The Current Landscape of Prostate-Specific Membrane Antigen (PSMA) Imaging Biomarkers for Aggressive Prostate Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Correlation between PSMA’s Expression and Prognosis in Localised Disease
2.1. Low or Negative Expression of PSMA
2.2. Heterogeneity in PSMA’s Expression
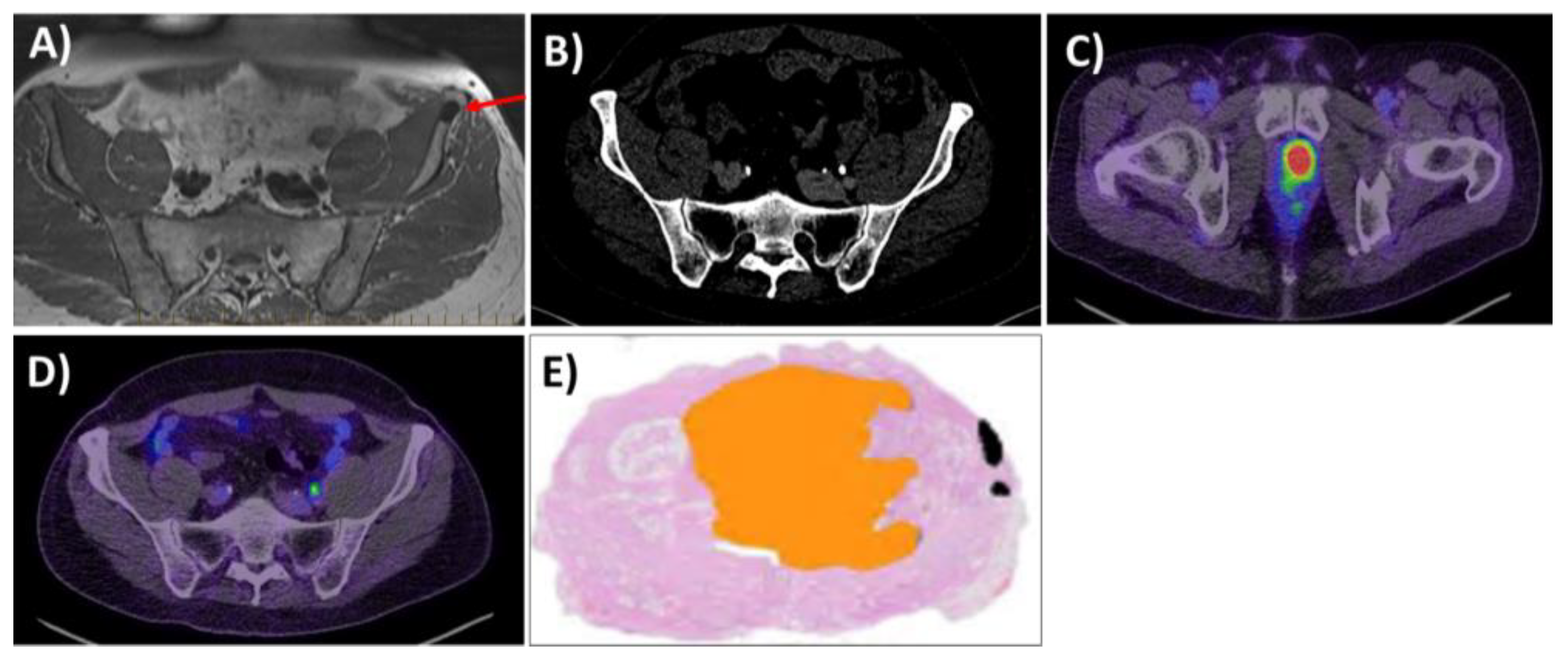
2.3. Expression of PSMA and Histological Correlations: Pretreatment Biomarker
2.4. Correlation between Pretreatment PSMA PET and BCR
3. PSMA PET Imaging-Directed Therapy and Measures of Treatment Response
3.1. PSMA PET/CT Directed External Beam Radiation Therapy (RT)
3.2. PSMA PET/CT and Hormonal Therapy—A Measure of Treatment Response
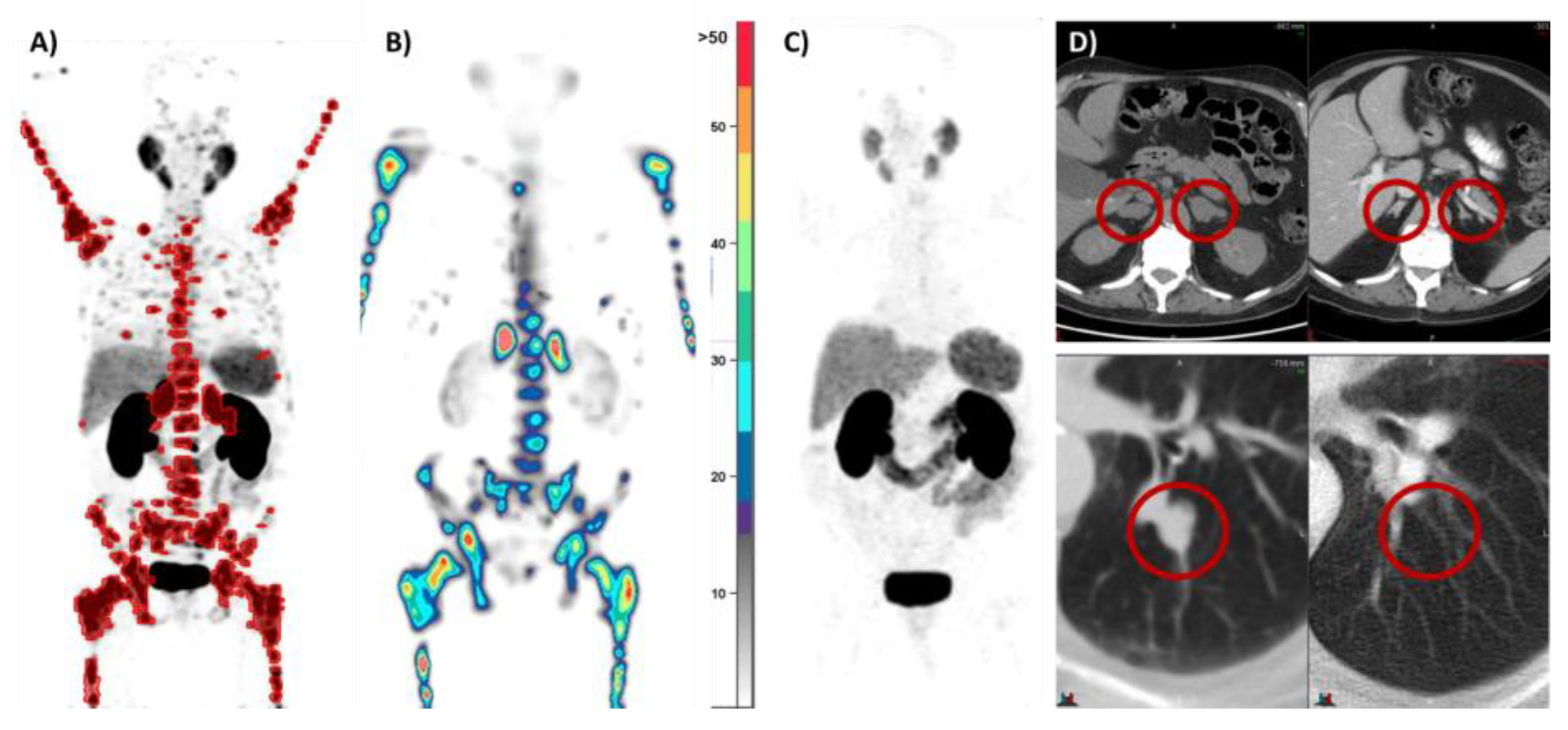
3.3. PSMA PET/CT SUVMax and Taxane Therapy
4. PSMA Radioligand Therapy (RLT)
| Title | Short Title | Trial Registration Code | Intervention | Disease Stage | Author | Trial Design | Primary Endpoint |
|---|---|---|---|---|---|---|---|
| Administering [177Lu]Lu-PSMA-617 Prior to Radical Prostatectomy in Men with High-Risk Localised Prostate Cancer (LuTectomy): A Single-Centre, Single-Arm, Phase 1/2 Study [14] | Lutectomy | NCT04430192 | Neoadjuvant [177Lu]Lu-PSMA-617 prior to robotic-assisted radical prostatectomy (RARP) | Localised PCa | Eapen | Single-centre, single-arm Phase I/II study | Dose of radiation absorbed by the tumour |
| Neoadjuvant 177Lu-PSMA-I&T Radionuclide Treatment in Patients with High-risk Prostate Cancer Before Radical Prostatectomy: A Single-Arm Phase 1 Trial [72] | NaLuProst | NCT04297410 | Neoadjuvant [177Lu]Lu-PSMA-I and -T prior to RARP | Localised PCa | Golan | Open-label, single-arm clinical trial | Safety, as defined by the rate of perioperative complications and the rate of functional toxicity |
| 177-Lutetium–PSMA Before Stereotactic Body Radiotherapy for the Treatment of Oligorecurrent Prostate Cancer (active) | LUNAR | NCT05496959 | [177Lu]Lu-PSMA-617, stereotactic ablative radiotherapy (SABR) | Oligorecurrent PCa | Kishan | Randomised prospective Phase II clinical trial | PSMA PET/CT radiological PFS |
| LuPSMA for Oligometastatic Prostate with STereotactic Ablative Radiotherapy: A Randomised Phase II Parallel Cohort Trial (POPSTAR II) (active) | POPSTAR II | NCT05560659 | [177Lu]Lu-PSMA-617, SABR | Oligometastatic PCa, mHSPC | Siva | Randomised prospective multicentre Phase II trial | Evaluating the PFS of SABR alone and SABR + 177Lu-PSMA |
| In Men With Metastatic Prostate Cancer, What is the Safety and Benefit of Lutetium-177 PSMA Radionuclide Treatment in Addition to Chemotherapy? (UpFrontPSMA) (active) | UpfrontPSMA | NCT04343885 | [177Lu]Lu-PSMA-617, docetaxel | Metastatic hormone-naive PCa | Azad | Randomised, two-arm, multicentre, Phase II clinical trial | Undetectable rate of PSA at 12 months after commencement of the protocol’s therapy |
| [177Lu]-PSMA-617 Radionuclide Treatment in Patients with Metastatic Castration-Resistant Prostate Cancer (LuPSMA trial): A Single-Centre, Single-Arm, Phase 2 study [29] | LuPSMA | ANZCTR 12615000912583 | [177Lu]Lu-PSMA-617 | mCRPC | Hofman | Single-arm, single-centre Phase II trial | PSA-50 response rate |
| Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer [62] | VISION | NCT03511664 | [177Lu]Lu-PSMA-617 | mCRPC | Sartor | International open-label Phase III trial | Imaging-based PFS and OS |
| [177Lu]Lu-PSMA-617 Versus Cabazitaxel in Patients with Metastatic Castration-Resistant Prostate Cancer (TheraP): A Randomised, Open-Label, Phase 2 trial [63] | TheraP | NCT03392428 | [177Lu]Lu-PSMA-617 | mCRPC | Hofman | Randomised, multicentre, unblinded Phase II trial | PSA-50 response rate |
| Prospective Phase 2 Trial of PSMA-Targeted Molecular Radiotherapy with 177Lu-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer (RESIST-PC): Efficacy Results of the UCLA Cohort [73] | RESIST-PC | NCT03042312 | [177Lu]Lu-PSMA-617 | mCRPC | Calais | Randomised prospective multicentre Phase II trial | PSA-50 response rate |
