that CSCs are characterised by increased motility, as well as enhanced migratory and invasive properties, compared with normal stem cells [78,79]. These cells show the ability to detach from the original tumour, migrate, and invade a new site, which explains their high metastatic potential [16]. The presence of high amounts of CSCs is associated with enhanced aggressiveness and poor outcomes. CSCs use ATP-dependent efflux pumps, such as multidrug resistance protein 1 (MDR1), which enable their survival in unfavourable conditions and make the cancer unresponsive to chemotherapy [80]. In addition to the activity of ATP binding cassette (ABC) efflux transporters, other resistance mechanisms in endometrial CSCs include resistance to DNA damage, aldehyde dehydrogenase (ALDH) activity, the activation of developmental pathways and microenvironmental stimuli, resistance to apoptosis, and autophagy [22,55,72].
Several hypotheses have been formulated concerning the formation of CSCs. According to one hypothesis, CSCs arise from normal/adult stem cells via the ongoing acquisition of epigenetic alterations and genetic mutations [81]. Another thesis concerning CSC formation (the “big bang” model) states that, following the initial transformation, cells with initiating mutations grow predominantly to form a single expansion populated by numerous intermixed sub-clones [82]. The growth of tumours is an evolutionary process; thus, it seems that information on their early development is encoded in the genome and is responsible for intra-tumour heterogeneity. New sub-clonal mutations are constantly generated as a result of replication errors; however, only the earliest are omnipresent, while later alterations appear increasingly in only smaller tumour sub-populations. Some sub-clonal mutations acquired during growth may confer survival advantages. Therefore, the timing of a mutation, rather than clonal selection, was suggested to determine tumour pervasiveness [82].
Mani et al. [83] suggested that CSCs are derived from differentiated cancer cells via EMT-mediated dedifferentiation. EMT regulates the expression of genes involved in various metabolic pathways and metabolic reprogramming [84]. The activation of EMT was demonstrated to be crucial for the formation of CSCs and is involved in the acquisition of a mesenchymal phenotype and loss of epithelial features. This process enables malignant cells to metastasise from a primary tumour [85]. The nuclear expression of EMT transcription factors, such as SLUG and TWIST in endometrial CSCs, links EMT with stemness and the expression of programmed death ligand 1 (PDL1), which enables immune evasion [86]. Numerous studies have shown that PDL1 expression affects stem-like characteristics, including cell growth, stemness, metastasis, and drug resistance [87,88,89]. The proto-oncogene MYC directly promotes PDL1 expression, thus contributing to immunosuppression. In turn, MYC downregulation results in immune cell infiltration [90]. In general, the MYC family regulates various cellular processes and is involved in tumorigenesis [91]. The co-expression of MYC and CD44 has been observed in endometrial CSCs [92]. The expression of MYC appeared necessary for SALL4-induced EMT, invasion, and resistance to antineoplastic drugs in endometrial cancer cells [93].
The formation of CSCs involves the expression and release of “stemness” molecules [94]. Guy et al. [75] observed the enhanced expression of genes related to stemness, including OCT4A, MYC, KRT18 (which encodes CK-18), SOX2, B lymphoma Mo-MLV insertion region 1 homolog (BMI1), ABCG2, NANOG, and NES (which encodes nestin) in CD133-positive and CXCR4-positive endometrial cancer cells. The results of other studies have demonstrated that the expression of OCT4 and SOX2 in endometrial CSCs could be associated with their self-renewal capacity [20,95]. Endometrial CSCs isolated from endometrial cancer expressing stemness markers, including SOX2, OCT4, MYC, ABCG2, NES, and NANOG, have been demonstrated to show greater expansion potential and colony-forming capabilities, as well as to express self-renewal genes [20,96]. OCT4, SOX2, and NANOG were suggested to contribute to pluripotency [97].
In endometrial cancer cells, stemness is modulated mostly via the WNT, Notch (which controls cell development and stimulation-triggered differentiation), and Hedgehog pathways [98]. Numerous studies have confirmed the activation of CSC-related signalling pathways, including WNT/β-catenin and Notch in various tumours, including endometrial cancer [99,100,101,102]. The self-renewal and maintenance of CSCs is also associated with the activation of the WNT/β-catenin, Notch, BMI1, and SHH pathways [103,104,105,106]. The WNT/β-catenin pathway controls endometrial proliferation and differentiation [107]. The downregulation of WNT signalling in endometrial cancer cells hindered CSC proliferation and migration, confirming the importance of this pathway in tumour development [96]. As aforementioned, Notch signalling has been found to be involved in CSC proliferation and viability. The use of various micro-RNAs (miRNAs) targeting the Notch pathway hindered the proliferation of the CD44/CD133-positive subpopulation in endometrial cancer (miRNA134), limited the expression of vimentin (VIM), reduced EMT, and decreased tumour formation and invasiveness (miRNA-34a) [108,109]. In turn, the Hedgehog signalling pathway is involved in cell differentiation and growth via the transmembrane protein smoothened (SMO) and the glioma transcriptional factor GLI1. Sonic hedgehog (SHH) and SMO were found to be overexpressed in endometrial cancer compared with endometrial hyperplasia and normal endometrial epithelium [110].
The PTEN-PI3K/AKT/mammalian target of rapamycin (mTOR) pathway is another important element contributing to CSC stemness via the upregulation of EMT triggers, including enhancer of zeste homolog 2 (EZH2), BMI1, SNAI1, and SLUG [15,111]. Other pathways involved in the upregulation of self-renewal and differentiation in CSCs include AKT, mTOR, Janus kinase (JAK)/signal transducer and activator of transcription (STAT), and nuclear factor-κB (NF-κB) [112,113,114].
Numerous studies have reported a correlation between the presence of CSCs and resistance to chemotherapy [43,115]. The upregulation of the drug efflux mechanism that enables drug efflux against the concentration gradient and results from a high expression of several specific transporter proteins, such as ABC drug transporters, especially ABCG2, is partly responsible for this phenomenon [116]. Liu et al. [93] demonstrated that the downregulation of MYC in endometrial cancer decreased cell invasion and drug resistance considerably.
The problems with cancer treatment have resulted in numerous studies trying to identify plausible new therapeutic targets. In one of them, Cao et al. [10], based on a tandem mass tag quantitative proteomic analysis, identified 5735 proteins in spheroid cells from endometrial cancer cell lines. They are observed to have an elevated expression of hexokinase 2 (HK2) and 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) (both of which encode key molecules in the HIF-1 pathway), as well as G Protein-coupled receptor class C group 5 member A (GPRC5A) in spheroid cells. The authors demonstrated that knockdown of HK2 in endometrial cancer cells reduced cell proliferation and the self-renewal ability [10]. The results of other studies indicated a role for PFKFB3 in the regulation of endothelial glycolysis, the promotion of angiogenesis, and the metastasis of malignant tumours [117,118]. PFKFB3 inhibition limited tumour invasion, intravasation, and metastasis [119]. Moreover, this was found to be unique for CSCs and enabled their differentiation from both non-stem cells and induced pluripotent stem cells, specifically under hypoxia [120]. In turn, GPRC5A was demonstrated to stimulate the self-renewal and metastasis of bladder CSCs [121,122].
Potential therapeutic targets are presented on Figure 1.
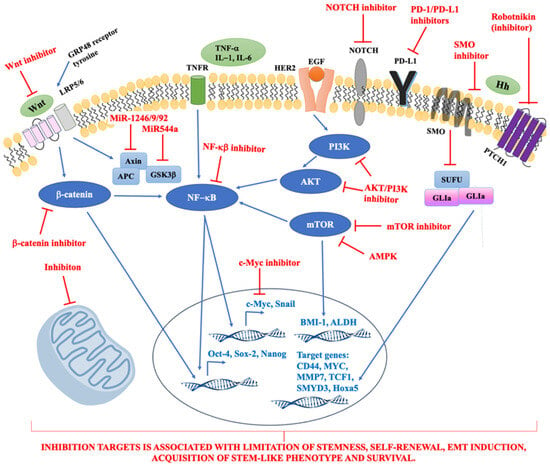
Figure 1.
The summary of potential therapeutic targets. Abbreviations: ALDH, aldehyde dehydrogenase; AMPK, 5′AMP-activated protein kinase; BMI-1, B-lymphoma Mo-MLV insertion region 1; CD44, cluster of differentiation 44; EGF, epidermal growth factor; GLI, transcription factor GLI; GRP48, G Protein Coupled Receptor 48; GSK3β, glycogen synthase kinase-3β; HER2, human epidermal growth factor receptor 2; Hh, hedgehog signalling pathway; Hoxa5, homeobox A5; IL-1,-6, interleukin-1,-6; LRP5/6, Low-density lipoprotein receptor-related protein 5; MMP-7, Matrix Metalloprotease-7; mTOR, mammalian target of rapamycin; MYC, MYC MYC transcription factor; NF-κB, nuclear factor κB; PD-1, programmed death receptor-1; PD-L1, programmed death receptor-1 ligand; PI3K, phosphatidylinositol 3-kinases; PTCH1, receptor called Patched 1; SMO, signal transducer called Smoothened; SMYD3, SET and MYND domain containing 3; SUFU, cytoplasmic protein; TCF1, T cell factor 1; TNF-α, tumour necrosis factor α; TNFR, tumour necrosis factor receptor; Wnt, Wnt signalling pathway.
