Exploring the Efficacy of Pembrolizumab in Combination with Carboplatin and Weekly Paclitaxel for Frail Patients with Advanced Non-Small-Cell Lung Cancer: A Key Investigative Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
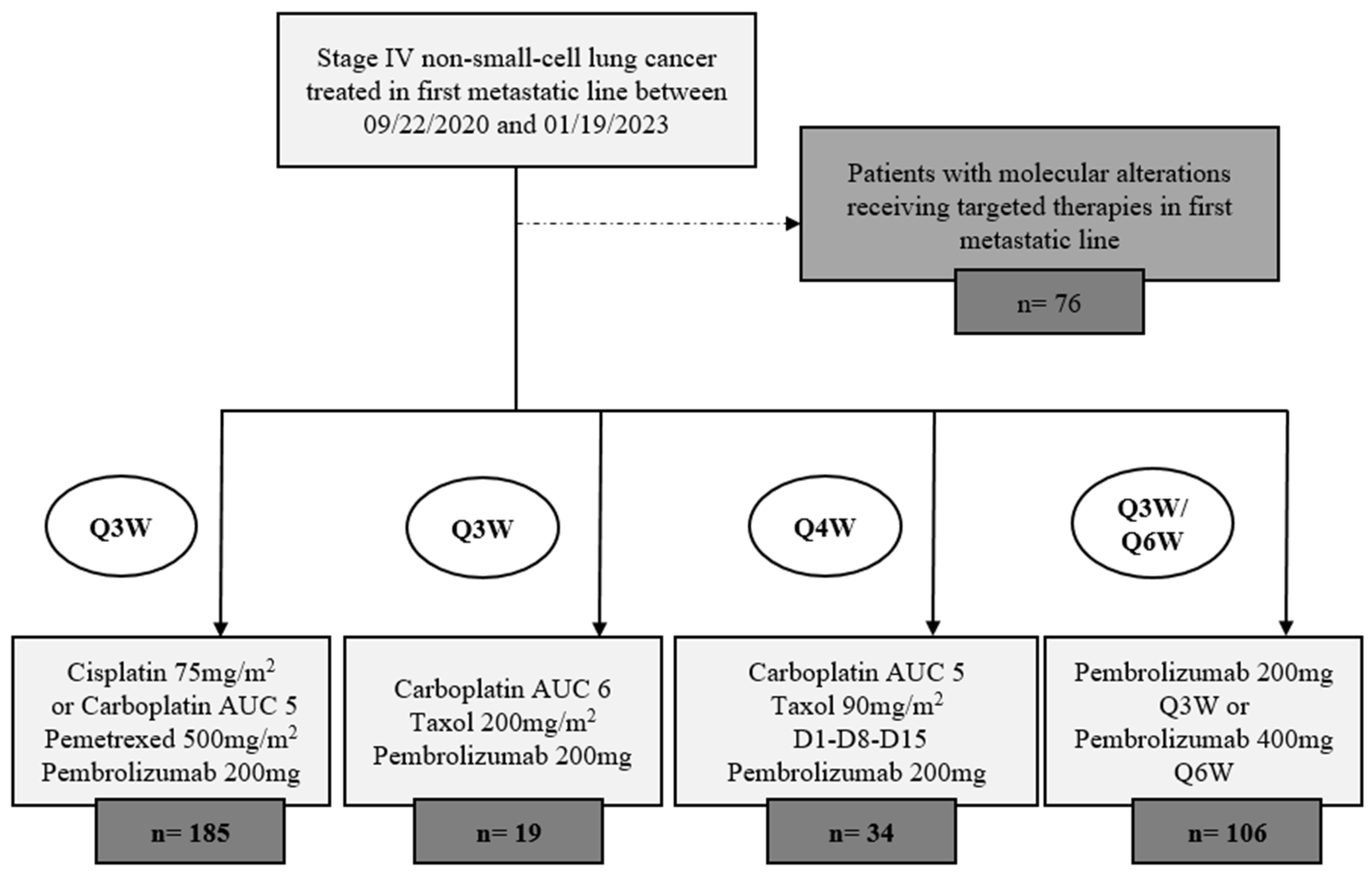
2.1. Study Design and Participant Selection
- Histologically proven NSCLC. Cytological evidence was authorized.
