Simple Summary
Researchers aim to assess the economic impact of testing predictive biomarkers for immunotherapy in solid tumour treatment using immune checkpoint inhibitors (ICIs). Despite recent advancements, concerns persist regarding the cost-effectiveness and budgetary implications of ICIs. This study systematically reviewed the economic evaluations of biomarker testing from various databases. The team assessed studies from June 2010 to February 2022, evaluating their quality and synthesising findings by tumour type. Understanding the economic implications of these tests could help drive future research, optimise treatment strategies, potentially influencing health care decisions and resource allocation in solid tumour therapy, impacting how we approach and fund immunotherapy for better patient outcomes.
Abstract
This study investigated the health economic evaluations of predictive biomarker testing in solid tumours treated with immune checkpoint inhibitors (ICIs). Searching PubMed, EMBASE, and Web of Science from June 2010 to February 2022, 58 relevant articles were reviewed out of the 730 screened. The focus was predominantly on non-small cell lung cancer (NSCLC) (65%) and other solid tumours (40%). Among the NSCLC studies, 21 out of 35 demonstrated cost-effectiveness, notably for pembrolizumab as first-line treatment when preceded by PD-L1 assessment, cost-effective at a threshold of $100,000/QALY compared to the standard of care. However, for bladder, cervical, and triple-negative breast cancers (TNBCs), no economic evaluations met the affordability threshold of $100,000/QALY. Overall, the review highlights a certain degree of uncertainty about the cost-effectiveness of ICI. In particular, we found PD-L1 expression associated with ICI treatment to be a cost-effective strategy, particularly in NSCLC, urothelial, and renal cell carcinoma. The findings suggest the potential value of predictive biomarker testing, specifically with pembrolizumab in NSCLC, while indicating challenges in achieving cost-effectiveness for certain other solid tumours.
1. Introduction
The advent of immunotherapy has significantly changed the therapeutic scenario of cancer patients. Immunotherapy drugs, so-called immune checkpoint inhibitors (ICIs), work by blocking checkpoint proteins from binding to their partners, representing a new weapon for cancer treatment in different settings [1]. Moreover, the association of a biomarker test to identify the best treatment choice has revolutionised patient management for many tumour types, driving towards a patient-tailored approach [2]. Since the FDA approval of ipilimumab (cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) inhibitor) in 2011, six more ICIs have been approved for cancer therapy: the programmed cell death-1 (PD-1) inhibitors including nivolumab, pembrolizumab, cemiplimab, and the programmed cell death ligand-1 (PD-L1) inhibitors including atezolizumab, durvalumab, and avelumab. These agents have become the standard of care in many solid tumours [1,2,3]. Thus, a predictive biomarker testing approach in this field may represent a virtuous model to invest in for treatment optimisation, with a possible impact also on the patients’ quality of life (QoL). Despite the successes achieved in recent years, there are many concerns about the cost-effectiveness (CE) and budget impact of the next wave of ICIs [3]. This systematic review, as part of a funded Italian National Research Project, aims to provide a snapshot of the current state-of-the-art regarding the cost-effectiveness, cost-utility, or net-monetary benefits of the use of predictive biomarkers in solid tumours treated with ICIs as tools for customising immunotherapy.
2. Materials and Methods
A systematic literature review was conducted in accordance with the PRISMA 2020 statement guidelines [4]. The review methodology was prospectively registered a priori in PROSPERO (CRD42020201549) as for the entire study protocol [5].
2.1. Data Sources and Searches
Searches of relevant studies were conducted across the following electronic bibliographic databases: Ovid MEDLINE, EMBASE, Web of Science, from June 2010 to February 2022 as per the date of the first ICI approval [6]. Search strategy and syntaxes are detailed in the Supplementary Box S1 and Supplementary Box S2 in the Supplementary Materials, respectively. References were collected with Reference Manager (Institute for Scientific Information, Berkeley, CA, USA, ver.12).
2.2. Study Selection, Data Extraction, and Quality Assessment
Predefined criteria for selecting studies related to economic evaluation (population, interventions and comparisons, outcomes, study design, [PICOS]) were set and published in the study protocol [5]. Data were processed by four researchers to identify potentially eligible studies and validated by five clinicians/researchers. The screening process is detailed in Figure 1. The quality of evidence was evaluated by the GRADE approach [7].
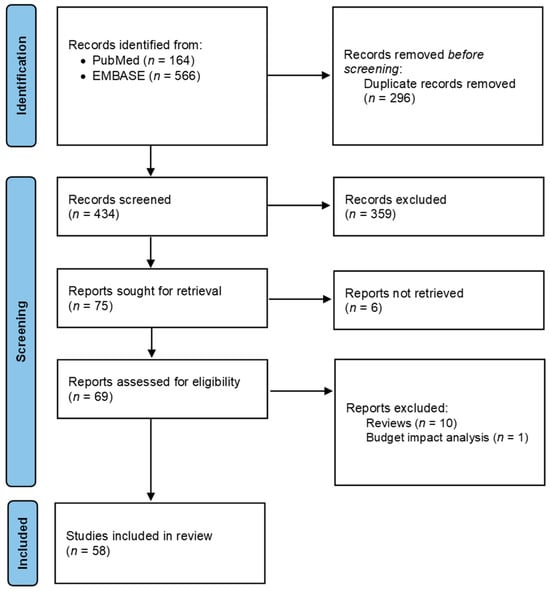
Figure 1.
PRISMA diagram of the review’s systematic searches.
2.3. Data Synthesis
Country, treatment line, comparators, biomarker test, willingness-to-pay (WTP) threshold, cost-effectiveness ratio, and affordability according to the WTP set were extracted from each study. Information was collected separately for non-small cell lung cancer (NSCLC) and other solid tumour diagnoses. A time-trend analysis was performed. After converting differences in costs to the 2021 US dollar, adjusting for both inflation and purchasing power parities [8] resources, adjusted differences in costs and QALYs were plotted on a CE plane, evaluated with a reference WTP threshold of $100,000/QALY.
2.4. Role of the Funding Source
This research was funded by the Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR) within the framework of the PRIN Project 2017, grant number 2017NR7W5K. This funding source had no role in the design, execution, analyses, interpretation of the data, and results of the present study.
3. Results
3.1. Study Identification
A total of 730 articles were identified through the systematic literature search across different bibliographic database queries. As reported in Figure 1, 295 (40.4%) duplicates were removed and 435 abstracts (59.6%) were screened, resulting in 76 studies potentially evaluable. After excluding six papers for which the full-text was not retrieved, a total of 70 full-text articles were examined. Among these papers, 10 systematic reviews and one paper based on a budget impact analysis model were excluded, for a total of 58 studies that met the inclusion criteria.