As aforementioned, the treatment of endometrial cancer is challenging. Figure 2 presents novel therapies that are being assessed for safety and efficacy in clinical trials.
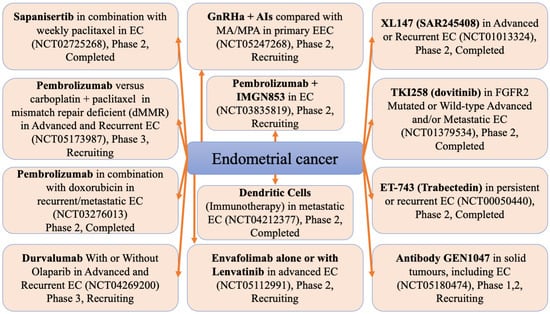
Figure 2.
Clinical trials of new endometrial cancer therapies. AIs, aromatase inhibitors; EC, endometrial carcinoma; EEC, early endometrial carcinoma; GnRHa, gonadotropin-releasing hormone analogue; IMGN853, mirvetuximab soravtansine; MA, megestrol acetate; MPA, medroxyprogesterone acetate.
5. CSC Markers
Cluster of differentiation (CD) markers, such as CD24, CD40, CD44, CD9, CD133, and CD 166, have been identified on the surface of CSCs [23,43,123]. An analysis of spheroid cells from endometrial cancer cell lines (Ishikawa and HEC1A) demonstrated the enhanced expression of CD90, CD117, CD133, and W5C5. In turn, other markers, such as CD29, CD44, and CD105, were found to not be uniquely expressed in these cells [10]. In endometrial cancer, specific cell surface markers, including CD44-antigen (CD44) and prominin-1 (CD133), have been identified as surrogate markers for CSCs [58]. These surface markers are frequently used to identify endometrial-carcinoma-derived stem-like cells [8]. The expression of these markers has been linked to tumorigenicity, invasiveness, and metastasis [124,125]. Moreover, CSCs exhibit high ALDH activity [126,127]. The increased expression of CSC-related markers, including CD44, CD133, ALDH1A1, and NANOG, in spheroid cells of endometrial cancer translates into enhanced tumorigenicity [55,128].
5.1. CD133
CD133 is a pentaspan transmembrane glycoprotein, the sub-cellular localisation of which enables its interaction with lipid rafts participating in the signalling cascade [129]. Mizrak et al. [130] suggested that this molecule could be involved in cell membrane organisation. Jaksch et al. [131] showed that the expression of CD133 in both CSCs and normal cells depended on the cell cycle. Various studies indicated that CSCs expressing CD133 comprised 5.7–27.4% of cells in primary endometrial tumours and their presence was associated with a worse prognosis [75]. The high expression of CD133 on the surface of CSCs correlates with a greater self-renewal ability, higher proliferative potential, lymphovascular invasion, and a poor prognosis [23,129]. Rutella et al. [75] demonstrated in a mouse model that cells expressing CD133 showed an increased ability to aggressively proliferate and form colonies compared with CD133-negative cells [75]. Moreover, CD133-positive cells had a greater ability to migrate from the primary mass to blood vessels and other organs and a higher resistance to chemotherapy than CD133-negative cells. Rutella et al. [75] suggested that CD133-positive populations of endometrial cancer cell lines form floating spheres and colonies that originate from clonal proliferation. Additionally, Kyo et al. [132] provided evidence that CD133-positive cells have a higher colony-forming ability in vitro and enhanced tumorigenicity in immunocompromised mice. A high CD133 expression in specimens of endometrioid-type endometrial cancer was associated with decreased overall survival compared with a low CD133 expression [132]. In addition to the CD133 expression, the FIGO stage and histological grade negatively influenced overall survival rates. The CD133 expression (hazard ratio (HR) 3.90; p < 0.045) and FIGO stage (HR 3.94; p < 0.042) were independent predictive factors for patient survival [132]. The expression of CD133 translates into higher rates of endometrial tumour relapse and worse survival [54,75,133]. A high expression of TGFB1 has been observed in the CD133-positive CSC subpopulation of endometrial cancer cells. TGF-β1 was found to activate EMT to trigger metastasis [75]. The TGF-β1/CD133 pathway was found to stimulate cell invasion, stem-like characteristics, and therapy resistance [134]. Moreover, TGF-β1 can stimulate genomic instability via hindering the repair of DNA double-strand breaks [135,136]. Nakamura et al. [137] suggested that CD133 was a risk factor for endometrial cancer due to the higher proliferative and tumorigenic potential and cisplatin and paclitaxel resistance. The results of another study revealed that CD33-positive cells have increased tumorigenic potential [125]. Aggressive tumour behaviour has also been linked with the upregulation of ABCG2 and matrix metalloproteinases (MMPs) in CD133-positive cells [132]. The expression of MMP14 (which encodes MT1-MMP) increases the invasive capacity of CD133-positive cells, whereas the knockdown of MMP14 with small interfering RNA (siRNA) mostly abolishes this effect.
The expression of CD133 in endometrial CSCs was found to be frequently accompanied by the upregulation of CD44 and NES, which enhanced proliferation and infiltration [75,124,138]. Bokhari et al. [139] confirmed that NES knockdown hindered cell growth and limited the invasive potential and colony formation abilities of endometrial cancer cell lines, whereas its overexpression was associated with a malignant phenotype.
5.2. CD44
CD44 is a transmembrane adhesion molecule suggested to be a useful marker of CSCs in endometrial cancer. This molecule is also involved in cancer invasion and metastasis [125,133]. CD44 expression was found to positively correlate with PDL1 expression in various tumours, as well as with immune infiltration [140]. This adhesion molecule has been demonstrated to be involved in tumour cell invasion and metastasis in endometrial cancer cell lines [133]. Zagorianakou et al. [141] linked CD44 expression with an enhanced proliferation of endometrial carcinomas of the endometrioid type, a higher tumour grade, and progesterone receptor status. Park et al. [124] found a close relationship between CD133 or CD44 expression and endometrial cancer progression and a poor prognosis.
5.3. SMOC2
Lu et al. [58] suggested that SPARC-related modular calcium binding 2 (SMOC2) could also be used as a signature gene of endometrial CSCs. SMOC2 protein, the levels of which are enhanced during embryogenesis and wound healing, shows angiogenic activity and is capable of stimulating endothelial cell proliferation and migration [58,142,143]. Lu et al. [58] found that SMOC2 expression was increased in sphere cultures and in CD133/CD44-positive cells compared with CD133/CD44-negative cells and also boosted the chemoresistance of endometrial cancer cells. Furthermore, SMOC2 silencing resulted in the diminished clonogenic potential of endometrial cancer and reduced the expression of stemness-related genes, including SOX2, OCT4, and NANOG [58]. In endometrial cancer, the SMOC2-related activation of WNT/β-catenin signalling promoted the progression of endometrial cancer since this pathway is vital for cell growth, differentiation, proliferation, and survival [58,144,145]. Furthermore, this pathway is involved in the formation and maintenance of stem cells and CSCs [144].
5.4. CXCR4
The invasive and metastatic phenotypes of CSCs have also been suggested to be mediated by the chemokine ligand/receptor axis [132]. In endometrial cancer, CXC motif chemokine receptor 4 (CXCR4) was demonstrated to be highly expressed by some tumour cells [146]. The activation of CXCR4, a stromal cell-derived factor-1 receptor, triggers signalling pathways promoting increased survival, enhanced proliferation, the degradation of the extracellular matrix, drug resistance, and angiogenesis in malignant tumour cells [98]. The expression of both CXCR4 and CD133 has been reported in all types of human tumours, including endometrial cancer [138]. Moreover, CXCR4 was found to be upregulated considerably in endometrial tumours compared with atypical, simple hyperplasia and normal endometrial tissue [125]. The results of several studies have found that the CXCL12/CXCR4 axis is partly responsible for tumour progression, angiogenesis, metastasis, and survival [147]. The actions of CXCR4 are associated with the induction of signalling pathways involved in gene transcription, survival, proliferation, and chemotaxis. Sun et al. [138] demonstrated that CD133/CXCR4-positive endometrial cancer cells comprised less than 10% of the total population, which is in agreement with the findings that CSCs constitute only a small percentage of cells in malignant tumours. Cioffi et al. [148] demonstrated considerably lower two-year survival rates in patients with a high expression of CD133 and CXCR4 compared with patients with a low CD133 and CXCR4 expression. The growth of CD133/CXCR4-positive cells in vitro was greater than that of CD133/CXCR4-negative cells, indicating enhanced proliferative properties. Sun et al. [138] also revealed that CD133/CXCR4-positive cells have an increased potential to form more spheres and colonies, which appear to be typical characteristics of stemness in various tumours [149,150]. CD133/CXCR4-positive cells also exhibited a higher expression of stemness genes [138]. The injection of CD133CXCR4-positive cells into nude mice was associated with tumour formation, and tumour formation was not observed following inoculation with CD133/CXCR4-negative cells [138].