- ECOG-PS ≤ 2.
- Stage IV NSCLC according to TNM 8th classification, UICC 2015.
- Absence of systemic anticancer therapy given as first-line treatment for advanced or metastatic disease.
- Small cell lung cancer (SCLC) or tumor with mixed histology, including a small cell component.
- Known EGFR activating mutation.
- Known ALK or ROS-1 gene rearrangement assessed by immunohistochemistry, FISH, or NGS sequencing.
- Polyneuropathy of CTCAE v5.0 grade ≥ 2.
2.2. Objectives of the Study
- Evaluating overall survival (OS), which was defined as the time from the initiation of treatment to death from any cause, with the last date of follow-up serving as the censoring point.
- Evaluating rwPFS and OS according to the histological type of NSCLC (sq-NSCLC and nsq-NSCLC)
- Identifying demographic characteristics of the treated patients.
- Assessing the toxicity profile of this combination therapy within predefined subgroups.
2.3. Data Collection and Statistical Analysis
3. Results
3.1. Population Demographics
3.2. Treatment Administration and Toxicites
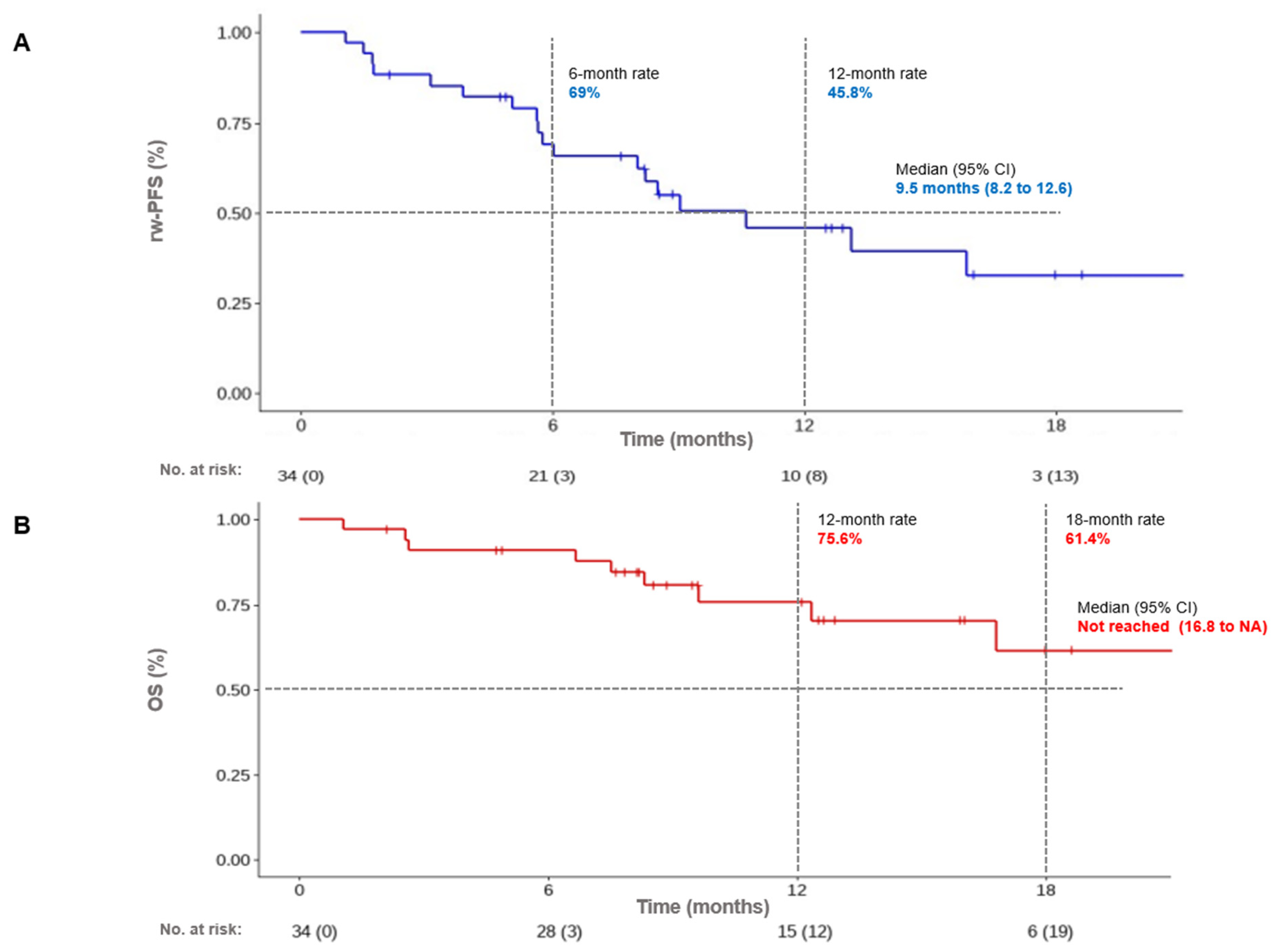
3.3. Survival Outcomes: Control Rate, Progression-Free Survival, and Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Total Cohort | Sq-NSCLC | Nsq-NSCLC | p-Value | |
|---|---|---|---|---|
| Adverse events—no. (%) | ||||
| Any event | 22 (64.7) | 15 (75.0) | 7 (50.0) | p = 0.20 |
| Event leading to dose reduction of chemotherapy | 9 (26.5) | 6 (30.0) | 3 (21.4) | p = 0.96 |
| Event leading to permanent discontinuation of chemotherapy | 5 (14.7) | 4 (20.0) | 1 (7.1) | p = 0.38 |
| Event leading to discontinuation of immunotherapy (temporary and permanent) | 6 (17.6) | 4 (20.0) | 2 (14.3) | p = 0.25 |
| Event leading to death | 1 (2.9) | 0 (0) | 1 (7.1) | p = 0.41 |
| Immune-related adverse events leading to discontinuation of ICBS—no. (%) | 6 (17.6) | 4 (20.0) | 2 (14.3) | p = 0.87 |
| Pneumonitis | 3 (8.8) | 2 (5.8) | 1 (2.9) | |
| Hepatitis | 1 (2.9) | 0 (0) | 1 (2.9) | |
| Colitis | 1 (2.9) | 1 (2.9) | 0 (0) | |
| Hypophisitis | 1 (2.9) | 1 (2.9) | 0 (0) |

References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Reck, M.; Remon, J.; Hellmann, M.D. First-Line Immunotherapy for Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 586–597. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. KEYNOTE-024 Investigators Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. KEYNOTE-407 Investigators Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. KEYNOTE-189 Investigators Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. IMpower150 Study Group Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Quoix, E.; Zalcman, G.; Oster, J.P.; Westeel, V.; Pichon, E.; Lavolé, A.; Dauba, J.; Debieuvre, D.; Souquet, P.J.; Bigay-Game, L.; et al. Intergroupe Francophone de Cancérologie Thoracique Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet 2011, 378, 1079–1088. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Garassino, M.C.; Gadgeel, S.; Speranza, G.; Felip, E.; Esteban, E.; Dómine, M.; Hochmair, M.J.; Powell, S.F.; Bischoff, H.G.; Peled, N.; et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J. Clin. Oncol. 2023, 41, 1992–1998. [Google Scholar] [CrossRef]
- Novello, S.; Kowalski, D.M.; Luft, A.; Gümüş, M.; Vicente, D.; Mazières, J.; Rodríguez-Cid, J.; Tafreshi, A.; Cheng, Y.; Lee, K.H.; et al. Pembrolizumab Plus Chemotherapy in Squamous Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study. J. Clin. Oncol. 2023, 41, 1999–2006. [Google Scholar] [CrossRef]
- Chan, O.T.M.; Yang, L.X. The immunological effects of taxanes. Cancer Immunol. Immunother. 2000, 49, 181–185. [Google Scholar] [CrossRef]
- Vacca, A.; Ribatti, D.; Iurlaro, M.; Merchionne, F.; Nico, B.; Ria, R.; Dammacco, F. Docetaxel versus paclitaxel for antiangiogenesis. J. Hematotherapy Stem Cell Res. 2002, 11, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Fayette, J.; Cropet, C.; Gautier, J.; Toullec, C.; Burgy, M.; Bruyas, A.; Sire, C.; Lagrange, A.; Clatot, F.; Calderon, B.; et al. Results of the multicenter phase II FRAIL-IMMUNE trial evaluating the efficacy and safety of durvalumab combined with weekly paclitaxel carboplatin in first-line in patients (pts) with recurrent/metastatic squamous cell carcinoma of the head and neck (R/M SCCHN) not eligible for cisplatin-based therapies. J. Clin. Oncol. 2023, 41, 16_suppl. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. KEYNOTE-048 Investigators Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Bringuier, M.; Carton, M.; Debieuvre, D.; Pasquier, D.; Perol, M.; Filleron, T.; Léna, H.; Quantin, X.; Simon, G.; Baldini, C. Enrolment of older adults with advanced or metastatic non-small cell lung cancer in first-line clinical trials in the multicentre ESME cohort. J. Geriatr. Oncol. 2023, 14, 101423. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. ESMO Guidelines Committee.Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 358–376. [Google Scholar] [CrossRef] [PubMed]
- Non-SmallCell Lung Cancer Version 2.2024—February 9. 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 23 February 2024).