3.2. Study Quality
Quality of evidence and risk of biases were assessed based on the GRADE scale for each study. Overall, 60% of economic evaluation (n = 35) was based on a Markov model and 26% on the partitioned survival model (n = 15). All of the methodological aspects of individual studies are shown in Supplementary Table S1 in the Supplementary Materials. Overall, most economic evaluations (62.1%, n = 36 studies) presented a moderate/high certainty of evidence and strength of recommendations. Next, 25.9% (n = 15) of the studies achieved a moderate certainty rating, 6.9% (n = 4) a high, and 5.2% (n = 3) a moderate/low certainty score (Supplementary Table S2 in the Supplementary Materials).
3.3. Study Characteristics
The geographical distribution of papers reporting an economic evaluation of an ICI associated to a biomarker test showed a marked majority of studies conducted in the US (56%; n = 33) and China (27%; n = 16). Figure 2 shows the worldwide distribution of studies included.
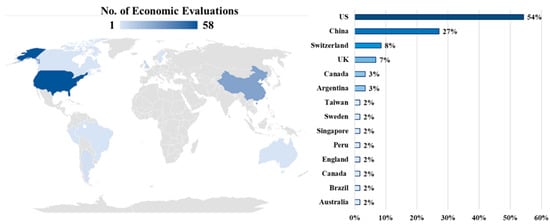
Figure 2.
Global distribution of economic evaluations of an ICI associated with a biomarker test.
In the overall analysis, 97% of the included studies (n = 56) assessed the cost-effectiveness ratio of an ICI with prior PD-L1 testing, whereas only 3% (n = 2) considered prior PD-1 testing. Among the overall 58 included studies, 60% (n = 35) included patients affected by NSCLC (Table 1) and 40% other solid tumours including 25.9% (n = 6) head and neck cancer squamous cell carcinoma (HNSCC), 15.5% (n = 9) urothelial, bladder cancer, and renal cell carcinoma (RCC), followed by a few studies on triple-negative breast cancer (5.2%; n = 3), melanoma including Merkel cell carcinoma (mMCC) (5.2%; n = 3), cervical, and gastric cancers (3.4%; n = 2) (Table 2). Following a bibliometric analysis, a huge increase in the number of papers on NSCLC from 2016 to 2021 was observed (Figure 3). This was confirmed by the time-trend estimates of the economic evaluations of ICIs associated with biomarker testing in NSCLC being considerably higher than in other solid tumours.

Table 1.
Characteristics of studies focusing on non-small cell lung cancer (NSCLC).

Table 2.
Characteristics of studies stratified by other solid tumours.
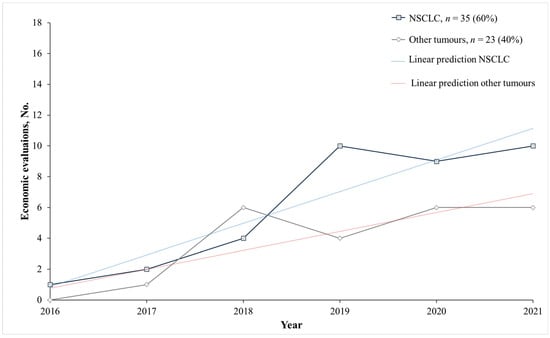
Figure 3.
Economic evaluations of an ICI associated with a biomarker test per year focused on solid tumour diagnoses. Other tumours: Urothelial, bladded and renal cell cancer, cervical cancer, gastric cancer, head and neck cancer squamous cell carcinoma; melanoma; Merkel cell carcinoma, triple-negative breast cancer. Abbreviations: Non-small cell lung cancer (NSCLC). Notes: Dotted lines are estimates of linear predictions on the yearly trend of economic evaluations of ICIs associated with biomarker testing in NSCLC (blue dotted line) and other solid tumours (red dotted line).
3.4. Non-Small Cell Lung Cancer (NSCLC)
Among the 35 studies on patients with NSCLC, slightly more than half of those studies (63%, n = 22) [9,10,11,12,13,14,20,21,22,23,29,30,31,32,37,38,39,42,67,68,69] suggested the cost-effectiveness of ICI treatment with biomarker testing; of these, n = 2 with previous PD-1 assessment and n = 20 with previous PD-L1 assessment. Particularly, when distinguishing between anti PD-1 and anti PD-L1 treatment and considering the multiple analyses performed as part of the selected studies, there were no differences in the proportion of analyses demonstrating the cost-effectiveness of ICIs (p-value = 1), Figure 4.
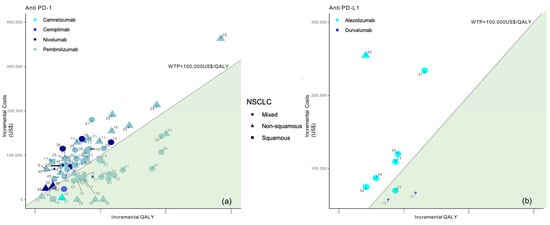
Figure 4.
Results from the different studies on a cost-effectiveness plane for NSCLC patients according to anti PD-1 (a) or anti PD-L1 therapy (b). Note: Studies in the figure are identified by reference number.
In more detail, pembrolizumab (n = 17) [9,10,11,13,21,23,24,29,30,31,32,37,38,39,42] was cost-effective with respect to chemotherapy (SoC) when associated with a previous PD-1/PD-L1 assessment, in the first-line treatment of NSCLC [9,10,11,13,21,23,24,29,30,31,32,34,37,38,42]. Moreover, four studies [14,22,39,40] demonstrated the cost-effectiveness of nivolumab with previous PD-L1 expression compared to chemotherapy in both first [22,40] and second [39] line treatment. Furthermore, durvalumab treatment in PD-L1-positive patients [20] was associated with a positive cost-effectiveness profile when compared with the placebo. The same evidence was recorded for atezolizumab from a US perspective [15]. Durvalumab was considered cost-effective with respect to the SoC in the US (WTP of $100,000–$150,000/QALY), but not-cost-effective in China (WTP of $33,210/QALY). Overall, 14 studies [16,17,18,19,25,26,27,28,33,35,36,40,41,43] showed that all ICI types associated with a previous biomarker test were not a cost-effective solution for NSCLC patients in settings where there was a WTP less than $100,000/QALY, also considering overall survival (OS) as most influential factor for the incremental cost-effectiveness ratio.
Figures show incremental QALY and incremental costs for different immune checkpoint inhibitors (ICI) among NSCLC patients according to NSCLC type (identified by the type of marker) and immune checkpoint inhibitor (identified by marker colour); the size of the marker identifies first-line (bigger) or second-line (smaller) treatment; the area under the WTP threshold indicates the cost-effective studies and is highlighted in green. Studies for which results are available for more than one country are represented more than once.