| 177Lu-PSMA-617 vs. Androgen Receptor-Directed Therapy in the Treatment of Progressive Metastatic Castration-Resistant Prostate Cancer (PSMAfore) (active) | PSMAfore | NCT04689828 | [177Lu]Lu-PSMA-617 | mCRPC or mHSPC in the taxane-naive setting | Sartor | Randomised open-label, multicenter Phase III clinical trial | Radiographic PFS |
| Enzalutamide With Lu PSMA-617 Versus Enzalutamide Alone in Men with Metastatic Castration-Resistant Prostate Cancer (ENZA-p) (active) | Enza-P | NCT04419402 | [177Lu]Lu-PSMA-617, enzalutamide | mCRPC | Emmett | Randomised prospective two-arm, multicentre Phase II clinical trial | PSA PFS |
| Evaluation of Radioligand Treatment in men with Metastatic Castration-Resistant Prostate Cancer With [161Tb]Tb-PSMA-I&T (VIOLET) (active) | VIOLET | NCT05521412 | [161Tb]Tb-PSMA-I and -T | mCRPC | Buteau | Prospective single-centre, single-arm, open-label Phase I/II trial | Dose-limiting toxicities, maximum tolerated dose, recommended Phase II dose, PSA-50 response rate |
| Cabazitaxel in Combination with 177Lu-PSMA-617 in Metastatic Castration-Resistant Prostate Cancer (LuCAB) (active) | LuCab | NCT05340374 | [177Lu]Lu-PSMA-617, cabazitaxel | mCRPC | Kostos | Prospective single-centre, single-arm, open-label Phase I/II trial | Dose-limiting toxicities, maximum tolerated dose, recommended phase II dose |
| Combination of Radium-223 and Lutetium-177 PSMA-I&T in Men with Metastatic Castration-Resistant Prostate Cancer (AlphaBet) (active) | Alphabet | NCT05383079 | [177Lu]Lu-PSMA-I and -T, radium-223 | mCRPC | Kostos | Prospective single-centre, single-arm, open-label Phase I/II trial | Dose-limiting toxicities, maximum tolerated dose, recommended Phase II dose, 50% PSA response rate |
| Single-Dose 177Lu-PSMA-617 Followed by Maintenance Pembrolizumab in Patients with Metastatic Castration-Resistant Prostate Cancer: An Open-Label, Dose-Expansion Phase 1 Trial (active) | N/A | NCT03805594 | [177Lu]Lu-PSMA-617, maintenance pembrolizumab | mCRPC | Aggarwal | Open-label, prospective, dose-expansion Phase I study | Part A: Phase II dose schedule of the treatment combination. Part B: objective response rate per investigator assessment by RECIST |
| PSMA-Lutetium Radionuclide Therapy and Immunotherapy in Prostate Cancer (active) | PRINCE | NCT03658447 | [177Lu]Lu-PSMA-617, pembrolizumab | mCRPC | Sandhu | Prospective single-centre Phase Ib/II study | PSA-50 response rate |
| Phase II Study of Radionuclide 177Lu-PSMA Therapy versus 177Lu-PSMA in Combination with Ipilimumab and Nivolumab for Men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) (active) | Evolution | NCT05150236 | [177Lu]Lu-PSMA-617, nivolumab, ipilimumab | mCRPC | Sandhu | Randomised prospective, multicentre Phase II study | PSA PFS at 1 year |
| 177 Lu-PSMA-617 Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Initial 254-Patient Results from a Prospective Registry (REALITY study) [74] | REALITY | NCT04833517 | [177Lu]Lu-PSMA-617 | mCRPC | Khreish | Registry-based study | PSA PFS, OS, caregiver-reported and patient-reported safety response to RLT |
| Prostate Cancer Theranostics and Imaging Centre of Excellence Compassionate Access Registry (active) | ProsTIC registry | NCT04769817 | [177Lu]Lu-PSMA-617 | mCRPC | Hofman | Registry-based study | N/A |
| 177Lu-PSMA-617 Therapy and Olaparib in Patients with Metastatic Castration Resistant Prostate Cancer (LuPARP) (active) | LuPARP | NCT03874884 | [177Lu]Lu-PSMA-617, olaparib | mCRPC | Sandhu | Open-label, multicentre, dose-escalation and dose-expansion Phase I study | Dose-limiting toxicities, maximum tolerated dose, recommended Phase II dose |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ARPIs | Androgen receptor pathway inhibitors |
| AS | Active surveillance |
| BCR | Biochemical recurrence |
| BCR-FS | Biochemical recurrence free survival |
| BgRT | Biology guided radiotherapy |
| CI | Conventional imaging |
| csPCa | Clinically significant prostate cancer |
| EANM | European Association of Nuclear Medicine |
| EAU | European Association of Urology |
| ePLND | Extended pelvic lymph node dissection |
| GBq | Gigabecquerel |
| GGG | Gleason grade group |
| Gy | Gray |
| IHC | Immunohistochemical |
| ISUP | International Society of Urological Pathologists |
| LN | Lymph node |
| MRI | Magnetic resonance imaging |