5.5. CD117
CD117 (c-kit) is a cell surface receptor tyrosine kinase that has been proposed as a CSC marker in various tumour types [15,43]. Following stimulation by stem cell factor, CD117 triggers cell replication, differentiation, and survival, and CSCs acquire stemness properties [151,152,153]. The results of some studies have demonstrated that endometrial CSCs expressing CD117 have increased the proliferative and colony-forming potential [15,154]. Zhang et al. [154] suggested that a high CD117 expression could be used as an independent prognostic factor. In addition to endometrial cancer, the expression of CD117 in CSCs has also been demonstrated in ovarian and lung cancer [152,155].
5.6. CD55
CD55 is an intrinsic cell surface complement inhibitor [43]. A high expression of CD55 has been reported in endometrial cancer cells and CSCs. Cells expressing this inhibitor displayed a higher self-renewal ability, as well as a higher resistance to chemotherapy, than CD55-negative cells [156]. CD55 expression in endometrioid ovarian and endometrial CSCs was demonstrated to enhance self-renewal properties and cisplatin resistance. In endometrial endometrioid cell lines, the inhibition of CD55 with saracatinib was associated with cisplatin resensitisation [156].
5.7. ALDH1
ALDH1 was demonstrated to be highly active during the early steps of stem cell differentiation [157,158]. Endometrial cancer cells with a high ALDH1 expression were found to be more tumorigenic and invasive, as well as resistant to cisplatin therapy and associated with a worse prognosis in patients with endometrial cancer (p = 0.01 for overall survival), compared with cells with a low expression of ALDH1. Additionally, Rahadiani et al. [159] observed that cells with a higher ALDH1 expression had higher tumorigenic potential, as well as increased invasiveness and resistance to cisplatin, compared with cells with a low ALDH1 expression. These features were associated with a poor prognosis in patients with endometrial cancer [159]. The expression of ALDH and CD133 in debulked primary tumour specimens was found to correlate with poor patient survival [157]. Mori at al. [160] observed that ALDH-dependent glycolytic activation mediated stemness and chemoresistance in spheroid uterine endometrial cancer derived from patients. The disulfiram and N,N-diethylaminobenzaldehyde (DEAB)-related inhibition of ALDH1 decreased the proliferation of spheroid CSCs [128]. Ran et al. [161] suggested that the ALDH expression in endometrial CSCs mediates autophagy, which contributes to CSC survival and chemoresistance, since they observed a decrease in cell growth and self-renewal potential following the use of an autophagy inhibitor (3-methyladenine or chloroquine).
5.8. NANOG
NANOG expression was suggested to be useful as a diagnostic marker, enabling the differentiation between true dysplasia and reactive lesions [23,162]. An increased expression of NANOG has been detected in precancerous lesions (high-grade dysplasia), as well as in cancerous tissue. In endometrial CSCs, NANOG expression was found to be regulated by transcription factor 3 (TCF3), OCT4, and SOX2 [15]. Grubelnik et al. [163] suggested that the high expression of NANOG is associated with a more advanced cancer, a higher cancer grade, resistance to treatment, and, thus, a worse prognosis. NANOG has also been suggested to be involved in the acquisition of stem-cell-like features, including self-renewal and immortality [23]. Al-Kaabi et al. [23] demonstrated the expression of NANOG in 88.37% of endometrial carcinoma cases. Moreover, they reported that NANOG expression correlated with deep myometrial invasion, a higher cancer grade, and a positive lymph node status, all of which indicated a worse prognosis. According to other studies, a high NANOG expression translates into the presence of poorly differentiated, advanced tumours and poor patient survival [164,165,166].
5.9. Other
Endometrial CSCs have also been reported to express the neuroendocrine marker synaptophysin [8]. Helweg et al. [8] observed that nuclear synaptophysin, CD133, CD44, and Nestin indicated endometrial-CSC-like characteristics. Additionally, the levels of CK-18, a structural protein normally present in many single-layer epithelia, were demonstrated to correlate with the clinical stage, number of positive lymph nodes, metastasis, and recurrence, as well as poorer overall and disease-free survival [167].
The utility of the aforementioned markers has been suggested in many studies; however, Tabuchi et al. [168] demonstrated that CSC populations in uterine endometrioid adenocarcinoma showed functional heterogeneity and suggested that there was no distinctive biomarker for the identification of CSCs as various colonies within the same tumour could express different markers. Spherical clones were found to be less tumorigenic but more resistant to chemotherapy. In turn, leukaemia-like clones show opposing features. Therefore, more studies are required to understand the functional mechanisms of CSCs and to target these cells in order to improve the effectiveness of cancer treatment and prevent its recurrence.
Table 1 summarises studies focused on possible interventions related to CSCs in endometrial cancer.

Table 1.
The summary of possible therapeutic interventions targeting endometrial cancer CSC described in the literature.
6. Conclusions and Future Directions
Cancer stem cells have recently emerged as a crucial target for cancer treatment. However, the available knowledge has been complicated by recent insights revealing the significant influence of the tumour microenvironment in modulating the properties of cancer stem cells (CSCs). This is of particular importance as the transformation of non-tumorigenic cancer cells into CSCs has been observed, leading to potential variations in the CSC population within a neoplasm over time. The plausible heterogeneity of CSCs justifies the existence of multiple biomarkers. Conflicting results from various studies regarding the diagnostic efficacy of these biomarkers highlight the necessity for thorough validation in order to develop successful, biology-oriented therapeutic strategies. Currently, only a limited number of clinical trials targeting CSCs with drugs have been initiated. While early-phase studies indicate the safety of these drugs, there is a lack of comprehensive data on their therapeutic efficacy.
Author Contributions
Conceptualisation, K.F. and B.B.; methodology, K.F. and B.B.; software, K.F.; validation, K.F.; formal analysis, K.F. and B.B.; investigation, K.F. and B.B.; resources, K.F.; data curation, K.F.; writing—original draft preparation, K.F.; writing—review and editing, K.F. and B.B.; visualisation, K.F.; supervision, K.F. and B.B.; project administration, K.F.; funding acquisition, K.F. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Medical University in Lublin.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2022. 2022. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf (accessed on 20 February 2024).
- Kavousi, S.; Maharlouei, N.; Rezvani, A.; Aliabad, H.A.; Vardanjani, H.M. Worldwide association of the gender inequality with the incidence and mortality of cervical, ovarian, endometrial, and breast cancers. SSM-Popul. Health 2024, 25, 101613. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: Diagnosis, treatment and follow-up. Int. J. Gynecol. Cancer 2016, 26, 16–41. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.T. Reproductive factors and the risk of endometrial cancer. Int. J. Gynecol. Cancer 2014, 24, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Cancer Stat Facts: Uterine Cancer. Available online: https://seer.cancer.gov/statfacts/html/corp.html (accessed on 20 February 2024).