3.5. Other Tumours
3.5.1. Urothelial, Bladder Cancer, and Renal Cell Carcinoma (RCC)
When considering other tumours, statistically significant differences emerged between anti PD-1 and anti PD-L1, with a clear indication of no cost-effectiveness of available evidence for the latter approach in those tumours (Figure 5).
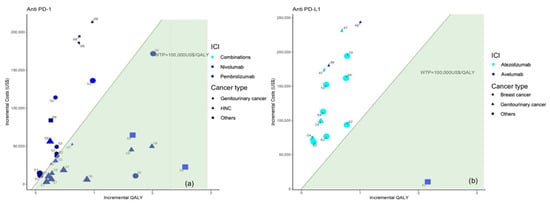
Figure 5.
Results from the different studies on a cost-effectiveness plane for patients with cancer other than NSCLC according to anti PD-1 (a) or anti PD-L1 therapy (b). Note: Studies in the figure are identified by reference number.
In more detail, five economic evaluations [44,45,46,47,49] were carried out on patients diagnosed with urothelial cancer, demonstrating that avelumab (n = 1) [45] and pembrolizumab (n = 2) [46,47] were cost-effective in first-line treatment when preceded by PD-L1 testing (with thresholds set in the US and Switzerland) with greater impact on the survival benefit of patients. Conversely, first-line treatment with atezolizumab (n = 1) [44] and second-line treatment with pembrolizumab [49] (n = 1) were not cost-effective with a threshold of $150,000/QALY in a US setting.
Regarding renal cell carcinoma from a US perspective, nivolumab was cost-effective compared to everolimus [50], and nivolumab with ipilimumab was cost-effective compared to sunitinib in both cases with a PD-L1 test, and a $150,000/QALY threshold [48] generated a gain of 0.34 QALYs and 0.98 QALYs, respectively.
Moreover, the two studies [51,52] conducted on bladder cancer showed that the second-line treatment with pembrolizumab and atezolizumab were not cost-effective compared to chemotherapy in a Canadian, British, and Australian perspective with a WTP less than $100,000/QALY (Figure 5).
3.5.2. Head and Neck Cancer Squamous Cell Carcinoma (HNSCC)
Four out of six studies [53,54,55,56,57,58] revealed that pembrolizumab [53,54,55] and nivolumab [57] were a cost-effective option compared with SoC in the US, China, Switzerland, and Argentina, while two found that nivolumab was not a cost-effective option compared to the SoC in the US [58] and docetaxel in Canada [56].
3.5.3. Triple-Negative Breast Cancer (TNBC)
The cost-effectiveness analysis of atezolizumab plus nab-paclitaxel versus nab-paclitaxel alone provided similar results. Two Markov models and a multi-country partitioned survival model [59,60] indicated that this combination (atezolizumab plus nab-paclitaxel) was not cost-effective compared to nab-paclitaxel alone, both in China and the US. Similarly, another Markov model-based study [61] corroborated these findings across the overall population (ICER $281,448/QALY; WTP of $200,000/QALY) (Figure 5).
3.5.4. Melanoma including Merkel Cell Carcinoma (mMCC)
Three cost-effectiveness analyses were retrieved on ICIs used for the treatment of melanoma and Merkel cell carcinoma (MCC) [62,63,64]. For metastatic MCC, avelumab treatment with previous PD-L1 assessment was cost-effective compared to best supportive care (ICER USD 44,885.06/QALY, WTP USD 53,333.33/QALY) and chemotherapy (ICER USD 42,993.06/QALY) [64] (Figure 5). Regarding patients with advanced melanoma, nivolumab was the most cost-effective treatment option in BRAF wild-type and BRAF mutant patients, as demonstrated by the Markov model developed to estimate the lifetime costs and benefits of nivolumab versus ipilimumab and dacarbazine (for BRAF wild-type) and versus ipilimumab, dabrafenib, and vemurafenib (for BRAF mutant patients) [62]. In patients affected by BRAF wild-type advanced melanoma, the discrete simulation event model of Tarhini et al. [63] found that the most cost-effective treatment sequences initiated with anti-PD-1 + anti-CTLA-4 (nivolumab + ipilimumab and chemotherapy through a mix of dacarbazine, temozolomide, paclitaxel, and carboplatin + paclitaxel), followed by either chemotherapy or anti-PD-1 monotherapy (nivolumab and pembrolizumab, assuming an equal share). This provided substantial quality-adjusted survival gains to patients with BRAF wild-type advanced melanoma.
3.5.5. Cervical and Gastric Cancers
Only one cost-effectiveness analysis for the treatment of patients with persistent, recurrent, or metastatic cervical cancers who had not received systemic chemotherapy and were not amenable to curative treatment was found [65]. Through a partitioned survival model over a 30-year lifetime horizon, Shi et al. [65] indicated that pembrolizumab was not cost-effective versus the placebo. With respect to patients in second-line therapy with metastatic gastric cancer, the most effective strategy was pembrolizumab for high microsatellite instability (MSI-H) patients and ramucirumab/paclitaxel for all other patients, but the Markov model resulted in a high ICER of $1,074,620/QALY. Among the following strategies, pembrolizumab monotherapy and ramucirumab/paclitaxel combination therapy for all patients and pembrolizumab for patients based on MSI status or PD-L1 expression, the only cost-effective one was paclitaxel monotherapy for all patients, with an ICER of $53,705/QALY (WTP USD 100,000/QALY in the US) [66] (Figure 5).
Figures show incremental QALY and incremental costs for different immune checkpoint inhibitors (ICI) for cancer other than NSCLC according to cancer type (identified by the type of marker) and immune checkpoint inhibitor (identified by marker colour); the size of the marker identifies first-line (bigger) or second-line (smaller) treatment; the area under the WTP threshold indicates the cost-effective studies and is highlighted in green. Studies for which results are available for more than one country are represented more than once.
4. Discussion
This systematic review examined the cost-effectiveness profile of ICI therapy in solid tumours associated with the biomarker test in oncology. Most of the studies [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] included focused on non-small cell lung cancer (NSCLC), followed by HNSCC and genitourinary cancers.
This review highlights that there is still no clear evidence regarding the cost-effectiveness of anti PD-L1 and PD-1 for treating NSCLC, while at present, the available evidence is generally not in favour of the cost-effectiveness of anti PD-L1 in other solid tumours.
Despite there being a significantly fewer number of studies retrieved for other solid tumours, the PD-L1 testing approach was also cost-effective in some settings for urothelial cancer patients first-line treated with avelumab and pembrolizumab but also for renal cell carcinoma treated with nivolumab. Moreover, this approach was also cost-effective in urothelial cancer and renal cell carcinoma.