| mCRPC | Metastatic castration-resistant prostate cancer |
| mHSPC | Metastatic hormone-sensitive prostate cancer |
| OS | Overall survival |
| PCa | Prostate cancer |
| PFS | Progression-free survival |
| PMCC | Peter MacCallum Cancer Centre |
| PIRAD | Prostate Imaging–Reporting and Data System |
| PSA | Prostate-specific antigen |
| PSMA | Prostate-specific membrane antigen |
| RLT | Radioligand therapy |
| RARP | Robotic-assisted radical prostatectomy |
| RP | Radical prostatectomy |
| RT | Radiation therapy |
| sRT | Salvage radiation |
| SUV | Standard uptake value |
| SUVMax | Maximum standard uptake value |
| TV | Tumour volume |
References
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries from 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-Specific Membrane Antigen PET-CT in Patients with High-Risk Prostate Cancer before Curative-Intent Surgery or Radiotherapy (ProPSMA): A Prospective, Randomised, Multicentre Study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.E.; Feldman, A.R.; Coyle, J.T. Prostate-Specific Membrane Antigen Is a Hydrolase with Substrate and Pharmacologic Characteristics of a Neuropeptidase. Proc. Natl. Acad. Sci. USA 1996, 93, 749–753. [Google Scholar] [CrossRef] [PubMed]
- de Galiza Barbosa, F.; Queiroz, M.A.; Nunes, R.F.; Costa, L.B.; Zaniboni, E.C.; Marin, J.F.G.; Cerri, G.G.; Buchpiguel, C.A. Nonprostatic Diseases on PSMA PET Imaging: A Spectrum of Benign and Malignant Findings. Cancer Imaging 2020, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Lauri, C.; Chiurchioni, L.; Russo, V.M.; Zannini, L.; Signore, A. PSMA Expression in Solid Tumors beyond the Prostate Gland: Ready for Theranostic Applications? J. Clin. Med. 2022, 11, 6590. [Google Scholar] [CrossRef] [PubMed]
- Tsui, P.; Rubenstein, M.; Guinan, P. Correlation Between PSMA and VEGF Expression as Markers for LNCaP Tumor Angiogenesis. J. Biomed. Biotechnol. 2005, 2005, 287–290. [Google Scholar] [CrossRef]
- Bakht, M.K.; Derecichei, I.; Li, Y.; Ferraiuolo, R.-M.; Dunning, M.; Oh, S.W.; Hussein, A.; Youn, H.; Stringer, K.F.; Jeong, C.W.; et al. Neuroendocrine Differentiation of Prostate Cancer Leads to PSMA Suppression. Endocr. Relat. Cancer 2019, 26, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.; Iravani, A.; Hofman, M.S.; Hicks, R.J. Intra-Individual Comparison of 68Ga-PSMA-11 and 18F-DCFPyL Normal-Organ Biodistribution. Cancer Imaging 2019, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ong, S.; McKenzie, D.; Mirabelli, A.; Chen, D.C.; Chengodu, T.; Murphy, D.G.; Hofman, M.S.; Lawrentschuk, N.; Perera, M. Comparison of 18F-Based PSMA Radiotracers with [68Ga]Ga-PSMA-11 in PET/CT Imaging of Prostate Cancer—A Systematic Review and Meta-Analysis. Prostate Cancer Prostatic Dis. 2023. [Google Scholar] [CrossRef]
- Trabulsi, E.J.; Rumble, R.B.; Jadvar, H.; Hope, T.; Pomper, M.; Turkbey, B.; Rosenkrantz, A.B.; Verma, S.; Margolis, D.J.; Froemming, A.; et al. Optimum Imaging Strategies for Advanced Prostate Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 1963–1996. [Google Scholar] [CrossRef]
- Emmett, L.; Buteau, J.; Papa, N.; Moon, D.; Thompson, J.; Roberts, M.J.; Rasiah, K.; Pattison, D.A.; Yaxley, J.; Thomas, P.; et al. The Additive Diagnostic Value of Prostate-Specific Membrane Antigen Positron Emission Tomography Computed Tomography to Multiparametric Magnetic Resonance Imaging Triage in the Diagnosis of Prostate Cancer (PRIMARY): A Prospective Multicentre Study [Formula Presented]. Eur. Urol. 2021, 80, 682–689. [Google Scholar] [CrossRef]
- Pienta, K.J.; Gorin, M.A.; Rowe, S.P.; Carroll, P.R.; Pouliot, F.; Probst, S.; Saperstein, L.; Preston, M.A.; Alva, A.S.; Patnaik, A.; et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY). J. Urol. 2021, 206, 52–61. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 Prostate-Specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer—Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-Specific Membrane Antigen-Avid Lesions: A Systematic Review and Meta-Analysis. Eur. Urol. 2020, 77, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Eapen, R.S.; Buteau, J.P.; Jackson, P.; Mitchell, C.; Oon, S.F.; Alghazo, O.; McIntosh, L.; Dhiantravan, N.; Scalzo, M.J.; O’Brien, J.; et al. Administering [177Lu]Lu-PSMA-617 Prior to Radical Prostatectomy in Men with High-Risk Localised Prostate Cancer (LuTectomy): A Single-Centre, Single-Arm, Phase 1/2 Study. Eur. Urol. 2023. [Google Scholar] [CrossRef]