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Helweg, L.P.; Windmöller, B.A.; Burghardt, L.; Storm, J.; Förster, C.; Wethkamp, N.; Wilkens, L.; Kaltschmidt, B.; Banz-Jansen, C.; Kaltschmidt, C. The Diminishment of Novel Endometrial Carcinoma-Derived Stem-like Cells by Targeting Mitochondrial Bioenergetics and MYC. Int. J. Mol. Sci. 2022, 23, 2426. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, M.; Lissoni, A.A.; Cormio, G.; Katsaros, D.; Pellegrino, A.; Selvaggi, L.; Ghezzi, F.; Scambia, G.; Zola, P.; Grassi, R.; et al. Modified radical hysterectomy versus extrafascial hysterectomy in the treatment of stage I endometrial cancer: Results from the ILIADE randomized study. Ann. Surg. Oncol. 2009, 16, 3431–3441. [Google Scholar] [CrossRef]
- Cao, M.; Liu, Z.; You, D.; Pan, Y.; Zhang, Q. TMT-based quantitative proteomic analysis of spheroid cells of endometrial cancer possessing cancer stem cell properties. Stem Cell Res. Ther. 2023, 14, 119. [Google Scholar] [CrossRef]
- Kurra, V.; Krajewski, K.M.; Jagannathan, J.; Giardino, A.; Berlin, S.; Ramaiya, N. Typical and atypical metastatic sites of recurrent endometrial carcinoma. Cancer Imaging 2013, 13, 113–122. [Google Scholar] [CrossRef]
- Lajer, H.; Jensen, M.B.; Kilsmark, J.; Albæk, J.; Svane, D.; Mirza, M.R.; Geertsen, P.F.; Reerman, D.; Hansen, K.; Milter, M.C. The value of gynecologic cancer follow-up: Evidence-based ignorance? Int. J. Gynecol. Cancer 2010, 20, 1307–1320. [Google Scholar]
- Fung-Kee-Fung, M.; Dodge, J.; Elit, L.; Lukka, H.; Chambers, A.; Oliver, T.; Cancer Care Ontario Program in Evidence-based Care Gynecology Cancer Disease Site Group. Follow-up after primary therapy for endometrial cancer: A systematic review. Gynecol. Oncol. 2006, 101, 520–529. [Google Scholar] [CrossRef]
- Plataniotis, G.; Castiglione, M. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21, v41–v45. [Google Scholar] [CrossRef] [PubMed]
- Giannone, G.; Attademo, L.; Scotto, G.; Genta, S.; Ghisoni, E.; Tuninetti, V.; Aglietta, M.; Pignata, S.; Valabrega, G. Endometrial Cancer Stem Cells: Role, Characterization and Therapeutic Implications. Cancers 2019, 11, 1820. [Google Scholar] [CrossRef]
- Takao, T.; Masuda, H.; Kajitani, T.; Miki, F.; Miyazaki, K.; Yoshimasa, Y.; Katakura, S.; Tomisato, S.; Uchida, S.; Uchida, H.; et al. Sorafenib targets and inhibits the oncogenic properties of endometrial cancer stem cells via the RAF/ERK pathway. Stem Cell Res. Ther. 2022, 13, 225. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; Rasco, D.; Vogelzang, N.J.; Brose, M.S.; Cohn, A.L.; Mier, J.; Di Simone, C.; Hyman, D.M.; Stepan, D.E.; Dutcus, C.E. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: An interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 711–718. [Google Scholar] [CrossRef]
- Mittica, G.; Ghisoni, E.; Giannone, G.; Aglietta, M.; Genta, S.; Valabrega, G. Checkpoint inhibitors in endometrial cancer: Preclinical rationale and clinical activity. Oncotarget 2017, 8, 90532. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, P.; Della Pepa, C.; Berardi, S.; Califano, D.; Scala, S.; Buonaguro, L.; Ciliberto, G.; Brauchli, P.; Pignata, S. Tumor genotype and immune microenvironment in POLE-ultramutated and MSI-hypermutated endometrial cancers: New candidates for checkpoint blockade immunotherapy? Cancer Treat. Rev. 2016, 48, 61–68. [Google Scholar] [CrossRef]
- Hubbard, S.A.; Friel, A.M.; Kumar, B.; Zhang, L.; Rueda, B.R.; Gargett, C.E. Evidence for cancer stem cells in human endometrial carcinoma. Cancer Res. 2009, 69, 8241–8248. [Google Scholar] [CrossRef]
- Zhu, P.; Fan, Z. Cancer stem cells and tumorigenesis. Biophys. Rep. 2018, 4, 178–188. [Google Scholar] [CrossRef]
- Tang, D.G. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012, 22, 457–472. [Google Scholar] [CrossRef]
- Al-Kaabi, M.; Noel, K.; Al-Rubai, A.J. Evaluation of immunohistochemical expression of stem cell markers (NANOG and CD133) in normal, hyperplastic, and malignant endometrium. J. Med. Life 2022, 15, 117–123. [Google Scholar] [CrossRef]
- Takebe, N.; Miele, L.; Harris, P.J.; Jeong, W.; Bando, H.; Kahn, M.; Yang, S.X.; Ivy, S.P. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: Clinical update. Nat. Rev. Clin. Oncol. 2015, 12, 445–464. [Google Scholar] [CrossRef]
- Stahl, M.; Kohrman, N.; Gore, S.D.; Kim, T.K.; Zeidan, A.M.; Prebet, T. Epigenetics in cancer: A hematological perspective. PLoS Genet. 2016, 12, e1006193. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, K.; Wang, Y.; Han, W. Targeting cancer stem cells by using chimeric antigen receptor-modified T cells: A potential and curable approach for cancer treatment. Protein Cell 2018, 9, 516–526. [Google Scholar] [CrossRef]
- Klapdor, R.; Wang, S.; Hacker, U.; Büning, H.; Morgan, M.; Dörk, T.; Hillemanns, P.; Schambach, A. Improved Killing of Ovarian Cancer Stem Cells by Combining a Novel Chimeric Antigen Receptor–Based Immunotherapy and Chemotherapy. Hum. Gene Ther. 2017, 28, 886–896. [Google Scholar] [CrossRef]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Wan, J.; Gao, Y.; Zeng, K.; Yin, Y.; Zhao, M.; Wei, J.; Chen, Q. The levels of the sex hormones are not different between type 1 and type 2 endometrial cancer. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lax, S.F. Pathology of Endometrial Carcinoma. Adv. Exp. Med. Biol. 2017, 943, 75–96. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N.; Endometrial Cancer Staging Subcommittee; et al. FIGO staging of endometrial cancer: 2023. Int. J. Gynecol. Obstet. 2023, 162, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.A.; The Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- López-Reig, R.; Fernández-Serra, A.; Romero, I.; Zorrero, C.; Illueca, C.; García-Casado, Z.; Poveda, A.; López-Guerrero, J.A. Prognostic classification of endometrial cancer using a molecular approach based on a twelve-gene NGS panel. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Church, D.N.; Stelloo, E.; Nout, R.A.; Valtcheva, N.; Depreeuw, J.; Ter Haar, N.; Noske, A.; Amant, F.; Tomlinson, I.P.; Wild, P.J. Prognostic significance of POLE proofreading mutations in endometrial cancer. J. Natl. Cancer Inst. 2015, 107, dju402. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Q.; McGrail, D.J.; Dai, H.; Li, K.; Lin, S.Y. CHD4 mutations promote endometrial cancer stemness by activating TGF-beta signaling. Am. J. Cancer Res. 2018, 8, 903–914. [Google Scholar]
- Le Gallo, M.; O’Hara, A.J.; Rudd, M.L.; Urick, M.E.; Hansen, N.F.; O’Neil, N.J.; Price, J.C.; Zhang, S.; England, B.M.; Godwin, A.K. Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nat. Genet. 2012, 44, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhong, F.; Li, J.; Yue, H.; Li, W.; Lu, X. The epigenetic factor CHD4 contributes to metastasis by regulating the EZH2/β-catenin axis and acts as a therapeutic target in ovarian cancer. J. Transl. Med. 2023, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Konno, Y.; Dong, P.; Xiong, Y.; Suzuki, F.; Lu, J.; Cai, M.; Watari, H.; Mitamura, T.; Hosaka, M.; Hanley, S.J. MicroRNA-101 targets EZH2, MCL-1 and FOS to suppress proliferation, invasion and stem cell-like phenotype of aggressive endometrial cancer cells. Oncotarget 2014, 5, 6049. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Kyo, S.; Nakamura, M.; Hashimoto, M.; Maida, Y.; Mizumoto, Y.; Takakura, M.; Ohno, S.; Kiyono, T.; Inoue, M. Expression of HER-2 affects patient survival and paclitaxel sensitivity in endometrial cancer. Br. J. Cancer 2010, 103, 889–898. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, J.N.; Temkin, S.M.; Mackay, H.J. Endometrial cancer: Not your grandmother’s cancer. Cancer 2016, 122, 2787–2798. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.M.; Overbeek-Wager, E.; Grumbo, R.J. Diagnosis and management of endometrial cancer. Am. Fam. Physician 2016, 93, 468–474. [Google Scholar] [PubMed]