Consistent with these findings, the European Society for Medical Oncology (ESMO) NSCLC guidelines emphasise the importance of molecular subtyping in guiding therapeutic decision-making, advocating its execution whenever feasible [70,71]. Hence, for patients with advanced NSCLC, ESMO recommends determining PD-L1 expression between others such as EGFR testing, BRAF mutations, and the analysis of ALK, ROS1, and NTRK rearrangements [71]. Generally, there is consensus across international guidelines around the need for PD-L1 testing and other biomarkers in advanced NSCLC. These have approved first-line targeted therapies in Europe [72,73,74].
Hence, multiple studies have highlighted the potential of PD-L1 overexpression as a crucial and extensively studied predictive biomarker for assessing the response to PD-L1 antibodies, resulting in improved clinical outcomes [75,76,77,78]. However, the implementation of these novel and more effective therapies has been acknowledged as both essential and cost-prohibitive [79]. To investigate this, the economic evaluations included in this review [9,10,13,21,24,29,30,31,32,34,37,42] confirmed that NSCLC patients with high tumour PD-L1 levels with a proportional score ≥ 50% for first-line therapy with pembrolizumab, exhibited superior response rates to immunotherapy and experienced prolonged survival compared to those who underwent conventional chemotherapy. Building upon these widely recognised clinical findings, this systematic review unequivocally demonstrated that the treatment approach in question was not only clinically effective but also cost-effective. The economic evaluations indicated a favourable cost threshold, falling within the range of $100,000/QALY to $150,000/QALY. Contrary to the majority of pharmacoeconomic assessments, the pivotal result of this review revealed that a distinct subset of studies failed to demonstrate the cost-effectiveness of PD-L1 testing and pembrolizumab for the first-line treatment of non-small cell lung cancer (NSCLC). Crucially, this divergence in outcomes emerged exclusively within a specific setting characterised by a willingness-to-pay (WTP) threshold falling below $100,000/QALY. This singular observation underscores the significance of the WTP threshold as a decisive factor in determining the cost-effectiveness of this therapeutic approach for NSCLC [17,23,26,28,35,36,38,40,41]. Additionally, of paramount importance is that biomarker testing prior to nivolumab treatment is not deemed cost-effective when administered as a second-line treatment [27], in combination with another monoclonal antibody [18], or when the WTP threshold falls below $100,000/QALY [27,43].
Regarding other solid tumours such as in the case of bladder [51,52], cervical [65], and triple-negative breast cancer (TNBC) [59,60,61], no economic evaluation has deemed the costs of the biomarker testing strategy acceptable within a setting where the threshold is less than $100,000/QALY. These findings diverge from the FDA’s approval of pembrolizumab, for instance, in patients with recurrent metastatic cervical cancer and platinum failure, but limited to those whose tumours exhibit biomarker test positivity [80]. To shed light on these results, we can analyse the outcomes of the clinical trials KEYNOTE-826 [81], designed to assess progression-free survival (PFS) and overall survival (OS) in patients with cervical cancer following genetic testing, and EMPOWER [82], which focused on evaluating OS. While both trials achieved their primary endpoints, subgroup analyses raised discussions on the benefits of ICIs for all cervical cancer patients [83]. Despite the limitations of PD-L1 expression as a predictive biomarker, it still informs clinical decision-making and can contribute to pushing advances in immunotherapy research [84]. Therefore, it can be concluded that a combination of biomarkers should be employed to identify patients who derive the greatest benefit [83].
Strengths and Limitations
This study has several strengths including a comprehensive literature search and the pioneering nature of the objective, as the economic impact and sustainability of health systems regarding immunotherapy and biomarker testing have not been thoroughly investigated yet. Furthermore, the review was executed using a systematic approach, providing a comprehensive overview of the cost-effectiveness of biomarker testing worldwide and over a wide range of tumours.
Nevertheless, this review did have certain limitations. The primary limitation is related to the inclusion of studies involving prior PD-1 testing constituting only 3% (n = 2) of the overall analysis. These specific studies are confined to pembrolizumab treatment in NSCLC. Due to this limited representation and the statistical insignificance of PD-1 in a broader context, we faced challenges in conducting a separate analysis based on PD-1 versus PD-L1 biomarkers. Another limitation is inherently associated with the methodology employed for conducting cost-effectiveness analyses (CEAs). Indeed, no study can fully encompass all of the potential cost-related factors or account for uncertainties surrounding the factors under investigation such as global economic and market forces, variations in practice and referral patterns, or reimbursements specific to individual insurance companies. Additionally, the set willingness-to-pay (WTP) thresholds do not incorporate other tax-related factors such as the financial impact on patients and supportive care providers, the utilisation of other costly cancer therapies, or indirect cost components such as the ability to return to work and contribute to the workforce and economy [3].
5. Conclusions
This systematic review highlights that PD-1 and PD-L1 overexpression, when used in combination with immune checkpoint inhibitor (ICI) therapy, could represent a cost-effective strategy for treating NSCLC as a first-line treatment with pembrolizumab and with both first- and second-line nivolumab, but also for renal cell and urothelial carcinoma. However, the cost-effectiveness is diminished when the willingness-to-pay (WTP) threshold falls below $100,000/QALY. Therefore, the economic impact of biomarkers upstream of the choice of the specific therapy represents an imperative to validate its effectiveness, the eventual relationship with the quality of life, and economic sustainability. A biomarker testing approach is therefore a virtuous model to invest in, providing the patient with a greater chance of receiving increasingly effective therapy and minimising adverse events due to the administration of untargeted therapies, resulting in an undoubted improvement in quality of life, while also optimising the management of health care resources.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16050995/s1, Box S1: Search strategy; Box S2: Search syntaxes; Table S1: Key features of economic methods for the overall included studies; Table S2: Quality appraisal of individual included studies according to the GRADE scale.
Author Contributions
Conceptualisation, E.M. and V.L.; Methodology, E.M., S.M. and V.L.; Software, S.M., V.O., V.L. and I.T.; Formal analysis, S.M., V.L., and I.T.; Investigation, S.M., V.L., I.T., V.O. and E.M.; Data curation, S.M. and V.O.; Writing—original draft preparation, S.M. and E.M.; Writing—review and editing, M.D.R., A.C., G.T., R.D. and V.O.; Supervision, E.M.; Funding acquisition, E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR) within the framework of the PRIN Project 2017, grant number 2017NR7W5K.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Food and Drug Administration (FDA). Table of Pharmacogenomic Biomarkers in Drug Labeling. 2020. Available online: https://www.fda.gov/drugs/science-and-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling (accessed on 24 May 2023).