- Wright, G.L.; Mayer Grob, B.; Haley, C.; Grossman, K.; Newhall, K.; Petrylak, D.; Troyer, J.; Konchuba, A.; Schellhammer, P.F.; Moriarty, R. Upregulation of Prostate-Specific Membrane Antigen after Androgen-Deprivation Therapy. Urology 1996, 48, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xu, T.; Wang, X.; Yu, Y.-B.; Fan, Z.-Y.; Li, D.-X.; Luo, L.; Yang, X.-C.; Jiao, W.; Niu, H.-T. Diagnostic Performance of 68Gallium Labelled Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography and Magnetic Resonance Imaging for Staging the Prostate Cancer with Intermediate or High Risk Prior to Radical Prostatectomy: A Systematic Review and Meta-Analysis. World J. Men’s Health 2020, 38, 208. [Google Scholar] [CrossRef]
- Chow, K.M.; So, W.Z.; Lee, H.J.; Lee, A.; Yap, D.W.T.; Takwoingi, Y.; Tay, K.J.; Tuan, J.; Thang, S.P.; Lam, W.; et al. Head-to-Head Comparison of the Diagnostic Accuracy of Prostate-Specific Membrane Antigen Positron Emission Tomography and Conventional Imaging Modalities for Initial Staging of Intermediate- to High-Risk Prostate Cancer: A Systematic Review and Meta-Analysis. Eur. Urol. 2023, 84, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Jansen, B.H.E.; Bodar, Y.J.L.; Zwezerijnen, G.J.C.; Meijer, D.; van der Voorn, J.P.; Nieuwenhuijzen, J.A.; Wondergem, M.; Roeleveld, T.A.; Boellaard, R.; Hoekstra, O.S.; et al. Pelvic Lymph-Node Staging with 18F-DCFPyL PET/CT Prior to Extended Pelvic Lymph-Node Dissection in Primary Prostate Cancer—The SALT Trial. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 509–520. [Google Scholar] [CrossRef]
- Rischpler, C.; Beck, T.I.; Okamoto, S.; Schlitter, A.M.; Knorr, K.; Schwaiger, M.; Gschwend, J.; Maurer, T.; Meyer, P.T.; Eiber, M. 68Ga-PSMA-HBED-CC Uptake in Cervical, Celiac, and Sacral Ganglia as an Important Pitfall in Prostate Cancer PET Imaging. J. Nucl. Med. 2018, 59, 1406–1411. [Google Scholar] [CrossRef]
- Vetrone, L.; Mei, R.; Bianchi, L.; Giunchi, F.; Farolfi, A.; Castellucci, P.; Droghetti, M.; Presutti, M.; Degiovanni, A.; Schiavina, R.; et al. Histology and PSMA Expression on Immunohistochemistry in High-Risk Prostate Cancer Patients: Comparison with 68Ga-PSMA PET/CT Features in Primary Staging. Cancers 2023, 15, 1716. [Google Scholar] [CrossRef]
- Veerman, H.; Donswijk, M.; Bekers, E.; olde Heuvel, J.; Bodar, Y.J.L.; Boellaard, T.N.; van Montfoort, M.L.; van Moorselaar, R.J.A.; Oprea-Lager, D.E.; van Leeuwen, P.J.; et al. The Clinical Characteristics of Patients with Primary Non-Prostate-Specific Membrane Antigen-Expressing Prostate Cancer on Preoperative Positron Emission Tomography/Computed Tomography. BJU Int. 2022, 129, 314–317. [Google Scholar] [CrossRef]
- Rüschoff, J.H.; Ferraro, D.A.; Muehlematter, U.J.; Laudicella, R.; Hermanns, T.; Rodewald, A.K.; Moch, H.; Eberli, D.; Burger, I.A.; Rupp, N.J. What’s behind 68Ga-PSMA-11 Uptake in Primary Prostate Cancer PET? Investigation of Histopathological Parameters and Immunohistochemical PSMA Expression Patterns. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4042–4053. [Google Scholar] [CrossRef] [PubMed]
- Cytawa, W.; Kircher, S.; Kübler, H.; Werner, R.A.; Weber, S.; Hartrampf, P.; Bandurski, T.; Lass, P.; Połom, W.; Matuszewski, M.; et al. Diverse PSMA Expression in Primary Prostate Cancer: Reason for Negative [68Ga]Ga-PSMA PET/CT Scans? Immunohistochemical Validation in 40 Surgical Specimens. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3938–3949. [Google Scholar] [CrossRef] [PubMed]
- Bronsert, P.; Reichel, K.; Ruf, J. Loss of PSMA Expression in Non-Neuroendocrine Dedifferentiated Acinar Prostate Cancer. Clin. Nucl. Med. 2018, 43, 526–528. [Google Scholar] [CrossRef]