- Pathak, R.; De Lima Lopes, G.; Yu, H.; Aryal, M.R.; Ji, W.; Frumento, K.S.; Wallis CJ, D.; Klaassen, Z.; Park, H.S.; Goldberg, S.B. Comparative efficacy of chemoimmunotherapy versus immunotherapy for advanced non-small cell lung cancer: A network meta-analysis of randomized trials. Cancer 2021, 127, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Akinboro, O.; Vallejo, J.J.; Nakajima, E.C.; Ren, Y.; Mishra-Kalyani, P.S.; Larkins, E.A.; Vellanki, P.; Drezner, N.; Mathieu, L.; Boeri Donoghue, M.; et al. Outcomes of anti–PD-(L)1 therapy with or without chemotherapy (chemo) for first-line (1L) treatment of advanced non–small cell lung cancer (NSCLC) with PD-L1 score ≥ 50%: FDA pooled analysis. J. Clin. Oncol. 2022, 40 (Suppl. 16), 9000. [Google Scholar] [CrossRef]
- Socinski, M.A.; Ivanova, A.; Bakri, K.; Wall, J.; Baggstrom, M.Q.; Hensing, T.A.; Mears, A.; Tynan, M.; Beaumont, J.; Peterman, A.H.; et al. A randomized phase II trial comparing every 3-weeks carboplatin/paclitaxel with every 3-weeks carboplatin and weekly paclitaxel in advanced non-small cell lung cancer. Ann. Oncol. 2006, 17, 104–109. [Google Scholar] [CrossRef]
- Belani, C.P.; Ramalingam, S.; Perry, M.C.; LaRocca, R.V.; Rinaldi, D.; Gable, P.S.; Tester, W.J. Randomized, phase III study of weekly paclitaxel in combination with carboplatin versus standard every-3-weeks administration of carboplatin and paclitaxel for patients with previously untreated advanced non-small-cell lung cancer. J. Clin. Oncol. 2008, 26, 468–473. [Google Scholar] [CrossRef]
- Schuette, W.; Blankenburg, T.; Guschall, W.; Dittrich, I.; Schroeder, M.; Schweisfurth, H.; Chemaissani, A.; Schumann, C.; Dickgreber, N.; Appel, T.; et al. Multicenter randomized trial for stage IIIB/IV non-small-cell lung cancer using every-3-week versus weekly paclitaxel/carboplatin. Clin. Lung Cancer 2006, 7, 338–343. [Google Scholar] [CrossRef]
- Ramalingam, S.; Perry, M.C.; La Rocca, R.V.; Rinaldi, D.; Gable, P.S.; Tester, W.J.; Belani, C.P. Comparison of outcomes for elderly patients treated with weekly paclitaxel in combination with carboplatin versus the standard 3-weekly paclitaxel and carboplatin for advanced nonsmall cell lung cancer. Cancer 2008, 113, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Lala, M.; Li, T.R.; de Alwis, D.P.; Sinha, V.; Mayawala, K.; Yamamoto, N.; Siu, L.L.; Chartash, E.; Aboshady, H.; Jain, L. A six-weekly dosing schedule for pembrolizumab in patients with cancer based on evaluation using modelling and simulation. Eur. J. Cancer 2020, 131, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.; Kang, S.P.; Rasco, D.; Papadopoulos, K.P.; Elassaiss-Schaap, J.; Beeram, M.; Drengler, R.; Chen, C.; Smith, L.; Espino, G.; et al. Phase I Study of Pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2015, 21, 4286–4293. [Google Scholar] [CrossRef] [PubMed]