- Gavrielatou, N.; Doumas, S.; Economopoulou, P.; Foukas, P.G.; Psyrri, A. Biomarkers for immunotherapy response in head and neck cancer. Cancer Treat. Rev. 2020, 84, 101977. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Sprave, T.; Haque, W.; Simone, C.B., II; Chang, J.Y.; Welsh, J.W.; Thomas, C.R., Jr. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J. Immunother. Cancer 2018, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Mucherino, S.; Lorenzoni, V.; Orlando, V.; Triulzi, I.; Del Re, M.; Capuano, A.; Danesi, R.; Turchetti, G.; Menditto, E. Cost-effectiveness of treatment optimisation with biomarkers for immunotherapy in solid tumours: A systematic review protocol. BMJ Open 2021, 11, e048141. [Google Scholar] [CrossRef] [PubMed]
- Dobosz, P.; Dzieciątkowski, T. The Intriguing History of Cancer Immunotherapy. Front. Immunol. 2019, 10, 2965. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.C.; Schünemann, H.J.; Oxman, A.D.; Pottie, K.; Meerpohl, J.J.; Coello, P.A.; Rind, D.; Montori, V.M.; Brito, J.P.; Norris, S.; et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. J. Clin. Epidemiol. 2013, 66, 726–735. [Google Scholar] [CrossRef]
- CCEMG-EPPI. Centre Cost Converter v.1.4. Available online: https://eppi.ioe.ac.uk/costconversion/default.aspx (accessed on 24 May 2023).
- Barbier, M.C.; Pardo, E.; Panje, C.M.; Gautschi, O.; Lupatsch, J.E.; Swiss Group for Clinical Cancer Research (SAKK). A cost-effectiveness analysis of pembrolizumab with or without chemotherapy for the treatment of patients with metastatic, non-squamous non-small cell lung cancer and high PD-L1 expression in Switzerland. Eur. J. Health Econ. HEPAC Health Econ. Prev. Care 2021, 22, 669–677. [Google Scholar] [CrossRef]
- Qiao, L.; Zhou, Z.; Zeng, X.; Tan, C. Cost-Effectiveness of Domestic PD-1 Inhibitor Camrelizumab Combined With Chemotherapy in the First-Line Treatment of Advanced Nonsquamous Non-Small-Cell Lung Cancer in China. Front. Pharmacol. 2021, 12, 728440. [Google Scholar] [CrossRef]
- Insinga, R.P.; Feliciano, J.L.; Qiao, N.; Vandormael, K.; Zhang, Y. Cost-effectiveness of pembrolizumab + chemotherapy versus chemotherapy and pembrolizumab monotherapy in first line treatment of NSCLC in the US—Updated analyses with additional trial follow-up. J. Med. Econ. 2021, 24, 792–805. [Google Scholar] [CrossRef]
- Hu, H.; She, L.; Liao, M.; Shi, Y.; Yao, L.; Ding, D.; Zhu, Y.; Zeng, S.; Carbone, D.P.; Huang, J. Cost-Effectiveness Analysis of Nivolumab Plus Ipilimumab vs. Chemotherapy as First-Line Therapy in Advanced Non-Small Cell Lung Cancer. Front. Oncol. 2020, 10, 1649. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Z.; Luo, X.; Yi, L.; Peng, L.; Wan, X.; Tan, C.; Zeng, X. First-Line ICI Monotherapies for Advanced Non-small-cell Lung Cancer Patients With PD-L1 of at Least 50%: A Cost-Effectiveness Analysis. Front. Pharmacol. 2021, 12, 788569. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, B.; Kiff, C.; Ling, C.; Brodtkorb, T.H. Cost Effectiveness of Nivolumab in Patients with Advanced, Previously Treated Squamous and Non-squamous Non-small-cell Lung Cancer in England. PharmacoEconomics–Open 2021, 5, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Pei, R.; Li, J.; Li, B.; Tang, L.; Yin, T.; Liu, S. Atezolizumab compared to chemotherapy for first-line treatment in non-small cell lung cancer with high PD-L1 expression: A cost-effectiveness analysis from US and Chinese perspectives. Ann. Transl. Med. 2021, 9, 1481. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Kang, S.; Wang, X.; Shang, F. Cost-Effectiveness Analysis of Atezolizumab Versus Chemotherapy as First-Line Treatment for Metastatic Non-Small-Cell Lung Cancer With Different PD-L1 Expression Status. Front. Oncol. 2021, 11, 669195. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Hui, W.; Zhu, M.; Zhang, M.; Gao, Z.; Wu, H. Cost-Effectiveness Analysis of Pembrolizumab Plus Pemetrexed and Platinum Versus Chemotherapy Alone as First-Line Treatment in Metastatic Non-Squamous Non-Small Cell Lung Cancer: A Reconstruction of Partitioned Survival Model Based on Time Dependent Pricing Mechanism of Patient Assistance Program. Front. Oncol. 2021, 11, 768035. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Kunst, N.; Gross, C.P.; Wang, J.D.; Su, W.C.; Wang, S.Y. Cost-Effectiveness of Nivolumab Plus Ipilimumab With and Without Chemotherapy for Advanced Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 760686. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zeng, X.; Peng, L.; Liu, Q.; Yi, L.; Luo, X.; Li, S.; Wang, L.; Qin, S.; Wan, X.; et al. First-Line Atezolizumab for Metastatic NSCLC with High PD-L1 Expression: A United States-Based Cost-Effectiveness Analysis. Adv. Ther. 2021, 38, 2447–2457. [Google Scholar] [CrossRef] [PubMed]
- Panje, C.M.; Lupatsch, J.E.; Barbier, M.; Pardo, E.; Lorez, M.; Dedes, K.J.; Aebersold, D.M.; Plasswilm, L.; Gautschi, O.; Schwenkglenks, M.; et al. A cost-effectiveness analysis of consolidation immunotherapy with durvalumab in stage III NSCLC responding to definitive radiochemotherapy in Switzerland. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 501–506. [Google Scholar] [CrossRef]
- Weng, X.; Luo, S.; Lin, S.; Zhong, L.; Li, M.; Xin, R.; Huang, P.; Xu, X. Cost-Utility Analysis of Pembrolizumab Versus Chemotherapy as First-Line Treatment for Metastatic Non-Small Cell Lung Cancer With Different PD-L1 Expression Levels. Oncol. Res. 2020, 28, 117–125. [Google Scholar] [CrossRef]
- Li, J.; Zhang, T.; Xu, Y.; Lu, P.; Zhu, J.; Liang, W.; Jiang, J. Cost-effectiveness analysis of nivolumab plus ipilimumab versus chemotherapy as first-line treatment in advanced NSCLC. Immunotherapy 2020, 12, 1067–1075. [Google Scholar] [CrossRef]
