Physical Deconditioning in Lung Cancer Patients Who Underwent Lung Resection Surgery in Spain: A Prospective Observational Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cancer-Related Symptoms
2.2. Upper Limb Exercise Capacity
2.3. Lower Limb Exercise Capacity
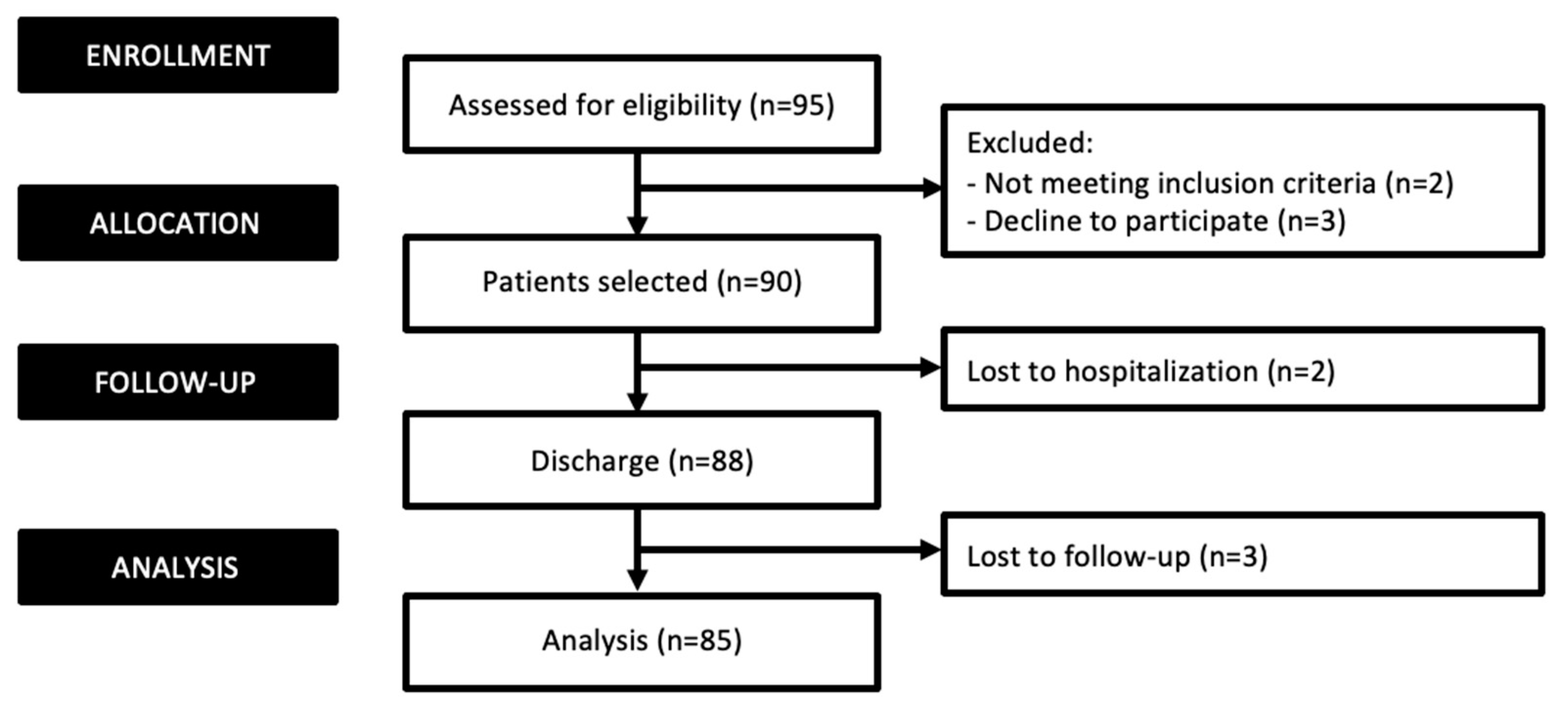
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cáncer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare (AIHW). Australian Cancer Incidence and Mortality (ACIM) Books; Australian Institute of Health and Welfare: Darlinghurst, Australia, 2017. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Gridelli, C.; Perrone, F.; Gallo, C.; Cigolari, S.; Rossi, A.; Piantedosi, F.; Barbera, S.; Ferrau, F.; Piazza, E.; Rosetti, F.; et al. MILES Investigators. Chemotherapy for elderly patients with advanced non-small-cell lung cancer: The Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J. Natl. Cancer Inst. 2003, 95, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, R.J.; Rubinstein, L.V. Randomized trial of lobectomy versus limited resection for T1 No non-small cell lung cancer. Lung Cancer Study group. Ann. Thorac. Surg. 1995, 60, 615–622. [Google Scholar] [CrossRef]
- Srisomboon, C.; Koizumi, K.; Haraguchi, S.; Mikami, I.; Iijima, Y.; Shimizu, K. Thoracoscopic surgery for non-small-cell lung cancer: Elderly vs. octogenarians. Asian Cardiovasc. Thorac Ann. 2013, 21, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Fiore, J.F., Jr.; Bejjani, J.; Conrad, K.; Niculiseanu, P.; Landry, L.; Lee, l.; Ferri, L.E.; Feldman, L.S. Systematic review of the influence of enhanced recovery pathways in elective lung resection. J. Thorac. Cardiovasc. Surg. 2016, 151, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Bolliger, C.T.; Jordan, P.; Soler, M.; Stulz, P.; Gradel, E.; Skarvan, K.; Elsasser, S.; Gonon, M.; Wyser, C.; Tamm, M. Exercise Capacity as a predictor of postoperative complications in lung resection candidates. Am. J. Respir. Crit. Care Med. 1995, 151, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Agostini, P.; Naidu, B.; Rajesh, P.; Steyn, R.; Bishay, E.; Kalkat, M.; Singh, S. Potentially modifiable factors contribute to limitation in physical activity following thoracotomy and lung resection: A prospective observational study. J. Cardiothorac. Surg. 2014, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Landreneau, R.J.; Mack, M.J.; Hazelrigg, S.R.; Naunheim, K.; Dowling, R.D.; Ritter, P.; Magee, M.J.; Nunchuck, S.; Keenan, R.J.; Person, P.F. Prevalence of chronic pain after pulmonary resection by thoracotomy or video-assisted thoracic surgery. J. Thorac. Cardiovasc. Surg. 1994, 107, 1079–1085. [Google Scholar] [CrossRef]
- Nishiyama, O.; Taniguchi, H.; Kondoh, Y.; Kimura, T.; Ogawa, T.; Watanabe, F.; Arizono, S. Quadriceps weakness is related to exercise capacity in idiopathic pulmonary fibrosis. Chest 2005, 127, 2028–2033. [Google Scholar] [CrossRef]