- Storer, B.E. Design and analysis of phase I clinical trials. Biometrics 1989, 45, 925–937. [Google Scholar] [CrossRef]
- Le Tourneau, C.; Lee, J.J.; Siu, L.L. Dose escalation methods in phase I cancer clinical trials. J. Natl. Cancer Inst. 2009, 101, 708–720. [Google Scholar] [CrossRef]
- Postel-Vinay, S.; Aspeslagh, S.; Lanoy, E.; Robert, C.; Soria, J.C.; Marabelle, A. Challenges of phase 1 clinical trials evaluating immune checkpoint-targeted antibodies. Ann. Oncol. 2016, 27, 214–224. [Google Scholar] [CrossRef]
- Patil, V.M.; Noronha, V.; Menon, N.; Rai, R.; Bhattacharjee, A.; Singh, A.; Nawale, K.; Jogdhankar, S.; Tambe, R.; Dhumal, S.; et al. Low-Dose Immunotherapy in Head and Neck Cancer: A Randomized Study. J. Clin. Oncol. 2023, 41, 222–232. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
| Total Cohort (n = 34) | Sq-NSCLC (n = 20) | Nsq-NSCLC (n = 14) | p-Value | |
|---|---|---|---|---|
| Age at date of first treatment cycle (yr) | ||||
| Median (range) | 75.5 (61; 81) | 74.5 (61; 81) | 78.0 (67; 81) | 0.09 |
| ≥70 yr—no. (%) | 27 (79.4) | 15 (75.0) | 12 (85.7) | 0.67 |
| Male sex—no. (%) | 29 (85.3) | 17 (85.0) | 12 (85.7) | 1 |
| Smoking statuts—no. (%) | 1 | |||
| Current or former | 34 (100) | 20 (100) | 14 (100) | |
| Never | 0 (0) | 0 (0) | 0 (0) | |
| ECOG performance status score—no. (%) | (n = 31) | (n = 18) | (n = 13) | 0.42 |
| 0 | 7 (22.6) | 4 (22.2) | 3 (23.1) | |
| 1 | 11 (35.5) | 8 (44.4) | 3 (23.1) | |
| 2 | 13 (41.9) | 6 (33.3) | 7 (53.8) | |
| Renal function GFR (mL/min/m2)—no. (%) | 0.07 | |||
| ≥60 | 27 (79.4) | 18 (90.0) | 9 (64.3) | |
| 45–60 | 4 (11.8) | 2 (10.0) | 2 (14.3) | |
| 30–45 | 3 (8.8) | 0 (0) | 3 (21.4) | |
| Brain metastases—no. (%) | (n = 33) | (n = 20) | (n = 13) | 1 |
| 4 (12.1) | 3 (15.0) | 1 (7.7) | ||
| PD-L1 tumor proportion score—no. (%) | (n = 32) | (n = 18) | (n = 14) | 0.40 |
| <1% | 17 (53.1) | 8 (44.4) | 9 (64.3) | |
| 1–49% | 13 (40.6) | 8 (44.4) | 5 (35.7) | |
| ≥50% | 2 (6.3) | 2 (11.1) | 0 (0) | |
| Previous therapy for non-metastatic disease | 0.26 | |||
| De novo metastatic | 25 (73.5) | 16 (80.0) | 9 (64.3) | |
| Surgery | 3 (8.8) | 1 (5.0) | 2 (14.3) | |
| Stereotactic radiotherapy | 2 (5.9) | 0 (0.0) | 2 (14.3) | |
| Radiochemotherapy | 4 (11.8) | 3 (15.0) | 1 (7.1) |
| Total Cohort (n = 34) | Sq-NSCLC (n = 20) | Nsq-NSCLC (n = 14) | p-Value | |
|---|---|---|---|---|
| Chemotherapy regimen at baseline—no. (%) | p = 0.86 | |||
| Carboplatin AUC 5 | 28 (82.3) | 17 (85.0) | 11 (78.6) | |
| Carboplatin AUC 4 | 6 (17.7) | 3 (15.0) | 3 (21.4) | |