- Veerman, H.; Donswijk, M.; Bekers, E.; Bodar, Y.J.L.; Meijer, D.; van Moorselaar, R.J.A.; Oprea-Lager, D.E.; van der Noort, V.; van Leeuwen, P.J.; Vis, A.N.; et al. The Oncological Characteristics of non-prostate-specific Membrane Antigen (PSMA)-expressing Primary Prostate Cancer on Preoperative PSMA Positron Emission Tomography/Computed Tomography. BJU Int. 2022, 130, 750–753. [Google Scholar] [CrossRef]
- Heetman, J.G.; Lavalaye, J.; Polm, P.D.; Soeterik, T.F.W.; Wever, L.; Paulino Pereira, L.J.; van der Hoeven, E.J.R.J.; van Melick, H.H.E.; van den Bergh, R.C.N. Gallium-68 Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography in Active Surveillance for Prostate Cancer Trial (PASPoRT). Eur. Urol. Oncol. 2023. [Google Scholar] [CrossRef]
- Liu, J.; Santucci, J.; Woon, D.T.S.; Catterwell, R.; Perera, M.; Murphy, D.G.; Lawrentschuk, N. A Systematic Review on Prostate-Specific Membrane Antigen Positron Emission Tomography (PSMA PET) Evaluating Localized Low- to Intermediate-Risk Prostate Cancer: A Tool to Improve Risk Stratification for Active Surveillance? Life 2024, 14, 76. [Google Scholar] [CrossRef]
- Mannweiler, S.; Amersdorfer, P.; Trajanoski, S.; Terrett, J.A.; King, D.; Mehes, G. Heterogeneity of Prostate-Specific Membrane Antigen (PSMA) Expression in Prostate Carcinoma with Distant Metastasis. Pathol. Oncol. Res. 2009, 15, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [177Lu]-PSMA-617 Radionuclide Treatment in Patients with Metastatic Castration-Resistant Prostate Cancer (LuPSMA Trial): A Single-Centre, Single-Arm, Phase 2 Study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Thang, S.P.; Violet, J.; Sandhu, S.; Iravani, A.; Akhurst, T.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; Williams, S.G.; Hicks, R.J.; et al. Poor Outcomes for Patients with Metastatic Castration-Resistant Prostate Cancer with Low Prostate-Specific Membrane Antigen (PSMA) Expression Deemed Ineligible for 177Lu-Labelled PSMA Radioligand Therapy. Eur. Urol. Oncol. 2019, 2, 670–676. [Google Scholar] [CrossRef]
- Paschalis, A.; Sheehan, B.; Riisnaes, R.; Rodrigues, D.N.; Gurel, B.; Bertan, C.; Ferreira, A.; Lambros, M.B.K.; Seed, G.; Yuan, W.; et al. Prostate-Specific Membrane Antigen Heterogeneity and DNA Repair Defects in Prostate Cancer. Eur. Urol. 2019, 76, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Vaz, S.; Hadaschik, B.; Gabriel, M.; Herrmann, K.; Eiber, M.; Costa, D. Influence of Androgen Deprivation Therapy on PSMA Expression and PSMA-Ligand PET Imaging of Prostate Cancer Patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.A.; Truillet, C.; Ehman, E.C.; Afshar-Oromieh, A.; Aggarwal, R.; Ryan, C.J.; Carroll, P.R.; Small, E.J.; Evans, M.J. 68Ga-PSMA-11 PET Imaging of Response to Androgen Receptor Inhibition: First Human Experience. J. Nucl. Med. 2017, 58, 81–84. [Google Scholar] [CrossRef]
- Unterrainer, L.; Farolfi, A.; Rosar, F.; Santina Denis, C.; Emmett, L.; de Kouchkovsky, I.; Hope, T.A.; Hotta, M.; Gafita, A.; Djaileb, L. 5063 Poster Session Early Changes of PSMA PET Signal after Initiation of Androgen Receptor Signaling Inhibitors in MCRPC: An International Multicenter Retrospective Study. J. Clin. Oncol. 2023, 41, 5063. [Google Scholar] [CrossRef]
- Ceci, F.; Oprea-Lager, D.E.; Emmett, L.; Adam, J.A.; Bomanji, J.; Czernin, J.; Eiber, M.; Haberkorn, U.; Hofman, M.S.; Hope, T.A.; et al. E-PSMA: The EANM Standardized Reporting Guidelines v1.0 for PSMA-PET. Eur. J. Pediatr. 2021, 48, 1626–1638. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer. JAMA Oncol. 2019, 5, 856. [Google Scholar] [CrossRef]
- Uprimny, C.; Kroiss, A.S.; Decristoforo, C.; Fritz, J.; von Guggenberg, E.; Kendler, D.; Scarpa, L.; di Santo, G.; Roig, L.G.; Maffey-Steffan, J.; et al. 68Ga-PSMA-11 PET/CT in Primary Staging of Prostate Cancer: PSA and Gleason Score Predict the Intensity of Tracer Accumulation in the Primary Tumour. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 941–949. [Google Scholar] [CrossRef]
- Xue, A.L.; Kalapara, A.A.; Ballok, Z.E.; Levy, S.M.; Sivaratnam, D.; Ryan, A.; Ramdave, S.; O’Sullivan, R.; Moon, D.; Grummet, J.P.; et al. 68Ga-Prostate-Specific Membrane Antigen Positron Emission Tomography Maximum Standardized Uptake Value as a Predictor of Gleason Pattern 4 and Pathological Upgrading in Intermediate-Risk Prostate Cancer. J. Urol. 2022, 207, 341–349. [Google Scholar] [CrossRef]