- Jones, L.W.; Eves, N.D.; Haykowsky, M.; Freedland, S.J.; Mackey, J.R. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009, 10, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Titz, C.; Hummler, S.; Schmidt, M.E.; Thomas, M.; Steins, M.; Wiskemann, J. Exercise Behavior and physical fitness in patients with advanced lung cancer. Support. Care Cancer 2018, 26, 2725–2736. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.; Higginson, I.J. Pain experienced by lung cancer patients: A review of prevalence, causes and pathophysiology. Lung Cancer 2004, 43, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Carnio, S.; Di Stefano, R.F.; Novello, S. Fatigue in lung cancer patients: Symptom burden and management of challenges. Lung Cancer 2016, 7, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.Y.; Yorke, J.; Harle, A.; Smith, J.; Blackhall, F.; Pilling, M.; Molassiotis, A. Assessment of breathlessness in lung cancer: Psychometric properties of the Dyspnea-12 questionnaire. J. Pain. Symptom Manage 2017, 53, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; Haes, J.C.J.M.D.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Jones, L.W.; Hornsby, W.E.; Goetzinger, A.; Forbes, L.M.; Sherrard, E.L.; Quist, M.; Lane, A.T.; West, M.; Eves, N.D.; Gradison, M.; et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer 2012, 76, 248–252. [Google Scholar] [CrossRef]
- Wang, J.S.; Abboud, R.T.; Evans, K.G.; Finley, R.J.; Graham, B.L. Role of CO diffusing capacity during exercise in the preoperative evaluation for lung resection. Am. J. Respir. Crit. Care Med. 2000, 162 Pt 1, 1435–1444. [Google Scholar] [CrossRef]
- Brutsche, M.H.; Spiliopoulos, A.; Bolliger, C.T.; Licker, M.; Frey, J.G.; Tschopp, J.M. Exercise capacity and extent of resection as predictors of surgical risk in lung cancer. EurRespir J. 2000, 15, 828–832. [Google Scholar] [CrossRef]
- Almadana-Pacheco, V.; Luque-Crespo, E.; Gómez-Bastero-Fernández, A.P.; López- Porras, M.; Benito-Bernáldez, C.; Valido-Morales, A. Impacto de los niveles de actividad física en las complicaciones postquirúrgicas del cáncer de pulmón. Rev. Esp. Patol. Torac. 2018, 30, 181–188. [Google Scholar]
- Rodríguez-Torres, J.; Lucena-Aguilera, M.D.M.; Cabrera-Martos, I.; López-López, L.; Torres-Sánchez, I.; Valenza, M.C. Musculoskeletal Signs Associated with Shoulder Pain in Patients Undergoing Video-Assisted Thoracoscopic Surgery. Pain. Med. 2019, 20, 1997–2003. [Google Scholar] [CrossRef] [PubMed]