- Wu, B.; Lu, S. The effect of PD-L1 categories-directed pembrolizumab plus chemotherapy for newly diagnosed metastatic non-small-cell lung cancer: A cost-effectiveness analysis. Transl. Lung Cancer Res. 2020, 9, 1770–1784. [Google Scholar] [CrossRef] [PubMed]
- Loong, H.H.; Wong, C.K.H.; Leung, L.K.S.; Dhankhar, P.; Insinga, R.P.; Chandwani, S.; Hsu, D.C.; Lee, M.Y.K.; Huang, M.; Pellissier, J.; et al. Cost Effectiveness of PD-L1-Based Test-and-Treat Strategy with Pembrolizumab as the First-Line Treatment for Metastatic NSCLC in Hong Kong. PharmacoEconomics-Open 2020, 4, 235–247. [Google Scholar] [CrossRef]
- Wan, N.; Zhang, T.T.; Hua, S.H.; Lu, Z.L.; Ji, B.; Li, L.X.; Lu, L.Q.; Huang, W.J.; Jiang, J.; Li, J. Cost-effectiveness analysis of pembrolizumab plus chemotherapy with PD-L1 test for the first-line treatment of NSCLC. Cancer Med. 2020, 9, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Criss, S.D.; Palazzo, L.; Watson, T.R.; Paquette, A.M.; Sigel, K.; Wisnivesky, J.; Kong, C.Y. Cost-effectiveness of pembrolizumab for advanced non-small cell lung cancer patients with varying comorbidity burden. PLoS ONE 2020, 15, e0228288. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, X.; Peng, L.; Yi, L.; Wan, X.; Zeng, X.; Tan, C. Nivolumab Versus Docetaxel for Previously Treated Advanced Non-Small Cell Lung Cancer in China: A Cost-Effectiveness Analysis. Clin. Drug Investig. 2020, 40, 129–137. [Google Scholar] [CrossRef]
- Aziz, M.I.A.; Tan, L.E.; Tan, W.H.G.; Toh, C.K.; Loke, L.P.Y.; Pearce, F.; Ng, K. Cost-effectiveness analysis of pembrolizumab monotherapy versus chemotherapy for previously untreated advanced non-small cell lung cancer. J. Med. Econ. 2020, 23, 952–960. [Google Scholar] [CrossRef] [PubMed]
- She, L.; Hu, H.; Liao, M.; Xia, X.; Shi, Y.; Yao, L.; Ding, D.; Zhu, Y.; Zeng, S.; Shen, L.; et al. Cost-effectiveness analysis of pembrolizumab versus chemotherapy as first-line treatment in locally advanced or metastatic non-small cell lung cancer with PD-L1 tumor proportion score 1% or greater. Lung Cancer 2019, 138, 88–94. [Google Scholar] [CrossRef]
- Bhadhuri, A.; Insinga, R.; Guggisberg, P.; Panje, C.; Schwenkglenks, M. Cost effectiveness of pembrolizumab vs chemotherapy as first-line treatment for metastatic NSCLC that expresses high levels of PD-L1 in Switzerland. Swiss Med. Wkly. 2019, 149, w20170. [Google Scholar] [CrossRef]
- Chouaid, C.; Bensimon, L.; Clay, E.; Millier, A.; Levy-Bachelot, L.; Huang, M.; Levy, P. Cost-effectiveness analysis of pembrolizumab versus standard-of-care chemotherapy for first-line treatment of PD-L1 positive (>50%) metastatic squamous and non-squamous non-small cell lung cancer in France. Lung Cancer 2019, 127, 44–52. [Google Scholar] [CrossRef]
- Huang, M.; Lopes, G.L.; Insinga, R.P.; Burke, T.; Ejzykowicz, F.; Zhang, Y.; Feliciano, J.L. Cost-effectiveness of pembrolizumab versus chemotherapy as first-line treatment in PD-L1-positive advanced non-small-cell lung cancer in the USA. Immunotherapy 2019, 11, 1463–1478. [Google Scholar] [CrossRef]
- Wan, X.; Luo, X.; Tan, C.; Zeng, X.; Zhang, Y.; Peng, L. First-line atezolizumab in addition to bevacizumab plus chemotherapy for metastatic, nonsquamous non-small cell lung cancer: A United States-based cost-effectiveness analysis. Cancer 2019, 125, 3526–3534. [Google Scholar] [CrossRef] [PubMed]
- Insinga, R.P.; Vanness, D.J.; Feliciano, J.L.; Vandormael, K.; Traore, S.; Ejzykowicz, F.; Burke, T. Cost-effectiveness of pembrolizumab in combination with chemotherapy versus chemotherapy and pembrolizumab monotherapy in the first-line treatment of squamous non-small-cell lung cancer in the US. Curr. Med. Res. Opin. 2019, 35, 1241–1256. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Jiang, C.; Li, Q. Cost-effectiveness analysis of pembrolizumab monotherapy and chemotherapy in the non-small-cell lung cancer with different PD-L1 tumor proportion scores. Lung Cancer 2019, 136, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Huang, J.; Hutton, D.; Li, Q. Cost-effectiveness analysis of first-line pembrolizumab treatment for PD-L1 positive, non-small cell lung cancer in China. J. Med. Econ. 2019, 22, 344–349. [Google Scholar] [CrossRef]
- Insinga, R.P.; Vanness, D.J.; Feliciano, J.L.; Vandormael, K.; Traore, S.; Burke, T. Cost-effectiveness of pembrolizumab in combination with chemotherapy in the 1st line treatment of non-squamous NSCLC in the US. J. Med. Econ. 2018, 21, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, M.; da Silveira Nogueira Lima, J.P.; Aguiar, P., Jr.; de Lima Lopes, G., Jr.; Haaland, B. Cost-effectiveness of pembrolizumab as first-line therapy for advanced non-small cell lung cancer. Lung Cancer 2018, 124, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, P.N., Jr.; Perry, L.A.; Penny-Dimri, J.; Babiker, H.; Tadokoro, H.; de Mello, R.A.; Lopes, G.L., Jr. The effect of PD-L1 testing on the cost-effectiveness and economic impact of immune checkpoint inhibitors for the second-line treatment of NSCLC. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 2256–2263. [Google Scholar] [CrossRef]
- Hu, X.; Hay, J.W. First-line pembrolizumab in PD-L1 positive non-small-cell lung cancer: A cost-effectiveness analysis from the UK health care perspective. Lung Cancer 2018, 123, 166–171. [Google Scholar] [CrossRef]
- Aguiar, P., Jr.; Giglio, A.D.; Perry, L.A.; Penny-Dimri, J.; Babiker, H.; Tadokoro, H.; Lopes, G., Jr.; De Mello, R.A. Cost-effectiveness and budget impact of lung cancer immunotherapy in South America: Strategies to improve access. Immunotherapy 2018, 10, 887–897. [Google Scholar] [CrossRef]