| Taxol 90 mg/m2 | 27 (79.4) | 15 (75.0) | 12 (85.7) | |
| Taxol 80 mg/m2 | 7 (20.6) | 5 (25.0) | 2 (14.3) | |
| Best response to treatment—no. (%) | ||||
| Complete response (CR) | 0 | 0 | 0 | |
| Partial response (PR) | 19 (55.9) | 16 (80.0) | 3 (21.4) | |
| Stable disease (SD) | 11 (32.4) | 3 (15.0) | 8 (57.2) | |
| Progression disease (PD) | 4 (11.7) | 1 (5.0) | 3 (21.4) | |
| Objective response rate (CR + PR) | 19 (55.9) | 16 (80.0) | 3 (21.4) | p = 0.001 |
| Disease control rate (CR + PR + SD) | 30 (88.2) | 19 (95.0) | 11 (78.6) | p = 0.28 |
| Immunotherapy: Number of maintenance cycles | p = 0.90 | |||
| Median (range) | 5.0 (0; 27) | 5.0 (0; 27) | 5.0 (0; 21) | |
| None | 8 (23.5) | 4 (20.0) | 4 (28.6) | |
| 0–5 | 9 (26.5) | 6 (30.0) | 3 (21.4) | |
| >5 | 17 (50.0) | 10 (50.0) | 7 (50.0) | |
| Number of subsequent lines of treatment—no. (%) | p = 0.67 | |||
| 0 | 19 (55.9) | 10 (50.0) | 9 (64.3) | |
| 1 | 12 (35.3) | 7 (35.0) | 5 (35.7) | |
| 2 | 2 (5.9) | 2 (10.0) | 0 (0.0) | |
| 3 | 1 (2.9) | 1 (5.0) | 0 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, Q.D.; Chaabouni, M.; Al herk, A.; Lefevbre, C.; Cavaillon, S.; Sinoquet, L.; Pouderoux, S.; Viala, M.; Roca, L.; Quantin, X. Exploring the Efficacy of Pembrolizumab in Combination with Carboplatin and Weekly Paclitaxel for Frail Patients with Advanced Non-Small-Cell Lung Cancer: A Key Investigative Study. Cancers 2024, 16, 992. https://doi.org/10.3390/cancers16050992
Thomas QD, Chaabouni M, Al herk A, Lefevbre C, Cavaillon S, Sinoquet L, Pouderoux S, Viala M, Roca L, Quantin X. Exploring the Efficacy of Pembrolizumab in Combination with Carboplatin and Weekly Paclitaxel for Frail Patients with Advanced Non-Small-Cell Lung Cancer: A Key Investigative Study. Cancers. 2024; 16(5):992. https://doi.org/10.3390/cancers16050992
Chicago/Turabian StyleThomas, Quentin Dominique, Mohamed Chaabouni, Anas Al herk, Cesar Lefevbre, Sarah Cavaillon, Léa Sinoquet, Stéphane Pouderoux, Marie Viala, Lise Roca, and Xavier Quantin. 2024. "Exploring the Efficacy of Pembrolizumab in Combination with Carboplatin and Weekly Paclitaxel for Frail Patients with Advanced Non-Small-Cell Lung Cancer: A Key Investigative Study" Cancers 16, no. 5: 992. https://doi.org/10.3390/cancers16050992
APA StyleThomas, Q. D., Chaabouni, M., Al herk, A., Lefevbre, C., Cavaillon, S., Sinoquet, L., Pouderoux, S., Viala, M., Roca, L., & Quantin, X. (2024). Exploring the Efficacy of Pembrolizumab in Combination with Carboplatin and Weekly Paclitaxel for Frail Patients with Advanced Non-Small-Cell Lung Cancer: A Key Investigative Study. Cancers, 16(5), 992. https://doi.org/10.3390/cancers16050992