- Lu, K.H.; Schorge, J.O.; Rodabaugh, K.J.; Daniels, M.S.; Sun, C.C.; Soliman, P.T.; White, K.G.; Luthra, R.; Gershenson, D.M.; Broaddus, R.R. Prospective determination of prevalence of lynch syndrome in young women with endometrial cancer. J. Clin. Oncol. 2007, 25, 5158–5164. [Google Scholar] [CrossRef] [PubMed]
- Therachiyil, L.; Hussein, O.J.; Uddin, S.; Korashy, H.M. Regulation of the aryl hydrocarbon receptor in cancer and cancer stem cells of gynecological malignancies: An update on signaling pathways. Semin. Cancer Biol. 2022, 86, 1186–1202. [Google Scholar] [CrossRef]
- Hwang, J.; Bae, H.; Choi, S.; Yi, H.; Ko, B.; Kim, N. Impact of air pollution on breast cancer incidence and mortality: A nationwide analysis in South Korea. Sci. Rep. 2020, 10, 5392. [Google Scholar] [CrossRef]
- Brody, J.G.; Moysich, K.B.; Humblet, O.; Attfield, K.R.; Beehler, G.P.; Rudel, R.A. Environmental pollutants and breast cancer: Epidemiologic studies. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2007, 109, 2667–2711. [Google Scholar] [CrossRef]
- Al-Dhfyan, A.; Alhoshani, A.; Korashy, H.M. Aryl hydrocarbon receptor/cytochrome P450 1A1 pathway mediates breast cancer stem cells expansion through PTEN inhibition and β-Catenin and Akt activation. Mol. Cancer 2017, 16, 1–18. [Google Scholar]
- Stanford, E.A.; Wang, Z.; Novikov, O.; Mulas, F.; Landesman-Bollag, E.; Monti, S.; Smith, B.W.; Seldin, D.C.; Murphy, G.J.; Sherr, D.H. The role of the aryl hydrocarbon receptor in the development of cells with the molecular and functional characteristics of cancer stem-like cells. BMC Biol. 2016, 14, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Liu, S.; Shi, Y.; Liu, N.; Chen, L.; Wang, X.; Xiao, D.; Liu, X.; Mao, C.; Jiang, Y. Activation of AhR with nuclear IKKα regulates cancer stem-like properties in the occurrence of radioresistance. Cell Death Dis. 2018, 9, 490. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yu, S.; Tan, Q.; Guo, P.; Liu, H. Role of AhR in regulating cancer stem cell–like characteristics in choriocarcinoma. Cell Cycle 2018, 17, 2309–2320. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Takao, T.; Tsunematsu, R.; Morokuma, S.; Fukushima, K.; Kobayashi, H.; Saito, T.; Furue, M.; Wake, N.; Asanoma, K. Inhibition of AHR transcription by NF1C is affected by a single-nucleotide polymorphism, and is involved in suppression of human uterine endometrial cancer. Oncogene 2013, 32, 4950–4959. [Google Scholar] [CrossRef] [PubMed]
- Vergis, R.; Corbishley, C.M.; Norman, A.R.; Bartlett, J.; Jhavar, S.; Borre, M.; Heeboll, S.; Horwich, A.; Huddart, R.; Khoo, V. Intrinsic markers of tumour hypoxia and angiogenesis in localised prostate cancer and outcome of radical treatment: A retrospective analysis of two randomised radiotherapy trials and one surgical cohort study. Lancet Oncol. 2008, 9, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Mimeault, M.; Batra, S.K. Hypoxia-inducing factors as master regulators of stemness properties and altered metabolism of cancer-and metastasis-initiating cells. J. Cell. Mol. Med. 2013, 17, 30–54. [Google Scholar] [CrossRef]
- Luo, Y.; Cui, X.; Zhao, J.; Han, Y.; Li, M.; Lin, Y.; Jiang, Y.; Lan, L. Cells susceptible to epithelial-mesenchymal transition are enriched in stem-like side population cells from prostate cancer. Oncol. Rep. 2014, 31, 874–884. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Friel, A.M.; Sergent, P.A.; Patnaude, C.; Szotek, P.P.; Oliva, E.; Scadden, D.T.; Seiden, M.V.; Foster, R.; Rueda, B.R. Functional analyses of the cancer stem cell-like properties of human endometrial tumor initiating cells. Cell Cycle 2008, 7, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.J.; Laranjo, M.; Abrantes, A.M.; Casalta-Lopes, J.; Sarmento-Santos, D.; Costa, T.; Serambeque, B.; Almeida, N.; Gonçalves, T.; Mamede, C.; et al. Endometrial Cancer Spheres Show Cancer Stem Cells Phenotype and Preference for Oxidative Metabolism. Pathol. Oncol. Res. 2019, 25, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E.; Chan, R.W.; Schwab, K.E. Hormone and growth factor signaling in endometrial renewal: Role of stem/progenitor cells. Mol. Cell. Endocrinol. 2008, 288, 22–29. [Google Scholar] [CrossRef]
- Gargett, C.E.; Nguyen, H.P.; Ye, L. Endometrial regeneration and endometrial stem/progenitor cells. Rev. Endocr. Metab. Disord. 2012, 13, 235–251. [Google Scholar] [CrossRef]
- Lu, H.; Ju, D.D.; Yang, G.D.; Zhu, L.Y.; Yang, X.M.; Li, J.; Song, W.W.; Wang, J.H.; Zhang, C.C.; Zhang, Z.G.; et al. Targeting cancer stem cell signature gene SMOC-2 Overcomes chemoresistance and inhibits cell proliferation of endometrial carcinoma. EBioMedicine 2019, 40, 276–289. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Kolios, G.; Moodley, Y. Introduction to stem cells and regenerative medicine. Respiration 2012, 85, 3–10. [Google Scholar] [CrossRef]
- Poliwoda, S.; Noor, N.; Downs, E.; Schaaf, A.; Cantwell, A.; Ganti, L.; Kaye, A.D.; Mosel, L.I.; Carroll, C.B.; Viswanath, O.; et al. Stem cells: A comprehensive review of origins and emerging clinical roles in medical practice. Orthop. Rev. 2022, 14, 37498. [Google Scholar] [CrossRef]
- Hong, I.S. Endometrial Stem Cells: Orchestrating Dynamic Regeneration of Endometrium and Their Implications in Diverse Endometrial Disorders. Int. J. Biol. Sci. 2024, 20, 864–879. [Google Scholar] [CrossRef]
- Dekoninck, S.; Blanpain, C. Stem cell dynamics, migration and plasticity during wound healing. Nat. Cell Biol. 2019, 21, 18–24. [Google Scholar] [CrossRef]
- Olovnikov, A.M. A theory of marginotomy: The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J. Theor. Biol. 1973, 41, 181–190. [Google Scholar] [CrossRef]
- Sfeir, A.; de Lange, T. Removal of shelterin reveals the telomere end-protection problem. Science 2012, 336, 593–597. [Google Scholar] [CrossRef]
- Pucci, F.; Gardano, L.; Harrington, L. Short telomeres in ESCs lead to unstable differentiation. Cell Stem Cell 2013, 12, 479–486. [Google Scholar] [CrossRef]
- Pickett, H.A.; Cesare, A.J.; Johnston, R.L.; Neumann, A.A.; Reddel, R.R. Control of telomere length by a trimming mechanism that involves generation of t-circles. EMBO J. 2009, 28, 799–809. [Google Scholar] [CrossRef]
- Li, J.S.Z.; Denchi, E.L. How stem cells keep telomeres in check. Differentiation 2018, 100, 21–25. [Google Scholar] [CrossRef]
- Zhu, X.; Péault, B.; Yan, G.; Sun, H.; Hu, Y.; Ding, L. Stem cells and endometrial regeneration: From basic research to clinical trial. Curr. Stem Cell Res. Ther. 2019, 14, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Li, Y.; Yang, X. SIX1 is overexpressed in endometrial carcinoma and promotes the malignant behavior of cancer cells through ERK and AKT signaling. Oncol. Lett. 2016, 12, 3435–3440. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.M.; Kumar, M.; Ghosh, A.; Tomasetig, F.; Ali, A.; Whan, R.M.; Alterman, D.; Tanwar, P.S. Endometrial Axin2+ cells drive epithelial homeostasis, regeneration, and cancer following oncogenic transformation. Cell Stem Cell 2020, 26, 64–80.e13. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.J.; Laranjo, M.; Abrantes, A.M.; Torgal, I.; Botelho, M.F.; Oliveira, C.F. Clinical translation for endometrial cancer stem cells hypothesis. Cancer Metastasis Rev. 2015, 34, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Weiswald, L.-B.; Bellet, D.; Dangles-Marie, V. Spherical cancer models in tumor biology. Neoplasia 2015, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rutella, S.; Bonanno, G.; Procoli, A.; Mariotti, A.; Corallo, M.; Prisco, M.G.; Eramo, A.; Napoletano, C.; Gallo, D.; Perillo, A. Cells with characteristics of cancer stem/progenitor cells express the CD133 antigen in human endometrial tumors. Clin. Cancer Res. 2009, 15, 4299–4311. [Google Scholar] [CrossRef]