- Demirci, E.; Kabasakal, L.; Şahin, O.E.; Akgün, E.; Gültekin, M.H.; Doǧanca, T.; Tuna, M.B.; Öbek, C.; Kiliç, M.; Esen, T.; et al. Can SUVmax Values of Ga-68-PSMA PET/CT Scan Predict the Clinically Significant Prostate Cancer? Nucl. Med. Commun. 2019, 40, 86–91. [Google Scholar] [CrossRef]
- Roberts, M.J.; Morton, A.; Donato, P.; Kyle, S.; Pattison, D.A.; Thomas, P.; Coughlin, G.; Esler, R.; Dunglison, N.; Gardiner, R.A.; et al. 68Ga-PSMA PET/CT Tumour Intensity Pre-Operatively Predicts Adverse Pathological Outcomes and Progression-Free Survival in Localised Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 477–482. [Google Scholar] [CrossRef]
- Buteau, J.P.; Moon, D.; Fahey, M.T.; Roberts, M.; Thompson, J.; Murphy, D.G.; Papa, N.; Mitchell, C.; Kasivisvanathan, V.; Stricker, P.; et al. PRIMARY2: A Phase III, Multi-Centre, Randomised Controlled Trial Investigating the Additive Diagnostic Value of [68Ga]Ga-PSMA-11 PET/CT in Men with Negative/Equivocal MRI in the Diagnosis of Clinically Significant Prostate Cancer. J. Clin. Oncol. 2023, 41, TPS397. [Google Scholar] [CrossRef]
- Hupe, M.C.; Philippi, C.; Roth, D.; Kümpers, C.; Ribbat-Idel, J.; Becker, F.; Joerg, V.; Duensing, S.; Lubczyk, V.H.; Kirfel, J.; et al. Expression of Prostate-Specific Membrane Antigen (PSMA) on Biopsies Is an Independent Risk Stratifier of Prostate Cancer Patients at Time of Initial Diagnosis. Front. Oncol. 2018, 8, 623. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Chen, M.; Yin, H.; Zhang, Q.; Li, H.; Guo, S.; Fu, Y.; Zang, S.; Ai, S.; Wang, F.; et al. Prediction of Biochemical Recurrence After Radical Prostatectomy Based on Preoperative 68Ga-PSMA-11 PET/CT. Front. Oncol. 2021, 11, 745530. [Google Scholar] [CrossRef]
- Cooperberg, M.R.; Pasta, D.J.; Elkin, E.P.; Litwin, M.S.; Latini, D.M.; Du Chane, J.; Carroll, P.R. The university of california, san francisco cancer of the prostate risk assessment score: A straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J. Urol. 2005, 173, 1938–1942. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Moul, J.; Carroll, P.R.; Sun, L.; Lubeck, D.; Chen, M.-H. Cancer-Specific Mortality After Surgery or Radiation for Patients With Clinically Localized Prostate Cancer Managed During the Prostate-Specific Antigen Era. J. Clin. Oncol. 2003, 21, 2163–2172. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Giatromanolaki, A.; Panteliadou, M.; Pouliliou, S.E.; Chondrou, P.S.; Mavropoulou, S.; Sivridis, E. Lactate Dehydrogenase 5 Isoenzyme Overexpression Defines Resistance of Prostate Cancer to Radiotherapy. Br. J. Cancer 2014, 110, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- De-Colle, C.; Yaromina, A.; Hennenlotter, J.; Thames, H.; Mueller, A.-C.; Neumann, T.; Stenzl, A.; Scharpf, M.; Fend, F.; Ricardi, U.; et al. Ex Vivo ΓH2AX Radiation Sensitivity Assay in Prostate Cancer: Inter-Patient and Intra-Patient Heterogeneity. Radiother. Oncol. 2017, 124, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, M.; Chang, D.; Hardcastle, N.; Jackson, P.; Kron, T.; Hofman, M.S.; Siva, S. Feasibility of Biology-Guided Radiotherapy Using PSMA-PET to Boost to Dominant Intraprostatic Tumour. Clin. Transl. Radiat. Oncol. 2022, 35, 84–89. [Google Scholar] [CrossRef]
- Zschaeck, S.; Lohaus, F.; Beck, M.; Habl, G.; Kroeze, S.; Zamboglou, C.; Koerber, S.A.; Debus, J.; Hölscher, T.; Wust, P.; et al. PSMA-PET Based Radiotherapy: A Review of Initial Experiences, Survey on Current Practice and Future Perspectives. Radiat. Oncol. 2018, 13, 90. [Google Scholar] [CrossRef]
- Djaïleb, L.; Armstrong, W.R.; Thompson, D.; Gafita, A.; Farolfi, A.; Rajagopal, A.; Grogan, T.R.; Nguyen, K.; Benz, M.R.; Hotta, M.; et al. Presurgical 68Ga-PSMA-11 Positron Emission Tomography for Biochemical Recurrence Risk Assessment: A Follow-up Analysis of a Multicenter Prospective Phase 3 Imaging Trial. Eur. Urol. 2023, 84, 588–596. [Google Scholar] [CrossRef]