- Huang, M.; Lou, Y.; Pellissier, J.; Burke, T.; Liu, F.X.; Xu, R.; Velcheti, V. Cost Effectiveness of Pembrolizumab vs. Standard-of-Care Chemotherapy as First-Line Treatment for Metastatic NSCLC that Expresses High Levels of PD-L1 in the United States. PharmacoEconomics 2017, 35, 831–844. [Google Scholar] [CrossRef]
- Matter-Walstra, K.; Schwenkglenks, M.; Aebi, S.; Dedes, K.; Diebold, J.; Pietrini, M.; Klingbiel, D.; von Moos, R.; Gautschi, O.; Swiss Group for Clinical Cancer Research. A Cost-Effectiveness Analysis of Nivolumab versus Docetaxel for Advanced Nonsquamous NSCLC Including PD-L1 Testing. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2016, 11, 1846–1855. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Yi, L.; Li, S.; Tan, C.; Zeng, X.; Wang, L.; Peng, Y.; Wan, X. Cost-Effectiveness of Atezolizumab Plus Chemotherapy as First-Line Therapy for Metastatic Urothelial Cancer. Adv. Ther. 2021, 38, 3399–3408. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; She, Z.; Peng, L.; Liu, Q.; Yi, L.; Luo, X.; Li, S.; Wang, L.; Qin, S.; Wan, X.; et al. Cost-Effectiveness of Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma in the United States. Adv. Ther. 2021, 38, 5710–5720. [Google Scholar] [CrossRef] [PubMed]
- Hale, O.; Patterson, K.; Lai, Y.; Meng, Y.; Li, H.; Godwin, J.L.; Homet Moreno, B.; Mamtani, R. Cost-effectiveness of Pembrolizumab versus Carboplatin-based Chemotherapy as First-line Treatment of PD-L1-positive Locally Advanced or Metastatic Urothelial Carcinoma Ineligible for Cisplatin-based Therapy in the United States. Clin. Genitourin. Cancer 2021, 19, e17–e30. [Google Scholar] [CrossRef]
- Patterson, K.; Prabhu, V.; Xu, R.; Li, H.; Meng, Y.; Zarabi, N.; Zhong, Y.; Batteson, R.; Pellissier, J.; Keefe, S.; et al. Cost-effectiveness of Pembrolizumab for Patients with Advanced, Unresectable, or Metastatic Urothelial Cancer Ineligible for Cisplatin-based Therapy. Eur. Urol. Oncol. 2019, 2, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Reinhorn, D.; Sarfaty, M.; Leshno, M.; Moore, A.; Neiman, V.; Rosenbaum, E.; Goldstein, D.A. A Cost-Effectiveness Analysis of Nivolumab and Ipilimumab Versus Sunitinib in First-Line Intermediate- to Poor-Risk Advanced Renal Cell Carcinoma. Oncol. 2019, 24, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Criss, S.D.; Weaver, D.T.; Sheehan, D.F.; Lee, R.J.; Pandharipande, P.V.; Kong, C.Y. Effect of PD-L1 testing on the cost-effectiveness and budget impact of pembrolizumab for advanced urothelial carcinoma of the bladder in the United States. Urol. Oncol. 2019, 37, 180.e11–180.e18. [Google Scholar] [CrossRef]
- Sarfaty, M.; Leshno, M.; Gordon, N.; Moore, A.; Neiman, V.; Rosenbaum, E.; Goldstein, D.A. Cost Effectiveness of Nivolumab in Advanced Renal Cell Carcinoma. Eur. Urol. 2018, 73, 628–634. [Google Scholar] [CrossRef]
- Parmar, A.; Richardson, M.; Coyte, P.C.; Cheng, S.; Sander, B.; Chan, K.K.W. A cost-utility analysis of atezolizumab in the second-line treatment of patients with metastatic bladder cancer. Curr. Oncol. 2020, 27, e386–e394. [Google Scholar] [CrossRef]
- Sarfaty, M.; Hall, P.S.; Chan, K.K.W.; Virik, K.; Leshno, M.; Gordon, N.; Moore, A.; Neiman, V.; Rosenbaum, E.; Goldstein, D.A. Cost-effectiveness of Pembrolizumab in Second-line Advanced Bladder Cancer. Eur. Urol. 2018, 74, 57–62. [Google Scholar] [CrossRef]
- Wurcel, V.; Chirovsky, D.; Borse, R.; Altuna, J.I.; Carabajal, F.; Gandhi, J. Cost-Effectiveness of Pembrolizumab Regimens for the First-Line Treatment of Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma in Argentina. Adv. Ther. 2021, 38, 2613–2630. [Google Scholar] [CrossRef]
- Zhou, K.; Li, Y.; Liao, W.; Zhang, M.; Bai, L.; Li, Q. Pembrolizumab alone or with chemotherapy for squamous cell carcinoma of the head and neck: A cost-effectiveness analysis from Chinese perspective. Oral Oncol. 2020, 107, 104754. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Han, S.; Zheng, B.; Cai, H.; Yang, J.; Zhuang, Q.; Li, N. Cost-Effectiveness Analysis Of Pembrolizumab In The Treatment Of Advanced Recurrent Metastatic Head And Neck Squamous Cell Carcinoma In China And The United States. Cancer Manag. Res. 2019, 11, 9483–9493. [Google Scholar] [CrossRef]
- Zargar, M.; McFarlane, T.; Chan, K.K.W.; Wong, W.W.L. Cost-Effectiveness of Nivolumab in Recurrent Metastatic Head and Neck Squamous Cell Carcinoma. Oncologist 2018, 23, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Hirschmann, A.; Lupatsch, J.E.; Schwenkglenks, M.; Panje, C.M.; Matter-Walstra, K.; Espeli, V.; Dedes, K.J.; Siano, M.; Swiss Group of Clinical Cancer Research (SAKK). Cost-effectiveness of nivolumab in the treatment of head and neck cancer. Oral Oncol. 2018, 87, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.C.; Shah, C.; Adelstein, D.J.; Geiger, J.L.; Miller, J.A.; Koyfman, S.A.; Singer, M.E. Cost-effectiveness of nivolumab for recurrent or metastatic head and neck cancer. Oral Oncol. 2017, 74, 49–55. [Google Scholar] [CrossRef]
- Liu, X.; Lang, Y.; Liao, Y.; Zhu, Y. Atezolizumab Plus Chemotherapy vs. Chemotherapy in Advanced or Metastatic Triple-Negative Breast Cancer: A Cost-Effectiveness Analysis. Front. Public Health 2021, 9, 756899. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Huang, X.; Li, H.; Lin, S.; Rao, X.; Guo, X.; Huang, P. First-Line Treatment With Atezolizumab Plus Nab-Paclitaxel for Advanced Triple-Negative Breast Cancer: A Cost-Effectiveness Analysis. Am. J. Clin. Oncol. 2020, 43, 340–348. [Google Scholar] [CrossRef]
- Wu, B.; Ma, F. Cost-effectiveness of adding atezolizumab to first-line chemotherapy in patients with advanced triple-negative breast cancer. Ther. Adv. Med. Oncol. 2020, 12, 1758835920916000. [Google Scholar] [CrossRef]