- Stefanelli, F.; Meoli, I.; Cobuccio, R.; Curcio, C.; Amore, D.; Casazza, D.; Tracey, M.; Rocco, G. High-intensity training and cardiopulmonary exercise testing in patients with chronic obstructive pulmonary disease and non-small-cell lung cancer undergoing lobectomy. Eur. J. Cardiothorac. Surg. 2013, 44, e260–e265. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, A.; Kim, A.W.; Berger, K.I.; Addrizzo-Harris, D.J. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. S5), e166S–e190S. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, A.; Charloux, A.; Bolliger, C.T.; Rocco, G.; Sculier, J.P.; Varela, G.; Licker, M.; Ferguson, M.K.; Faivre-Finn, C.; Huber, R.M.; et al. European Respiratory Society and European Society of Thoracic Surgeons joint tasforce on fitness for radical therapy. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur. Respir J. 2009, 34, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Baldwin, D.; Beckles, M.; Duffy, J.; Entwisle, J.; Faivre-Finn, C.; Kerr, K.; Macfie, A.; McGuigan, J.; Padley, S.; et al. Guidelines on the radical management of patients with lung cancer. Tórax 2010, 65, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.; Pirl, W.F.; et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N. Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Shi, Q.; Lu, C.; Basch, E.M.; Johnson, V.E.; Mendoza, T.R.; Mobley, G.M.; Cleeland, C.S. Prognostic value of the burden of symptoms for overall survival in patients receiving chemotherapy for advanced non-small cell lung cancer. Cancer 2010, 116, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Birim, O.; Maat, A.P.; Kappetein, A.P.; van Meerbeeck, J.P.; Damhuis, R.A.; Bogers, A.J. Validation of the Charlson comorbidity index in patients with operated primary non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2003, 23, 30–34. [Google Scholar] [CrossRef]
- Fillenbaum, G.G. Screening the elderly. A brief instrumental activities of daily living measure. J. Am. Geriatr. Soc. 1985, 33, 698–706. [Google Scholar] [CrossRef]
- Osoba, D.; Rodrigues, G.; Myles, J.; Zee, B.; Pater, J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 1998, 16, 139–144. [Google Scholar] [CrossRef]
- Tittle, M.B.; McMillan, S.C.; Hagan, S. Validating the brief pain inventory for use with surgical patients with cancer. Oncol. Nurs. Forum 2003, 30, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Birring, S.S.; Prudon, B.; Carr, A.J.; Singh, S.J.; Morgan, M.D.; Pavord, I.D. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003, 58, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Munoz, G.; Buxó, M.; de Gracia, J.; Olveira, C.; Martinez-Garcia, M.A.; Giron, R.; Polverino, E.; Alvarez, A.; Birring, S.S.; Vendrell, M. Validation of a Spanish version of the Leicester Cough Questionnaire in non-cystic fibrosis bronchiectasis. Chron. Respir. Dis. 2016, 13, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Krupp, L.B.; LaRocca, N.G.; Muir-Nash, J.; Steinberg, A.D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989, 46, 1121–1123. [Google Scholar] [CrossRef]
- O’Shea, S.D.; Taylor, N.F.; Paratz, J.D. Measuring muscle strength for people with chronic obstructive pulmonary disease: Retest reliability of hand-held dynamometry. Arch. Phys. Med. Rehabil. 2007, 88, 32–36. [Google Scholar] [CrossRef]