- Wang, H.; Amiel, T.; Würnschimmel, C.; Langbein, T.; Steiger, K.; Rauscher, I.; Horn, T.; Maurer, T.; Weber, W.; Wester, H.-J.; et al. PSMA-Ligand Uptake Can Serve as a Novel Biomarker in Primary Prostate Cancer to Predict Outcome after Radical Prostatectomy. EJNMMI Res. 2021, 11, 76. [Google Scholar] [CrossRef]
- Spohn, S.K.B.; Farolfi, A.; Schandeler, S.; Vogel, M.M.E.; Ruf, J.; Mix, M.; Kirste, S.; Ceci, F.; Fanti, S.; Lanzafame, H.; et al. The Maximum Standardized Uptake Value in Patients with Recurrent or Persistent Prostate Cancer after Radical Prostatectomy and PSMA-PET-Guided Salvage Radiotherapy—A Multicenter Retrospective Analysis. Eur. J. Nucl. Med. Mol. Imaging 2022, 50, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Sonni, I.; Dal Pra, A.; O’Connell, D.P.; Ells, Z.; Benz, M.; Nguyen, K.; Yoon, S.M.; Deng, J.; Smith, C.; Grogan, T.; et al. 68Ga-PSMA PET/CT–Based Atlas for Prostate Bed Recurrence After Radical Prostatectomy: Clinical Implications for Salvage Radiation Therapy Contouring Guidelines. J. Nucl. Med. 2023, 64, 902–909. [Google Scholar] [CrossRef]
- Schiller, K.; Stöhrer, L.; Düsberg, M.; Borm, K.; Devecka, M.; Vogel, M.M.E.; Tauber, R.; Heck, M.M.; Rauscher, I.; Eiber, M.; et al. PSMA-PET/CT–Based Lymph Node Atlas for Prostate Cancer Patients Recurring After Primary Treatment: Clinical Implications for Salvage Radiation Therapy. Eur. Urol. Oncol. 2021, 4, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Smith-Jones, P.M.; Wongvipat, J.; Navarro, V.; Kim, S.; Bander, N.H.; Larson, S.M.; Sawyers, C.L. Noninvasive Measurement of Androgen Receptor Signaling with a Positron-Emitting Radiopharmaceutical That Targets Prostate-Specific Membrane Antigen. Proc. Natl. Acad. Sci. USA 2011, 108, 9578–9582. [Google Scholar] [CrossRef]
- Emmett, L.; Yin, C.; Crumbaker, M.; Hruby, G.; Kneebone, A.; Epstein, R.; Nguyen, Q.; Hickey, A.; Ihsheish, N.; O’Neill, G.; et al. Rapid Modulation of PSMA Expression by Androgen Deprivation: Serial 68Ga-PSMA-11 PET in Men with Hormone-Sensitive and Castrate-Resistant Prostate Cancer Commencing Androgen Blockade. J. Nucl. Med. 2019, 60, 950–954. [Google Scholar] [CrossRef]
- Vlachostergios, P.J.; Niaz, M.J.; Sun, M.; Mosallaie, S.A.; Thomas, C.; Christos, P.J.; Osborne, J.R.; Molina, A.M.; Nanus, D.M.; Bander, N.H.; et al. Prostate-Specific Membrane Antigen Uptake and Survival in Metastatic Castration-Resistant Prostate Cancer. Front. Oncol. 2021, 11, 630589. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Woo, S.; Kim, Y.; Lee, J.-L.; Wibmer, A.G.; Schoder, H.; Ryu, J.-S.; Vargas, H.A. Concordance between Response Assessment Using Prostate-Specific Membrane Antigen PET and Serum Prostate-Specific Antigen Levels after Systemic Treatment in Patients with Metastatic Castration Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Shagera, Q.A.; Artigas, C.; Karfis, I.; Critchi, G.; Chanza, N.M.; Sideris, S.; Peltier, A.; Paesmans, M.; Gil, T.; Flamen, P. 68Ga-PSMA PET/CT for Response Assessment and Outcome Prediction in Metastatic Prostate Cancer Patients Treated with Taxane-Based Chemotherapy. J. Nucl. Med. 2022, 63, 1191–1198. [Google Scholar] [CrossRef]
- Seifert, R.; Kessel, K.; Schlack, K.; Weber, M.; Herrmann, K.; Spanke, M.; Fendler, W.P.; Hadaschik, B.; Kleesiek, J.; Schäfers, M.; et al. PSMA PET Total Tumor Volume Predicts Outcome of Patients with Advanced Prostate Cancer Receiving [177Lu]Lu-PSMA-617 Radioligand Therapy in a Bicentric Analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1200–1210. [Google Scholar] [CrossRef]
- Emmett, L.; Willowson, K.; Violet, J.; Shin, J.; Blanksby, A.; Lee, J. Lutetium 177 PSMA Radionuclide Therapy for Men with Prostate Cancer: A Review of the Current Literature and Discussion of Practical Aspects of Therapy. J. Med. Radiat. Sci. 2017, 64, 52–60. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus Cabazitaxel in Patients with Metastatic Castration-Resistant Prostate Cancer (TheraP): A Randomised, Open-Label, Phase 2 Trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Hofman, M.S.; Emmett, L.; Calais, J.; Osborne, J.R.; Iravani, A.; Koo, P.; Lindenberg, L.; et al. Joint EANM/SNMMI Procedure Guideline for the Use of 177Lu-Labeled PSMA-Targeted Radioligand-Therapy (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2830–2845. [Google Scholar] [CrossRef]