- Meng, Y.; Hertel, N.; Ellis, J.; Morais, E.; Johnson, H.; Philips, Z.; Roskell, N.; Walker, A.; Lee, D. The cost-effectiveness of nivolumab monotherapy for the treatment of advanced melanoma patients in England. Eur. J. Health Econ. HEPAC Health Econ. Prev. Care 2018, 19, 1163–1172. [Google Scholar] [CrossRef]
- Tarhini, A.; Benedict, A.; McDermott, D.; Rao, S.; Ambavane, A.; Gupte-Singh, K.; Sabater, J.; Ritchings, C.; Aponte-Ribero, V.; Regan, M.M.; et al. Sequential treatment approaches in the management of BRAF wild-type advanced melanoma: A cost-effectiveness analysis. Immunotherapy 2018, 10, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Lin, A.Y.; Hsu, J.C.; Wu, C.E.; Goh, C.; Chou, P.; Kuo, K.; Chang, A.; Palencia, R. A cost-utility analysis of avelumab for metastatic Merkel cell carcinoma in Taiwan. Cancer Rep. 2021, 4, e1399. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Shi, B.; Liu, A. Cost-effectiveness analysis of pembrolizumab for treatment of US patients with persistent, recurrent, or metastatic cervical cancer. Gynecol. Oncol. 2022, 164, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Lauren, B.; Ostvar, S.; Silver, E.; Ingram, M.; Oh, A.; Kumble, L.; Laszkowska, M.; Chu, J.N.; Hershman, D.L.; Manji, G.; et al. Cost-Effectiveness Analysis of Biomarker-Guided Treatment for Metastatic Gastric Cancer in the Second-Line Setting. J. Oncol. 2020, 2020, 2198960. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Bardhan, K.; Boussiotis, V.A.; Patsoukis, N. The PD-1 Interactome. Adv. Biol. 2021, 5, e2100758. [Google Scholar] [CrossRef]
- Paver, E.C.; Cooper, W.A.; Colebatch, A.J.; Ferguson, P.M.; Hill, S.K.; Lum, T.; Shin, J.S.; O’Toole, S.; Anderson, L.; Scolyer, R.A.; et al. Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: A guide to immunohistochemistry implementation and interpretation. Pathology 2021, 53, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Vesely, M.D.; Zhang, T.; Chen, L. Resistance Mechanisms to Anti-PD Cancer Immunotherapy. Annu. Rev. Immunol. 2022, 40, 45–74. [Google Scholar] [CrossRef]
- Kerr, K.M.; Bibeau, F.; Thunnissen, E.; Botling, J.; Ryška, A.; Wolf, J.; Öhrling, K.; Burdon, P.; Malapelle, U.; Büttner, R. The evolving landscape of biomarker testing for non-small cell lung cancer in Europe. Lung Cancer 2021, 154, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29 (Suppl. S4), iv192–iv237. [Google Scholar] [CrossRef]
- Kalemkerian, G.P.; Narula, N.; Kennedy, E.B.; Biermann, W.A.; Donington, J.; Leighl, N.B.; Lew, M.; Pantelas, J.; Ramalingam, S.S.; Reck, M.; et al. Molecular Testing Guideline for the Selection of Patients With Lung Cancer for Treatment With Targeted Tyrosine Kinase Inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 911–919. [Google Scholar] [CrossRef]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med. 2018, 142, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Planchard, D.; Lu, S.; Sun, H.; Yamamoto, N.; Kim, D.W.; Tan, D.S.W.; Yang, J.C.; Azrif, M.; Mitsudomi, T.; et al. Pan-Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: A CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 171–210. [Google Scholar] [CrossRef]
- Incorvaia, L.; Fanale, D.; Badalamenti, G.; Barraco, N.; Bono, M.; Corsini, L.R.; Galvano, A.; Gristina, V.; Listì, A.; Vieni, S.; et al. Programmed Death Ligand 1 (PD-L1) as a Predictive Biomarker for Pembrolizumab Therapy in Patients with Advanced Non-Small-Cell Lung Cancer (NSCLC). Adv. Ther. 2019, 36, 2600–2617. [Google Scholar] [CrossRef] [PubMed]
- Del Re, M.; van Schaik, R.H.N.; Fogli, S.; Mathijssen, R.H.J.; Cucchiara, F.; Capuano, A.; Scavone, C.; Jenster, G.W.; Danesi, R. Blood-based PD-L1 analysis in tumor-derived extracellular vesicles: Applications for optimal use of anti-PD-1/PD-L1 axis inhibitors. Biochim. Et Biophys. Acta. Rev. Cancer 2021, 1875, 188463. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Ancevski Hunter, K.; Socinski, M.A.; Villaruz, L.C. PD-L1 Testing in Guiding Patient Selection for PD-1/PD-L1 Inhibitor Therapy in Lung Cancer. Mol. Diagn. Ther. 2018, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tartari, F.; Santoni, M.; Burattini, L.; Mazzanti, P.; Onofri, A.; Berardi, R. Economic sustainability of anti-PD-1 agents nivolumab and pembrolizumab in cancer patients: Recent insights and future challenges. Cancer Treat. Rev. 2016, 48, 20–24. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). FDA Approves Pembrolizumab for Advanced Cervical Cancer with Disease Progression during or After Chemotherapy|FDA. 2018. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-advanced-cervical-cancer-disease-progression-during-or-after-chemotherapy (accessed on 24 May 2023).
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Hoyos Usta, E.; Yañez, E.; et al. KEYNOTE-826 Investigators. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef]
- Tewari, K.S.; Monk, B.J.; Vergote, I.; Miller, A.; de Melo, A.C.; Kim, H.S.; Kim, Y.M.; Lisyanskaya, A.; Samouëlian, V.; Lorusso, D.; et al. Investigators for GOG Protocol 3016 and ENGOT Protocol En-Cx9 Survival with Cemiplimab in Recurrent Cervical Cancer. N. Engl. J. Med. 2022, 386, 544–555. [Google Scholar] [CrossRef]
- Berberabe, A. Is PD-L1 an Appropriate Biomarker in Cervical Cancer? Target Ther. Oncol. 2022, 11, 73. [Google Scholar]
- Valiullina, A.K.; Zmievskaya, E.A.; Ganeeva, I.A.; Zhuravleva, M.N.; Garanina, E.E.; Rizvanov, A.A.; Petukhov, A.V.; Bulatov, E.R. Evaluation of CAR-T Cells’ Cytotoxicity against Modified Solid Tumor Cell Lines. Biomedicines 2023, 11, 626. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).