- Takahashi, T.; Jenkins, S.C.; Strauss, G.R.; Watson, C.P.; Lake, F.R. A new unsupported upper limb exercise test for patients with chronic obstructive pulmonary disease. J. Cardiopulm. Rehabil. 2003, 23, 430–437. [Google Scholar] [CrossRef]
- Borg, G. Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. Int. J. Sports Med. 1982, 3, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.J.; Yule, V.; Syddall, H.E.; Dennison, E.M.; Cooper, C.; Aihie Sayer, A. Is hand-held dynamometry useful for the measurement of quadriceps strength in older people? Gerontology 2006, 52, 154–159. [Google Scholar] [CrossRef]
- Dall, P.M.; Kerr, A. Frequency of the sit to stand task: An observational study of free-living adults. Appl. Ergon. 2010, 41, 58–61. [Google Scholar] [CrossRef]
- Nagamatsu, Y.; Maeshiro, K.; Kimura, N.Y.; Nishi, T.; Shima, I.; Yamana, H.; Shirouzu, K. Long-term recovery of exercise capacity and pulmonary function after lobectomy. J. Thorac. Cardiovasc. Surg. 2007, 134, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Schulte, T.; Schniewind, B.; Dohrmann, P.; Küchler, T.; Kurdow, R. The extent of lung parenchyma resection significantly impacts long-term quality of life in patients with non-small cell lung cancer. Chest 2009, 135, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Andy, J.R., Jr.; Asaph, J.W.; Skokan, L.; Reed, C.E.; Koh, S.; Brooks, G.; Charles, D.E.; Andrew, T.; Garry, O.; Gerard, S. What happens to patients undergoing lung cancer surgery? Outcomes and quality of life before and after surgery. Chest 2002, 122, 21–30. [Google Scholar]
- Kehlet, H.; Jensen, T.S.; Woolf, C.J. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006, 367, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Vagvolgyi, A.; Rozgonyi, Z.; Kerti, M.; Vadasz, P.; Varga, J. Effectiveness of perioperative pulmonary rehabilitation in thoracic surgery. J. Thorac. Dis. 2017, 9, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Poornima, K.N.; Karthick, N.; Sitalakshmi, R. Study of the effect of stress on skeletal muscle function in Geriatrics. J. Clin. Diagn. Res. 2014, 8, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Izawa, K.; Kasahara, Y.; Hiraki, K.; Hirano, Y.; Watanabe, S. Relation between the disability of the arm, shoulder and hand score and muscle strength in post-cardiac surgery patients. Diseases 2017, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Bobbio, A.; Chetta, A.; Carbognani, P.; Internullo, E.; Verduri, A.; Sansebastian, G.; Rusca, M.; Olivieri, D. Changes in pulmonary function test and cardio-pulmonary exercise capacity in COPD patients after lobar pulmonary resection. Eur. J. Cardiothorac. Surg. 2005, 28, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Barata, A.T.; Santos, C.; Cravo, M.; Vinhas, M.D.C.; Morais, C.; Carolino, E.; Vieira, J.R.; Fonseca, J. Handgrip dynamometry and Patient-Generated Subjective Global Assessment in patients with non resectable lung cancer. Nutr. Cancer 2017, 69, 154–158. [Google Scholar] [CrossRef]