- Buteau, J.P.; Martin, A.J.; Emmett, L.; Iravani, A.; Sandhu, S.; Joshua, A.M.; Francis, R.J.; Zhang, A.Y.; Scott, A.M.; Lee, S.-T.; et al. PSMA and FDG-PET as Predictive and Prognostic Biomarkers in Patients given [177Lu]Lu-PSMA-617 versus Cabazitaxel for Metastatic Castration-Resistant Prostate Cancer (TheraP): A Biomarker Analysis from a Randomised, Open-Label, Phase 2 Trial. Lancet Oncol. 2022, 23, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.; Kendi, A.T.; Sartor, O. Status of PSMA-Targeted Radioligand Therapy in Prostate Cancer: Current Data and Future Trials. Ther. Adv. Med. Oncol. 2023, 15, 175883592311576. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, S.; Yaginuma, K.; Meguro, S.; Onagi, A.; Matsuoka, K.; Hata, J.; Sato, Y.; Akaihata, H.; Kataoka, M.; Ogawa, S.; et al. PSMA Targeted Molecular Imaging and Radioligand Therapy for Prostate Cancer: Optimal Patient and Treatment Issues. Curr. Oncol. 2023, 30, 7286–7302. [Google Scholar] [CrossRef] [PubMed]
- Kaneda-Nakashima, K.; Shirakami, Y.; Kadonaga, Y.; Watabe, T.; Ooe, K.; Yin, X.; Haba, H.; Shirasaki, K.; Kikunaga, H.; Tsukada, K.; et al. Comparison of Nuclear Medicine Therapeutics Targeting PSMA among Alpha-Emitting Nuclides. Int. J. Mol. Sci. 2024, 25, 933. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Haberkorn, U.; Giesel, F.L. 225Ac-PSMA-617 for Therapy of Prostate Cancer. Semin. Nucl. Med. 2020, 50, 133–140. [Google Scholar] [CrossRef]
- Busslinger, S.D.; Tschan, V.J.; Richard, O.K.; Talip, Z.; Schibli, R.; Müller, C. [225Ac]Ac-SibuDAB for Targeted Alpha Therapy of Prostate Cancer: Preclinical Evaluation and Comparison with [225Ac]Ac-PSMA-617. Cancers 2022, 14, 5651. [Google Scholar] [CrossRef]
- Golan, S.; Frumer, M.; Zohar, Y.; Rosenbaum, E.; Yakimov, M.; Kedar, D.; Margel, D.; Baniel, J.; Steinmetz, A.P.; Groshar, D.; et al. Neoadjuvant 177Lu-PSMA-I&T Radionuclide Treatment in Patients with High-Risk Prostate Cancer Before Radical Prostatectomy: A Single-Arm Phase 1 Trial. Eur. Urol. Oncol. 2023, 6, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Calais, J.; Gafita, A.; Eiber, M.; Armstrong, W.R.; Gartmann, J.; Thin, P.; Nguyen, K.; Lok, V.; Gosa, L.; Grogan, T.; et al. Prospective Phase 2 Trial of PSMA-Targeted Molecular RadiothErapy with 177Lu-PSMA-617 for Metastatic Castration-ReSISTant Prostate Cancer (RESIST-PC): Efficacy Results of the UCLA Cohort. J. Nucl. Med. 2021, 62, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Khreish, F.; Ghazal, Z.; Marlowe, R.J.; Rosar, F.; Sabet, A.; Maus, S.; Linxweiler, J.; Bartholomä, M.; Ezziddin, S. 177 Lu-PSMA-617 Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Initial 254-Patient Results from a Prospective Registry (REALITY Study). Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1075–1085. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Saffar, H.; Chen, D.C.; Delgado, C.; Ingvar, J.; Hofman, M.S.; Lawrentschuk, N.; Perera, M.; Murphy, D.G.; Eapen, R. The Current Landscape of Prostate-Specific Membrane Antigen (PSMA) Imaging Biomarkers for Aggressive Prostate Cancer. Cancers 2024, 16, 939. https://doi.org/10.3390/cancers16050939
Al Saffar H, Chen DC, Delgado C, Ingvar J, Hofman MS, Lawrentschuk N, Perera M, Murphy DG, Eapen R. The Current Landscape of Prostate-Specific Membrane Antigen (PSMA) Imaging Biomarkers for Aggressive Prostate Cancer. Cancers. 2024; 16(5):939. https://doi.org/10.3390/cancers16050939
Chicago/Turabian StyleAl Saffar, Haidar, David C. Chen, Carlos Delgado, Jacob Ingvar, Michael S. Hofman, Nathan Lawrentschuk, Marlon Perera, Declan G. Murphy, and Renu Eapen. 2024. "The Current Landscape of Prostate-Specific Membrane Antigen (PSMA) Imaging Biomarkers for Aggressive Prostate Cancer" Cancers 16, no. 5: 939. https://doi.org/10.3390/cancers16050939
APA StyleAl Saffar, H., Chen, D. C., Delgado, C., Ingvar, J., Hofman, M. S., Lawrentschuk, N., Perera, M., Murphy, D. G., & Eapen, R. (2024). The Current Landscape of Prostate-Specific Membrane Antigen (PSMA) Imaging Biomarkers for Aggressive Prostate Cancer. Cancers, 16(5), 939. https://doi.org/10.3390/cancers16050939