- Ozsvari, B.; Bonuccelli, G.; Sanchez-Alvarez, R.; Foster, R.; Sotgia, F.; Lisanti, M.P. Targeting flavin-containing enzymes eliminates cancer stem cells (CSCs), by inhibiting mitochondrial respiration: Vitamin B2 (Riboflavin) in cancer therapy. Aging 2017, 9, 2610. [Google Scholar] [CrossRef]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef]
- Celià-Terrassa, T.; Jolly, M.K. Cancer stem cells and epithelial-to-mesenchymal transition in cancer metastasis. Cold Spring Harb. Perspect. Med. 2020, 10, a036905. [Google Scholar] [CrossRef]
- Ye, X.; Weinberg, R.A. Epithelial–mesenchymal plasticity: A central regulator of cancer progression. Trends Cell Biol. 2015, 25, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Nassar, D.; Blanpain, C. Cancer stem cells: Basic concepts and therapeutic implications. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 47–76. [Google Scholar] [CrossRef]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef]
- Sottoriva, A.; Kang, H.; Ma, Z.; Graham, T.A.; Salomon, M.P.; Zhao, J.; Marjoram, P.; Siegmund, K.; Press, M.F.; Shibata, D.; et al. A Big Bang model of human colorectal tumor growth. Nat. Genet. 2015, 47, 209–216. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.-J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Sciacovelli, M.; Frezza, C. Metabolic reprogramming and epithelial-to-mesenchymal transition in cancer. FEBS J. 2017, 284, 3132–3144. [Google Scholar] [CrossRef]
- Thiery, J.P. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Hsu, J.-M.; Xia, W.; Hsu, Y.-H.; Chan, L.-C.; Yu, W.-H.; Cha, J.-H.; Chen, C.-T.; Liao, H.-W.; Kuo, C.-W.; Khoo, K.-H. STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nat. Commun. 2018, 9, 1908. [Google Scholar] [CrossRef]
- Chen, L.; Gibbons, D.L.; Goswami, S.; Cortez, M.A.; Ahn, Y.-H.; Byers, L.A.; Zhang, X.; Yi, X.; Dwyer, D.; Lin, W. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun. 2014, 5, 5241. [Google Scholar] [CrossRef]
- Hong, W.; Xue, M.; Jiang, J.; Zhang, Y.; Gao, X. Circular RNA circ-CPA4/let-7 miRNA/PD-L1 axis regulates cell growth, stemness, drug resistance and immune evasion in non-small cell lung cancer (NSCLC). J. Exp. Clin. Cancer Res. 2020, 39, 1–19. [Google Scholar] [CrossRef]
- Gao, L.; Guo, Q.; Li, X.; Yang, X.; Ni, H.; Wang, T.; Zhao, Q.; Liu, H.; Xing, Y.; Xi, T. MiR-873/PD-L1 axis regulates the stemness of breast cancer cells. EBioMedicine 2019, 41, 395–407. [Google Scholar] [CrossRef]
- Marinkovic, D.; Marinkovic, T. The new role for an old guy: MYC as an immunoplayer. J. Cell. Physiol. 2021, 236, 3234–3243. [Google Scholar] [CrossRef] [PubMed]
- Eilers, M.; Eisenman, R.N. Myc’s broad reach. Genes Dev. 2008, 22, 2755–2766. [Google Scholar] [CrossRef]
- Witte, K.E.; Hertel, O.; Windmoeller, B.A.; Helweg, L.P.; Hoeving, A.L.; Knabbe, C.; Busche, T.; Greiner, J.F.; Kalinowski, J.; Noll, T. Nanopore sequencing reveals global transcriptome signatures of mitochondrial and ribosomal gene expressions in various human cancer stem-like cell populations. Cancers 2021, 13, 1136. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Yang, X.; Fang, C.; Xu, H.; Xi, X. SALL4 as an epithelial-mesenchymal transition and drug resistance inducer through the regulation of c-Myc in endometrial cancer. PLoS ONE 2015, 10, e0138515. [Google Scholar] [CrossRef]
- Allegra, A.; Alonci, A.; Penna, G.; Innao, V.; Gerace, D.; Rotondo, F.; Musolino, C. The cancer stem cell hypothesis: A guide to potential molecular targets. Cancer Investig. 2014, 32, 470–495. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, Y.-P.; Huang, G.-R.; Gong, B.-L.; Yang, B.; Zhang, D.-X.; Hu, P.; Xu, S.-R. Expression of the stem cell marker Nanog in human endometrial adenocarcinoma. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2011, 30, 262. [Google Scholar] [CrossRef]
- Kusunoki, S.; Kato, K.; Tabu, K.; Inagaki, T.; Okabe, H.; Kaneda, H.; Suga, S.; Terao, Y.; Taga, T.; Takeda, S. The inhibitory effect of salinomycin on the proliferation, migration and invasion of human endometrial cancer stem-like cells. Gynecol. Oncol. 2013, 129, 598–605. [Google Scholar] [CrossRef]
- Boyer, L.A.; Lee, T.I.; Cole, M.F.; Johnstone, S.E.; Levine, S.S.; Zucker, J.P.; Guenther, M.G.; Kumar, R.M.; Murray, H.L.; Jenner, R.G.; et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005, 122, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Guy, M.S.; Qamar, L.; Behbakht, K.; Post, M.D.; Sheeder, J.; Sartorius, C.A.; Spillman, M.A. Progestin treatment decreases CD133+ cancer stem cell populations in endometrial cancer. Gynecol. Oncol. 2016, 140, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Song, Z.; Wang, X.; Ouyang, L. Ubiquitin C-terminal hydrolase L5 (UCHL5) accelerates the growth of endometrial cancer via activating the wnt/β-catenin signaling pathway. Front. Oncol. 2020, 10, 865. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, D.; Gao, J.; Shi, Z.; Chi, P.; Meng, Y.; Zou, C.; Wang, Y. Retracted: MicroRNA-373 promotes the development of endometrial cancer by targeting LATS2 and activating the Wnt/β-Catenin pathway. J. Cell. Biochem. 2019, 120, 8611–8618. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, Z.; Xu, X.; Fu, J.; Wang, K.; Liu, T.; Wu, C.; Kong, X.; Yang, Q.; Yan, G. Aurora-A induces chemoresistance through activation of the AKT/mTOR pathway in endometrial cancer. Front. Oncol. 2019, 9, 422. [Google Scholar] [CrossRef]
- Jiang, N.; Li, Q.-L.; Pan, W.; Li, J.; Zhang, M.-F.; Cao, T.; Su, S.-G.; Shen, H. PRMT6 promotes endometrial cancer via AKT/mTOR signaling and indicates poor prognosis. Int. J. Biochem. Cell Biol. 2020, 120, 105681. [Google Scholar] [CrossRef]
- Essex, A.; Pineda, J.; Acharya, G.; Xin, H.; Evans, J. Replication Study: Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Elife 2019, 8, e45426. [Google Scholar] [CrossRef]
- Clement, V.; Sanchez, P.; de Tribolet, N.; Radovanovic, I.; i Altaba, A.R. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr. Biol. 2007, 17, 165–172. [Google Scholar] [CrossRef]
- Farnie, G.; Clarke, R.B. Mammary stem cells and breast cancer—role of Notch signalling. Stem Cell Rev. 2007, 3, 169–175. [Google Scholar] [CrossRef]
- Borah, A.; Raveendran, S.; Rochani, A.; Maekawa, T.; Kumar, D. Targeting self-renewal pathways in cancer stem cells: Clinical implications for cancer therapy. Oncogenesis 2015, 4, e177. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; van der Zee, M.; Fodde, R.; Blok, L.J. Wnt/Β-catenin and sex hormone signaling in endometrial homeostasis and cancer. Oncotarget 2010, 1, 674–684. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, T.; Huang, Y. MicroRNA-134 suppresses endometrial cancer stem cells by targeting POGLUT1 and Notch pathway proteins. FEBS Lett. 2015, 589, 207–214. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Huang, K.; Wang, Y.; Li, J.; Yang, X. MicroRNA-34a inhibits cells proliferation and invasion by downregulating Notch1 in endometrial cancer. Oncotarget 2017, 8, 111258. [Google Scholar] [CrossRef]