- Martín-Salvador, A.; Torres-Sánchez, I.; Sáez-Roca, G.; López-Torres, I.; Rodríguez-Alzueta, E.; Valenza, M.C. Estudio del deterioro psicofísico y funcional en pacientes ingresados con neumonía. Análisis por grupos de edad. Arch. Bronconeumol. 2015, 51, 496–501. [Google Scholar] [CrossRef]
- Hsu, K.Y.; Lin, J.R.; Lin, M.S.; Chen, W.; Chen, Y.J.; Yan, Y.H. The modified Medical Research Council dyspnoea scale is a good indicator of health-related quality of life in patients with chronic obstructive pulmonary disease. Singap. Med. J. 2013, 54, 321–327. [Google Scholar] [CrossRef] [PubMed]
- McHorney, C.A.; Ware, J.E.; Lu, J.F.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med. Care. 1997, 31, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Hays, R.D.; Sherbourne, C.D.; Mazel, R.M. The RAND 36-Item Health Survey 1.0. Health Econ. 1993, 2, 217–227. [Google Scholar] [CrossRef]
- Ware, J., Jr.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef] [PubMed]
| Variables | Values (n = 88) | |
|---|---|---|
| Sex (% male) | 60.2 | |
| Age (years) | 59.33 ± 13.63 | |
| BMI (kg/m2) | 26.84 ± 4.42 | |
| Charlson index | 4.55 ± 2.51 | |
| FEV1% | 80.44 ± 23.63 | |
| Smoking habits n (%) | Non-smoker | 24 (27.3) |
| Smoker | 6 (7.0) | |
| Ex-smoker | 58 (65.7) | |
| Smoking habits frequency n (%) | <5 cigarettes/day | 36 (57.2) |
| >5 cigarettes/day | 28 (42.8) | |
| Type of tumor n (%) | Squamous carcinoma | 18 (20.5) |
| Adenocarcinoma | 41 (46.5) | |
| Metastatic | 21 (23.0) | |
| Unclassified | 8 (9.1) | |
| Type of intervention n (%) | Segmental resection | 36 (40.0) |
| Lobectomy | 50 (56.8) | |
| Pneumonectomy | 2 (2.2) | |
| Method of execution n (%) | VATS | 58 (65.9) |
| Thoracotomy | 30 (34.1) | |
| Type of anesthesia n (%) | General | 88 (100) |
| Local | 0 (0) | |
| Surgery duration (minutes) | 206.18 ± 71.52 | |
| Post-operating complications n (%) | 1 (1.1) | |
| Length of stay (days) | 6.82 ± 2.03 | |
| Variables | Pre-Surgery | Post-Surgery | p |
|---|---|---|---|
| Red blood cells | 4.65 ± 0.54 | 4.14 ± 0.79 | 0.016 * |
| Hemoglobin | 14.49 ± 4.08 | 11.82 ± 1.30 | 0.048 * |
| Hematocrit | 41.62 ± 5.27 | 36.37 ± 3.81 | 0.013 * |
| Leukocytes | 8.63 ± 4.69 | 12.49 ± 1.52 | 0.020 * |
| Platelet | 242.66 ± 66.05 | 221.71 ± 49.58 | 0.217 |
| pH | 7.38 ± 0.03 | 7.37 ± 0.05 | 0.423 |
| pCO2 | 43.46 ± 4.71 | 50.38 ± 16.80 | 0.031 * |
| pO2 | 105.31 ± 38.69 | 94.83 ± 32.41 | 0.247 |
| Pre-Surgery (n = 88) | Post-Surgery (n = 88) | p | ||
|---|---|---|---|---|
| Symptoms | ||||
| Dyspnea | 0.91 ± 2.09 | 1.61 ± 2.22 | 0.013 * | |
| Fatigue | 25.17 ± 19.05 | 30.65 ± 20.27 | 0.004 * | |
| Cough | 20.18 ± 2.24 | 18.38 ± 3.07 | <0.001 ** | |
| Pain | Severity | 2.89 ± 7.02 | 15.39 ± 9.91 | <0.001 ** |
| Interference | 4.08 ± 12.47 | 23.07 ± 20.61 | <0.001 ** | |