- Feng, Y.-Z.; Shiozawa, T.; Miyamoto, T.; Kashima, H.; Kurai, M.; Suzuki, A.; Ying-Song, J.; Konishi, I. Overexpression of hedgehog signaling molecules and its involvement in the proliferation of endometrial carcinoma cells. Clin. Cancer Res. 2007, 13, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Konno, Y.; Watari, H.; Hosaka, M.; Noguchi, M.; Sakuragi, N. The impact of microRNA-mediated PI3K/AKT signaling on epithelial-mesenchymal transition and cancer stemness in endometrial cancer. J. Transl. Med. 2014, 12, 1–9. [Google Scholar] [CrossRef]
- Kroon, P.; Berry, P.A.; Stower, M.J.; Rodrigues, G.; Mann, V.M.; Simms, M.; Bhasin, D.; Chettiar, S.; Li, C.; Li, P.-K. JAK-STAT blockade inhibits tumor initiation and clonogenic recovery of prostate cancer stem-like cells. Cancer Res. 2013, 73, 5288–5298. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Vazquez-Santillan, K.; Melendez-Zajgla, J.; Jimenez-Hernandez, L.; Martinez-Ruiz, G.; Maldonado, V. NF-κB signaling in cancer stem cells: A promising therapeutic target? Cell. Oncol. 2015, 38, 327–339. [Google Scholar] [CrossRef]
- Brasseur, K.; Gévry, N.; Asselin, E. Chemoresistance and targeted therapies in ovarian and endometrial cancers. Oncotarget 2017, 8, 4008. [Google Scholar] [CrossRef]
- Bomken, S.; Fišer, K.; Heidenreich, O.; Vormoor, J. Understanding the cancer stem cell. Br. J. Cancer 2010, 103, 439–445. [Google Scholar] [CrossRef]
- Xu, Y.; An, X.; Guo, X.; Habtetsion, T.G.; Wang, Y.; Xu, X.; Kandala, S.; Li, Q.; Li, H.; Zhang, C. Endothelial PFKFB3 plays a critical role in angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1231–1239. [Google Scholar] [CrossRef]
- Peng, F.; Li, Q.; Sun, J.-Y.; Luo, Y.; Chen, M.; Bao, Y. PFKFB3 is involved in breast cancer proliferation, migration, invasion and angiogenesis. Int. J. Oncol. 2018, 52, 945–954. [Google Scholar] [CrossRef]
- Cantelmo, A.R.; Conradi, L.-C.; Brajic, A.; Goveia, J.; Kalucka, J.; Pircher, A.; Chaturvedi, P.; Hol, J.; Thienpont, B.; Teuwen, L.-A. Inhibition of the glycolytic activator PFKFB3 in endothelium induces tumor vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell 2016, 30, 968–985. [Google Scholar] [CrossRef]
- Cieślar-Pobuda, A.; Jain, M.V.; Kratz, G.; Rzeszowska-Wolny, J.; Ghavami, S.; Wiechec, E. The expression pattern of PFKFB3 enzyme distinguishes between induced-pluripotent stem cells and cancer stem cells. Oncotarget 2015, 6, 29753. [Google Scholar] [CrossRef]
- Yang, Z.; Li, C.; Fan, Z.; Liu, H.; Zhang, X.; Cai, Z.; Xu, L.; Luo, J.; Huang, Y.; He, L. Single-cell sequencing reveals variants in ARID1A, GPRC5A and MLL2 driving self-renewal of human bladder cancer stem cells. Eur. Urol. 2017, 71, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Zhou, N.; Wang, Z.; Li, G.; Kou, Y.; Yu, S.; Feng, Y.; Chen, L.; Yang, J.; Tian, F. circGprc5a promoted bladder oncogenesis and metastasis through Gprc5a-targeting peptide. Mol. Ther.-Nucleic Acids 2018, 13, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.; Chen, M.; Darrow, M.A.; Li, C.-S.; Chiu, C.-L.; Monjazeb, A.M.; Murphy, W.J.; Canter, R.J. Alterations in cancer stem-cell marker CD44 expression predict oncologic outcome in soft-tissue sarcomas. J. Surg. Res. 2018, 223, 207–214. [Google Scholar] [CrossRef]
- Park, J.Y.; Hong, D.; Park, J.Y. Association between morphological patterns of myometrial invasion and cancer stem cell markers in endometrial endometrioid carcinoma. Pathol. Oncol. Res. 2019, 25, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Elbasateeny, S.S.; Salem, A.A.; Abdelsalam, W.A.; Salem, R.A. Immunohistochemical expression of cancer stem cell related markers CD44 and CD133 in endometrial cancer. Pathol.-Res. Pract. 2016, 212, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadegan, M.R.; Bagheri, V.; Razavi, M.S.; Momtazi, A.A.; Sahebkar, A.; Gholamin, M. Isolation, identification, and characterization of cancer stem cells: A review. J. Cell. Physiol. 2017, 232, 2008–2018. [Google Scholar] [CrossRef]
- Jaggupilli, A.; Elkord, E. Significance of CD44 and CD24 as cancer stem cell markers: An enduring ambiguity. Clin. Dev. Immunol. 2012, 2012, 708036. [Google Scholar] [CrossRef] [PubMed]
- Shiba, S.; Ikeda, K.; Suzuki, T.; Shintani, D.; Okamoto, K.; Horie-Inoue, K.; Hasegawa, K.; Inoue, S. Hormonal Regulation of Patient-Derived Endometrial Cancer Stem-like Cells Generated by Three-Dimensional Culture. Endocrinology 2019, 160, 1895–1906. [Google Scholar] [CrossRef] [PubMed]
- Glumac, P.M.; LeBeau, A.M. The role of CD133 in cancer: A concise review. Clin. Transl. Med. 2018, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mizrak, D.; Brittan, M.; Alison, M. CD133: Molecule of the moment. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2008, 214, 3–9. [Google Scholar] [CrossRef]
- Jaksch, M.; Múnera, J.; Bajpai, R.; Terskikh, A.; Oshima, R.G. Cell cycle–dependent variation of a CD133 epitope in human embryonic stem cell, colon cancer, and melanoma cell lines. Cancer Res. 2008, 68, 7882–7886. [Google Scholar] [CrossRef]
- Kyo, S.; Kato, K. Endometrial Cancer Stem Cell as a Potential Therapeutic Target. Semin. Reprod. Med. 2015, 33, 341–349. [Google Scholar] [CrossRef]
- Mirantes, C.; Espinosa, I.; Ferrer, I.; Dolcet, X.; Prat, J.; Matias-Guiu, X. Epithelial-to-mesenchymal transition and stem cells in endometrial cancer. Hum. Pathol. 2013, 44, 1973–1981. [Google Scholar] [CrossRef]
- Tabu, K.; Taga, T.; Tanaka, S. Tumor Stem Cells: CD133 Gene Regulation and Tumor Stemness. In Stem Cells and Cancer Stem Cells, Volume 2: Stem Cells and Cancer Stem Cells, Therapeutic Applications in Disease and Injury; Hayat, M.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 145–153. [Google Scholar]
- Comaills, V.; Kabeche, L.; Morris, R.; Buisson, R.; Yu, M.; Madden, M.W.; LiCausi, J.A.; Boukhali, M.; Tajima, K.; Pan, S.; et al. Genomic Instability Is Induced by Persistent Proliferation of Cells Undergoing Epithelial-to-Mesenchymal Transition. Cell Rep. 2016, 17, 2632–2647. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Pertot, A.; Shirole, N.H.; Yao, Z.; Anaparthy, N.; Garvin, T.; Cox, H.; Chang, K.; Rollins, F.; Kendall, J.; et al. TGF-β reduces DNA ds-break repair mechanisms to heighten genetic diversity and adaptability of CD44+/CD24− cancer cells. eLife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Zhang, X.; Mizumoto, Y.; Maida, Y.; Bono, Y.; Takakura, M.; Kyo, S. Molecular characterization of CD133+ cancer stem-like cells in endometrial cancer. Int. J. Oncol. 2014, 44, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yoshida, T.; Okabe, M.; Zhou, K.; Wang, F.; Soko, C.; Saito, S.; Nikaido, T. Isolation of Stem-Like Cancer Cells in Primary Endometrial Cancer Using Cell Surface Markers CD133 and CXCR4. Transl. Oncol. 2017, 10, 976–987. [Google Scholar] [CrossRef]
- Bokhari, A.A.; Baker, T.M.; Dorjbal, B.; Waheed, S.; Zahn, C.M.; Hamilton, C.A.; Maxwell, G.L.; Syed, V. Nestin suppression attenuates invasive potential of endometrial cancer cells by downregulating TGF-β signaling pathway. Oncotarget 2016, 7, 69733. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.; Ahn, R.; Yang, K.; Zhu, X.; Fu, Z.; Morin, G.; Bramley, R.; Cliffe, N.C.; Xue, Y.; Kuasne, H. CD44 promotes PD-L1 expression and its tumor-intrinsic function in breast and lung cancers. Cancer Res. 2020, 80, 444–457. [Google Scholar] [CrossRef]
- Zagorianakou, N.; Ioachim, E.; Mitselou, A.; Kitsou, E.; Zagorianakou, P.; Stefanaki, S.; Makrydimas, G.; Agnantis, N.J. Glycoprotein CD44 expression in normal, hyperplasic and neoplastic endometrium. An immunohistochemical study including correlations with p53, steroid receptor status and proliferative indices (PCNA, MIB1). Eur. J. Gynaecol. Oncol. 2003, 24, 500–504. [Google Scholar]
- Vannahme, C.; Gösling, S.; Paulsson, M.; Maurer, P.; Hartmann, U. Characterization of SMOC-2, a modular extracellular calcium-binding protein. Biochem. J. 2003, 373, 805–814. [Google Scholar] [CrossRef]
- Maier, S.; Paulsson, M.; Hartmann, U. The widely expressed extracellular matrix protein SMOC-2 promotes keratinocyte attachment and migration. Exp. Cell Res. 2008, 314, 2477–2487. [Google Scholar] [CrossRef]
- Wend, P.; Holland, J.D.; Ziebold, U.; Birchmeier, W. Wnt signaling in stem and cancer stem cells. Semin. Cell Dev. Biol. 2010, 21, 855–863. [Google Scholar] [CrossRef]
- Shi, J.; Chi, S.; Xue, J.; Yang, J.; Li, F.; Liu, X. Emerging role and therapeutic implication of Wnt signaling pathways in autoimmune diseases. J. Immunol. Res. 2016, 2016. [Google Scholar] [CrossRef]
- Gelmini, S.; Mangoni, M.; Castiglione, F.; Beltrami, C.; Pieralli, A.; Andersson, K.L.; Fambrini, M.; Taddei, G.L.; Serio, M.; Orlando, C. The CXCR4/CXCL12 axis in endometrial cancer. Clin. Exp. Metastasis 2009, 26, 261–268. [Google Scholar] [CrossRef]
- Zhang, S.S.; Han, Z.P.; Jing, Y.Y.; Tao, S.F.; Li, T.J.; Wang, H.; Wang, Y.; Li, R.; Yang, Y.; Zhao, X.; et al. CD133(+)CXCR4(+) colon cancer cells exhibit metastatic potential and predict poor prognosis of patients. BMC Med. 2012, 10, 85. [Google Scholar] [CrossRef]
- Cioffi, M.; D’Alterio, C.; Camerlingo, R.; Tirino, V.; Consales, C.; Riccio, A.; Ieranò, C.; Cecere, S.C.; Losito, N.S.; Greggi, S.; et al. Identification of a distinct population of CD133(+)CXCR4(+) cancer stem cells in ovarian cancer. Sci. Rep. 2015, 5, 10357. [Google Scholar] [CrossRef]
- Fang, D.; Nguyen, T.K.; Leishear, K.; Finko, R.; Kulp, A.N.; Hotz, S.; Van Belle, P.A.; Xu, X.; Elder, D.E.; Herlyn, M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005, 65, 9328–9337. [Google Scholar] [CrossRef]
- Grange, C.; Lanzardo, S.; Cavallo, F.; Camussi, G.; Bussolati, B. Sca-1 identifies the tumor-initiating cells in mammary tumors of BALB-neuT transgenic mice. Neoplasia 2008, 10, 1433–1443. [Google Scholar] [CrossRef]
- Edling, C.E.; Hallberg, B. c-Kit—A hematopoietic cell essential receptor tyrosine kinase. Int. J. Biochem. Cell Biol. 2007, 39, 1995–1998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Balch, C.; Chan, M.W.; Lai, H.-C.; Matei, D.; Schilder, J.M.; Yan, P.S.; Huang, T.H.; Nephew, K.P. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008, 68, 4311–4320. [Google Scholar] [CrossRef]
- Levina, V.; Marrangoni, A.; Wang, T.; Parikh, S.; Su, Y.; Herberman, R.; Lokshin, A.; Gorelik, E. Elimination of human lung cancer stem cells through targeting of the stem cell factor–c-kit autocrine signaling loop. Cancer Res. 2010, 70, 338–346. [Google Scholar] [CrossRef]
- Zhang, X.; Kyo, S.; Nakamura, M.; Mizumoto, Y.; Maida, Y.; Bono, Y.; Takakura, M.; Fujiwara, H. Imatinib sensitizes endometrial cancer cells to cisplatin by targeting CD117-positive growth-competent cells. Cancer Lett. 2014, 345, 106–114. [Google Scholar] [CrossRef]
- Mazzoldi, E.L.; Pavan, S.; Pilotto, G.; Leone, K.; Pagotto, A.; Frezzini, S.; Nicoletto, M.O.; Amadori, A.; Pastò, A. A juxtacrine/paracrine loop between C-Kit and stem cell factor promotes cancer stem cell survival in epithelial ovarian cancer. Cell Death Dis. 2019, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Saygin, C.; Wiechert, A.; Rao, V.S.; Alluri, R.; Connor, E.; Thiagarajan, P.S.; Hale, J.S.; Li, Y.; Chumakova, A.; Jarrar, A.; et al. CD55 regulates self-renewal and cisplatin resistance in endometrioid tumors. J. Exp. Med. 2017, 214, 2715–2732. [Google Scholar] [CrossRef] [PubMed]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Tanaka, K.; Tanaka, T.; Hara, A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget 2016, 7, 11018. [Google Scholar] [CrossRef] [PubMed]
- Rahadiani, N.; Ikeda, J.; Mamat, S.; Matsuzaki, S.; Ueda, Y.; Umehara, R.; Tian, T.; Wang, Y.; Enomoto, T.; Kimura, T.; et al. Expression of aldehyde dehydrogenase 1 (ALDH1) in endometrioid adenocarcinoma and its clinical implications. Cancer Sci. 2011, 102, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Yamawaki, K.; Ishiguro, T.; Yoshihara, K.; Ueda, H.; Sato, A.; Ohata, H.; Yoshida, Y.; Minamino, T.; Okamoto, K.; et al. ALDH-Dependent Glycolytic Activation Mediates Stemness and Paclitaxel Resistance in Patient-Derived Spheroid Models of Uterine Endometrial Cancer. Stem Cell Rep. 2019, 13, 730–746. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Zhou, P.; Zhang, K. Autophagy plays an important role in stemness mediation and the novel dual function of EIG121 in both autophagy and stemness regulation of endometrial carcinoma JEC cells. Int. J. Oncol. 2017, 51, 644–656. [Google Scholar] [CrossRef]
- de Vicente, J.C.; Rodríguez-Santamarta, T.; Rodrigo, J.P.; Allonca, E.; Vallina, A.; Singhania, A.; Donate-Pérez del Molino, P.; García-Pedrero, J.M. The emerging role of NANOG as an early cancer risk biomarker in patients with oral potentially malignant disorders. J. Clin. Med. 2019, 8, 1376. [Google Scholar] [CrossRef]
- Grubelnik, G.; Boštjančič, E.; Pavlič, A.; Kos, M.; Zidar, N. NANOG expression in human development and cancerogenesis. Exp. Biol. Med. 2020, 245, 456–464. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, J.; Chen, S.; Fang, C.; Zhang, X.; Luo, Z. Prognostic significance of NANOG expression in solid tumors: A meta-analysis. OncoTargets Ther. 2018, 11, 5515–5526. [Google Scholar] [CrossRef] [PubMed]
- Roudi, R.; Barodabi, M.; Madjd, Z.; Roviello, G.; Corona, S.P.; Panahi, M. Expression patterns and clinical significance of the potential cancer stem cell markers OCT4 and NANOG in colorectal cancer patients. Mol. Cell. Oncol. 2020, 7, 1788366. [Google Scholar] [CrossRef] [PubMed]
- Saravi, O.E.; Naghshvar, F.; Torabizadeh, Z.; Sheidaei, S. Immunohistochemical expression of nanog and its relation with clinicopathologic characteristics in breast ductal carcinoma. Iran. Biomed. J. 2019, 23, 184. [Google Scholar]
- Zhang, B.; Wang, J.; Liu, W.; Yin, Y.; Qian, D.; Zhang, H.; Shi, B.; Li, C.; Zhu, J.; Zhang, L.; et al. Cytokeratin 18 knockdown decreases cell migration and increases chemosensitivity in non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 2479–2487. [Google Scholar] [CrossRef]
- Tabuchi, Y.; Hirohashi, Y.; Hashimoto, S.; Mariya, T.; Asano, T.; Ikeo, K.; Kuroda, T.; Mizuuchi, M.; Murai, A.; Uno, S. Clonal analysis revealed functional heterogeneity in cancer stem-like cell phenotypes in uterine endometrioid adenocarcinoma. Exp. Mol. Pathol. 2019, 106, 78–88. [Google Scholar] [CrossRef]
- Kitson, S.J.; Rosser, M.; Fischer, D.P.; Marshall, K.M.; Clarke, R.B.; Crosbie, E.J. Targeting Endometrial Cancer Stem Cell Activity with Metformin Is Inhibited by Patient-Derived Adipocyte-Secreted Factors. Cancers 2019, 11, 653. [Google Scholar] [CrossRef]
- Li, Y.; Huo, J.; He, J.; Ma, X. LncRNA MONC suppresses the malignant phenotype of Endometrial Cancer Stem Cells and Endometrial Carcinoma Cells by regulating the MiR-636/GLCE axis. Cancer Cell Int. 2021, 21, 331. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, V.V.; Morrow, S.; Rahman Kawakibi, A.; Zhou, L.; Ralff, M.; Ray, J.; Jhaveri, A.; Ferrarini, I.; Lee, Y.; Parker, C.; et al. ONC201 and imipridones: Anti-cancer compounds with clinical efficacy. Neoplasia 2020, 22, 725–744. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).