| Physical Fitness | ||||
| Dynamometry | Dominant hand (N) | 303.22 ±100.65 | 270.57 ± 105.82 | <0.001 ** |
| Dominant leg (N) | 114.41 ± 47.34 | 102.69 ± 49.26 | 0.001 * | |
| UULEX | Time (seconds) | 362.4 ± 282.74 | 108 ± 180.83 | <0.001 ** |
| Dyspnea post-test | 1 ± 2.04 | 1.23 ± 2.42 | 0.513 | |
| Fatigue post-test | 6.23 ± 2.48 | 5.62 ± 3.70 | 0.639 | |
| 5STS | Time (seconds) | 13.95 ± 9.63 | 17.87 ± 11.73 | <0.001 ** |
| Dyspnea post-test | 1.02 ± 2.23 | 2.22 ± 2.65 | 0.001 * | |
| Fatigue post-test | 1.14 ± 2.20 | 1.37 ± 2.42 | 0.462 | |
| Pre-Surgery (n = 88) | 1-Month Follow-Up (n = 85) | p | ||
|---|---|---|---|---|
| Symptoms | ||||
| Dyspnea | 1.1 ± 2.27 | 2.22 ± 2.94 | 0.007 * | |
| Fatigue | 25.66 ± 18.06 | 0.93 ± 0.76 | <0.001 ** | |
| Cough | 19.75 ± 2.68 | 19.42 ± 2.90 | 0.518 | |
| Pain | Severity | 2.61 ± 6.61 | 6.63 ± 7.88 | 0.002 * |
| Interference | 3.32 ± 9.67 | 10.46 ± 16.80 | 0.005 * | |
| Physical Fitness | ||||
| Dynamometry | Dominant hand (N) | 309.23 ± 97.45 | 279.91 ± 94.98 | 0.001 * |
| Dominant leg (N) | 116.98 ± 48.51 | 128.95 ± 46.99 | 0.082 | |
| UULEX | Time (seconds) | 404.35 ± 264.15 | 339.13 ± 229.97 | 0.023 * |
| Dyspnea post-test | 1.95 ± 2.85 | 3.05 ± 2.97 | 0.151 | |
| Fatigue post-test | 6.14 ± 2.47 | 5.81 ± 1.99 | 0.624 | |
| 5STS | Time (seconds) | 14.29 ± 11.03 | 14.45 ± 8.14 | 0.876 |
| Dyspnea post-test | 1.48 ± 2.30 | 1.21 ± 0.41 | 0.529 | |
| Fatigue post-test | 1.18 ± 2.09 | 1.6 ± 2.63 | 0.242 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heredia-Ciuró, A.; Quero-Valenzuela, F.; Martín-Núñez, J.; Calvache-Mateo, A.; Valenza-Peña, G.; López-López, L.; Valenza, M.C. Physical Deconditioning in Lung Cancer Patients Who Underwent Lung Resection Surgery in Spain: A Prospective Observational Study. Cancers 2024, 16, 2790. https://doi.org/10.3390/cancers16162790
Heredia-Ciuró A, Quero-Valenzuela F, Martín-Núñez J, Calvache-Mateo A, Valenza-Peña G, López-López L, Valenza MC. Physical Deconditioning in Lung Cancer Patients Who Underwent Lung Resection Surgery in Spain: A Prospective Observational Study. Cancers. 2024; 16(16):2790. https://doi.org/10.3390/cancers16162790
Chicago/Turabian StyleHeredia-Ciuró, Alejandro, Florencio Quero-Valenzuela, Javier Martín-Núñez, Andrés Calvache-Mateo, Geraldine Valenza-Peña, Laura López-López, and Marie Carmen Valenza. 2024. "Physical Deconditioning in Lung Cancer Patients Who Underwent Lung Resection Surgery in Spain: A Prospective Observational Study" Cancers 16, no. 16: 2790. https://doi.org/10.3390/cancers16162790
APA StyleHeredia-Ciuró, A., Quero-Valenzuela, F., Martín-Núñez, J., Calvache-Mateo, A., Valenza-Peña, G., López-López, L., & Valenza, M. C. (2024). Physical Deconditioning in Lung Cancer Patients Who Underwent Lung Resection Surgery in Spain: A Prospective Observational Study. Cancers, 16(16), 2790. https://doi.org/10.3390/cancers16162790








